Sympathectomy Effects on Intra-Abdominal Organ Catecholamine Levels in a Streptozotocin-Induced Diabetic Rat Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Preparation
2.2. Experimental Design
2.3. Induction of Sympathectomy
2.4. Induction of Diabetes
2.5. Serum Biochemical Parameters
2.6. Catecholamine (CA) Analyses
2.7. Statistical Analysis
3. Results
3.1. Effect of Chemical Sympathectomy and DM on Blood Glucose, Triacylglycerides, Cholesterol, and Albumin
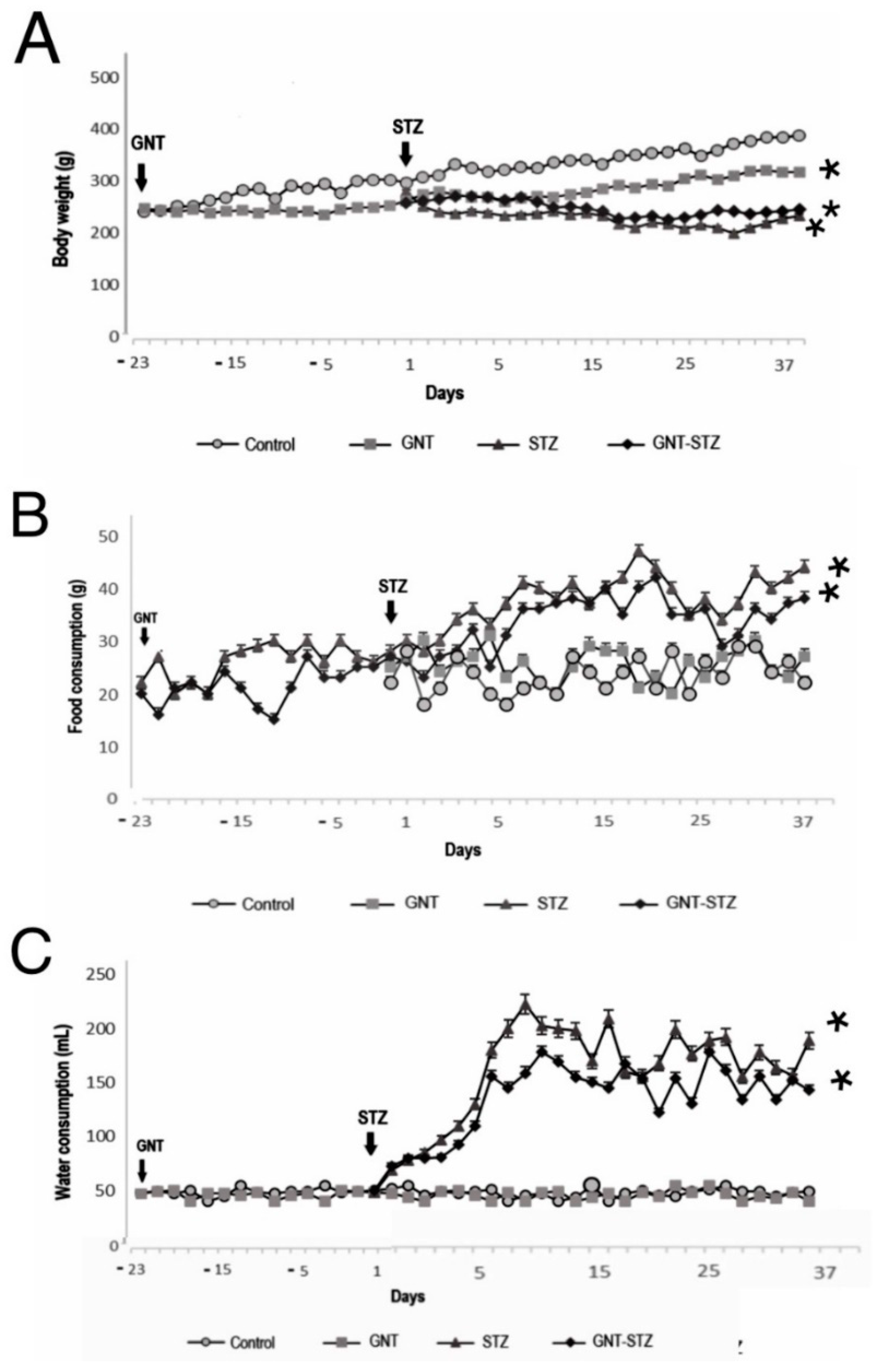
3.2. Effect of Chemical Sympathectomy and DM on Body Weight and Food and Water Intake
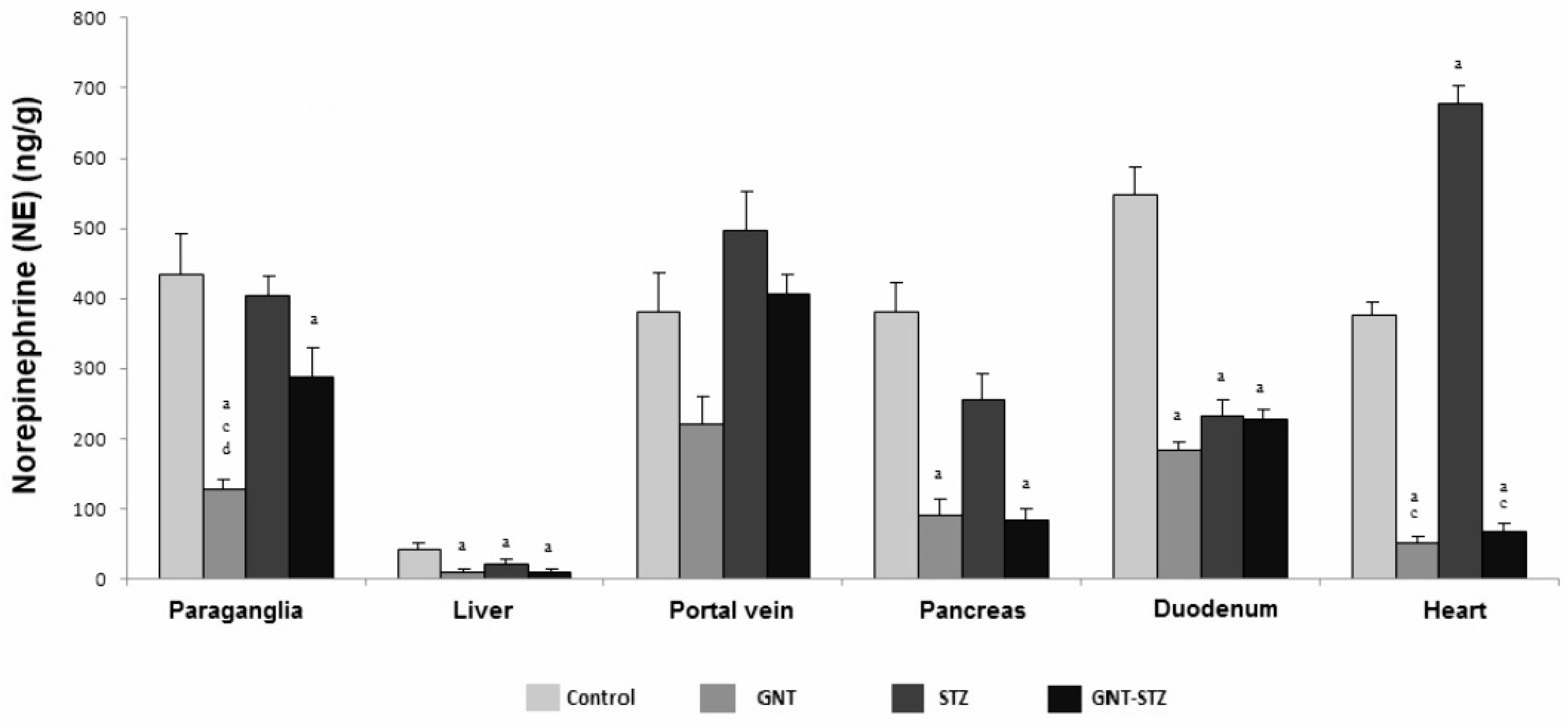
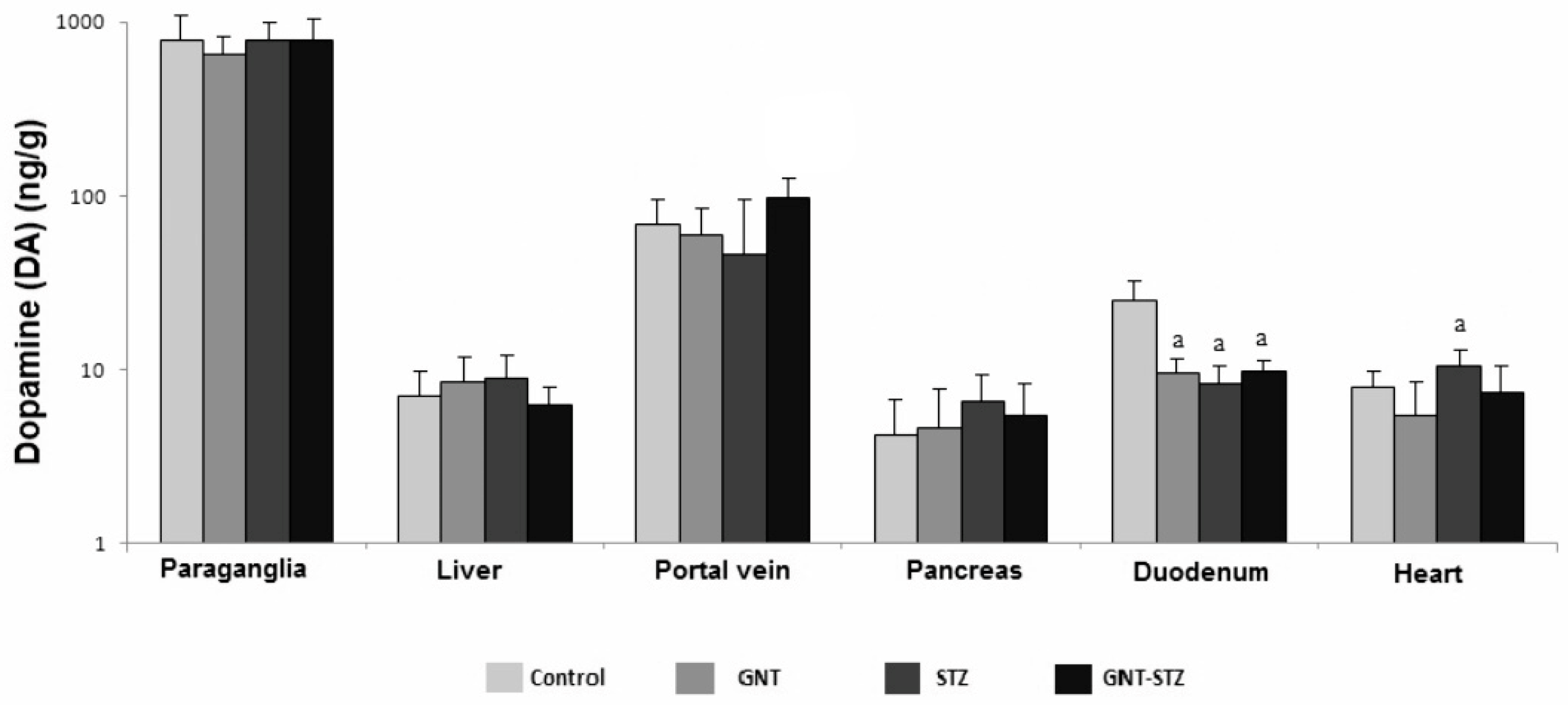
3.3. Concentration of CA in the Organs of the Splanchnic Area of Control, Guanetinized and Diabetic Rats
3.4. Concentration of CA in Adrenal Medulla
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. IDF Diabetes Atlas Committee. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.; Yang, X.; Sun, B.; Zhu, C. Perspectives of glycemic variability in diabetic neuropathy: A comprehensive review. Commun. Biol. 2021, 4, 1366. [Google Scholar] [CrossRef] [PubMed]
- Nalysnyk, L.; Hernandez-Medina, M.; Krishnarajah, G. Glycaemic variability and complications in patients with diabetes mellitus: Evidence from a systematic review of the literature. Diabetes Obes. Metab. 2010, 12, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Verrotti, A.; Prezioso, G.; Scattoni, R.; ChiarellI, F. Autonomic neuropathy in diabetes mellitus. Front. Endocrinol. 2014, 1, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jyotsna, V.P.; Sahoo, A.; Sreenivas, V.; Deepak, K.K. Prevalence and pattern of cardiac autonomic dysfunction in newly detected type 2 diabetes mellitus. Diabetes Res. Clin. Pract. 2009, 83, 83–88. [Google Scholar] [CrossRef]
- Paravati, S.; Warrington, S.J. Physiology, Catecholamines; StatPearls Publishing LLC: Treasure Island, FL, USA, 2019. [Google Scholar]
- Guy, D.A.; Sandoval, D.; Richardson, M.A.; Tate, D.; Flakoll, P.J.; Davis, S.N. Differing physiological effects of epinephrine in type 1 diabetes and nondiabetic humans. Am. J. Physiol. Endocrinol. Metab. 2005, 288, 178–186. [Google Scholar] [CrossRef]
- Thackeray, J.T.; Beanlands, R.S.; Dasilva, J.N. Altered sympathetic nervous system signaling in the diabetic heart: Emerging targets for molecular imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 314–334. [Google Scholar]
- Dhalla, N.S.; Ganguly, P.K.; Bhullar, S.K.; Tappia, P.S. Role of catecholamines in the pathogenesis of diabetic cardiomyopathy 1. Can. J. Physiol. Pharmacol. 2019, 97, 815–819. [Google Scholar] [CrossRef]
- Motataianu, A.; Barcutean, L.; Maier, S.; Balasa, A.; Stoian, A. Cardiac Autonomic Neuropathy in Diabetes Mellitus Patients Are We Aware of the Consequences? Acta Marisiensis Ser. Med. 2020, 66, 3–8. [Google Scholar] [CrossRef]
- Vinik, A.I.; Maser, R.E.; MitchelL, B.D.; Freeman, R. Diabetic autonomic neuropathy (Technical Review). Diabetes Care 2003, 26, 1553–1579. [Google Scholar] [CrossRef] [Green Version]
- Grassi, G.; Mark, A.; Esler, M. The sympathetic nervous system alterations in human hypertension. Circ. Res. 2015, 116, 976–990. [Google Scholar] [CrossRef] [PubMed]
- Bagyánszki, M.; Bódi, N. Diabetes-related alterations in the enteric nervous system and its microenvironment. World J. Diabetes 2012, 15, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Blommaart, P.J.; Ferwerda, G.; Kodde, A.; Tytgat, G.N.; Boeckxstaens, G.E. Nicotic acetylcholine receptor blocking effect of guanethidine in the rat gastric fundus. J. Pharmacol. 1999, 128, 903–908. [Google Scholar] [CrossRef] [Green Version]
- Akbarzadeh, A.; Norouzian, D.; Mehrabi, M.R.; Jamshidi, S.H.; Farhangi, A.; Verdi, A.A.; Mofidian, S.M.; Rad, B.L. Induction of diabetes by Streptozotocin in rats. Indian J. Clin. Biochem. 2007, 22, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- NORMA Oficial Mexicana NOM-062-ZOO-1999. Especificaciones Técnicas para La Producción, Cuidado y Uso de Los Animales de Laboratorio. 1999. Available online: http://www.fmvz.unam.mx/fmvz/principal/archivos/062ZOO.PDF (accessed on 3 January 2021).
- Eriksson, B.M.; Persson, B.A. Determination of catecholamines in rat heart tissue and plasma samples by liquid chromatography with electrochemical detection. J. Chromatogr. 1982, 228, 143–154. [Google Scholar] [CrossRef]
- Veeranjaneyulu, C.; Subrahmanyam, G.B. Rediscovered the induction of diabetogenic agents in the experimental animal model: Review. Int. J. Appl. Biol. Pharm. Technol. 2016, 7, 95–104. [Google Scholar] [CrossRef]
- Takada, J.; Machado, M.A.; Peres, S.B.; Brito, L.C.; Borges-Silva, C.N.; Costa, C.E.; Fonseca-Alaniz, M.H.; Andreotti, S.; Lima, F.B. Neonatal streptozotocin-induced diabetes mellitus: A model of insulin resistance associated with loss of adipose mass. Metabolism 2006, 56, 977–984. [Google Scholar] [CrossRef] [PubMed]
- Thackera, Y.J.; Radziuk, J.; Harper, M.E.; Suuronen, E.; Ascah, K.; Beanlands, R.; Dasilva, J. Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunction in a rat model of insulin resistance with hyperglycemia. Cardiovasc. Diabetol. 2011, 10, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.J.; Wu, J.S.; Lu, F.H.; Liu, I.M.; Chi, T.C.; Cheng, J.T. Sympathetic hyperactivity in wistar rats with insulin-resistence. J. Auton. Nerv. Syst. 1988, 11, 116–119. [Google Scholar] [CrossRef]
- Wang, J.; Wernette, C.M.; Judd, R.L.; Huggins, K.W.; White, B.D. Guanethidine treatment does not block the ability of central leptin administration to decrease blood glucose concentrations in streptozotocin-induced diabetic rats. J. Endocrinol. 2008, 198, 541–548. [Google Scholar] [CrossRef] [Green Version]
- Oettl, K.; Stauber, R.E. Physiological and pathological changes in the redox state of human serum albumin critically influence its binding properties. Br. J. Pharmacol. 2007, 15, 580–590. [Google Scholar] [CrossRef] [PubMed]
- Esler, M.; Rumantir, M.; Wiesner, G.; Kaye, D.; Hasting, J.; Lambert, G. Sympathetic nervous system and insulin resistance: From obesity to diabetes. Am. J. Hypertens. 2001, 14, 304–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorp, A.A.; Schlaich, M.P. Relevance of sympathetic nervous system activation in obesity and metabolic syndrome. J. Diabetes Res. 2015, 2015, 341583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brest, A.N.; Novack Kasparian-Hratch, P.; Moyer, J.H. Guanethidine. Chest 1962, 42, 359–363. [Google Scholar] [CrossRef]
- Demas, G.E.; Bartness, T.J. Novel method for localized, functional sympathetic nervous system denervation of peripheral tissue using guanethidine. J. Neurosci. Methods 2001, 112, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G.; Colombo, M.; Bolla, G.; Cattaneo, B.M.; Cavagnini, F.; Mancia, G. Body weight reduction, sympathetic nerve traffic, and arterial baroreflex in obese normotensive humans. Circulation 1998, 97, 2037–2042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambert, E.; Sari, C.I.; Dawood, T.; Nguyen, J.; Mcgrane, M.; Eikelis, N.; Chopra, R.; Wong, C.; Chatzivlastou, K.; Head, G.; et al. Sympathetic nervous system activity is associated with obesity-induced subclinical organ damage in young adults. Hypertension 2010, 56, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Bellush, L.L.; Reid, S.G.; North, D. The functional significance of biochemical alterations in streptozotocin-induced diabetes. Physiol. Behav. 1991, 50, 973–981. [Google Scholar] [CrossRef]
- Kassab, S.; Kato, T.; Wilkins, F.C.; Chen, R.; Hall, J.E.; Granger, J.P. Renal denervation attenuates the sodium retention and hypertension associated with obesity. Hypertension 1995, 25, 893–897. [Google Scholar] [CrossRef]
- Eisenhofer, G.; Aneman, A.; Hooper, D.; Holmes, C.; Goldstein, D.S.; Friberg, P. Production and metabolism of dopamine and norepinephrine in mesenteric organs and live of swine. Am. J. Physiol. 1995, 268, 641–649. [Google Scholar] [CrossRef] [Green Version]
- Eisenhofer, G.; Kopin, I.J.; Goldstein, D.S. Catecholamine metabolism: A contemporary view with implications for physiology and medicine. Pharmacol. Rev. 2004, 56, 331–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ross, L.L.; Pylipiw, A.; Mccarthy-Cosio, L. The effects of neonatal guanethidine treatment on the adrenal medulla of the rat. Annu. Meet. Soc. Neurosci. 1985, 11, 1154. Available online: https://eurekamag.com/research/029/343/029343039.php (accessed on 3 January 2021).
- Johnson, E.M.; O’Brien, F. Evaluation of the permanet sympathectomy produced by the administration of guanethidine to adult rats. J. Pharmacol. Exp. Ther. 1976, 196, 53–61. Available online: https://pubmed.ncbi.nlm.nih.gov/1517/ (accessed on 3 January 2021). [PubMed]
- Hall, J.E.; Brands, M.W.; Hildebrandt, D.A.; Kuo, J.; Fitzgerald, S. Role of sympathetic nervous system and neuropeptides in obesity hypertension. Braz. J. Med. Biol. Res. 2000, 33, 605–618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Frontoni, S.; Bracaglia, D.; Gigli, F. Relationship between autonomic dysfunction, insulin resistance and hypertension, in diabetes. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 441–449. [Google Scholar] [CrossRef] [PubMed]
- Grassi, G.; Seravalle, G. Autonomic imbalance and metabolic syndrome: Unravelling interactions, mechanisms and outcomes. J. Hypertens. 2006, 24, 47–49. [Google Scholar] [CrossRef]
- Berecek, K.H.; Brody, M.J. Evidence for a neurotransmitter role for epinephrine derived from the adrenal medulla. Am. J. Physiol. 1982, 242, 593–601. [Google Scholar] [CrossRef]
- Fuller, R.M.; Snoddy, H.D.; Perry, K.W. Dopamine accumulation alter dopamine B-hidroxilase inhibition in rat Herat as an index of norepinephrine turnover. Life Sci. 1982, 31, 563–570. [Google Scholar] [CrossRef]
- Pivonello, R.; Ferone, D.; DE Herder, W.W.; De Krijger, R.R.; Waaijers, M.; Mooij, D.M.; Van-Koetsveld, P.M.; Barreca, A.; De Caro, M.L.; Lombardi, G.; et al. Dopamine receptor expression and function in uman normal adrenal gland and adrenal tumors. J. Clin. Endocrinol. Metab. 2004, 89, 4493–4502. [Google Scholar] [CrossRef] [Green Version]
- Sonne, J.; Goyal, A.; Lopez-Ojeda, W. Dopamine; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Andén, N.E.; Grabowska-Andén, M.; Schwieler, J. Transfer of DOPA from the sympatho-adrenal system to the pancreas, liver and kidney via the blood circulation. Acta Physiol. Scand. 1989, 136, 75–79. [Google Scholar] [CrossRef] [PubMed]


| Group | Glucose (mg/dL) | Triacylglycerol (mg/dL) | Cholesterol (mg/dL) | Albumin (g/dL) |
|---|---|---|---|---|
| Control | 98 ± 0.9 b | 129.74 ± 2.8 b | 63.33 ± 0.9 b | 3.70 ± 0.15 a |
| GNT | 96 ± 1.2 b | 139.66 ± 7.4 b | 71.15 ± 5.9 b | 3.59 ± 0.19 a |
| STZ | 508 ± 2.8 a | 198.42 ± 3.2 a | 172.73 ± 2.4 a | 3.04 ± 0.28 b |
| GNT-STZ | 411 ± 1.9 a | 172.16 ± 9.6 a,b | 164.32 ± 7.0 a | 3.11 ± 0.13 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Juárez, A.; Aguirre-Pérez, A.G.; Barrientos-Alvarado, C. Sympathectomy Effects on Intra-Abdominal Organ Catecholamine Levels in a Streptozotocin-Induced Diabetic Rat Model. Life 2022, 12, 2147. https://doi.org/10.3390/life12122147
Pérez-Juárez A, Aguirre-Pérez AG, Barrientos-Alvarado C. Sympathectomy Effects on Intra-Abdominal Organ Catecholamine Levels in a Streptozotocin-Induced Diabetic Rat Model. Life. 2022; 12(12):2147. https://doi.org/10.3390/life12122147
Chicago/Turabian StylePérez-Juárez, Angélica, Andrea Giovanna Aguirre-Pérez, and Cornelio Barrientos-Alvarado. 2022. "Sympathectomy Effects on Intra-Abdominal Organ Catecholamine Levels in a Streptozotocin-Induced Diabetic Rat Model" Life 12, no. 12: 2147. https://doi.org/10.3390/life12122147
APA StylePérez-Juárez, A., Aguirre-Pérez, A. G., & Barrientos-Alvarado, C. (2022). Sympathectomy Effects on Intra-Abdominal Organ Catecholamine Levels in a Streptozotocin-Induced Diabetic Rat Model. Life, 12(12), 2147. https://doi.org/10.3390/life12122147





