Patterns of 25-Hydroxyvitamin D3, Calcium Status, and Anemia in the Saudi Population: A Cross-Sectional Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Statistical Analysis
3. Results
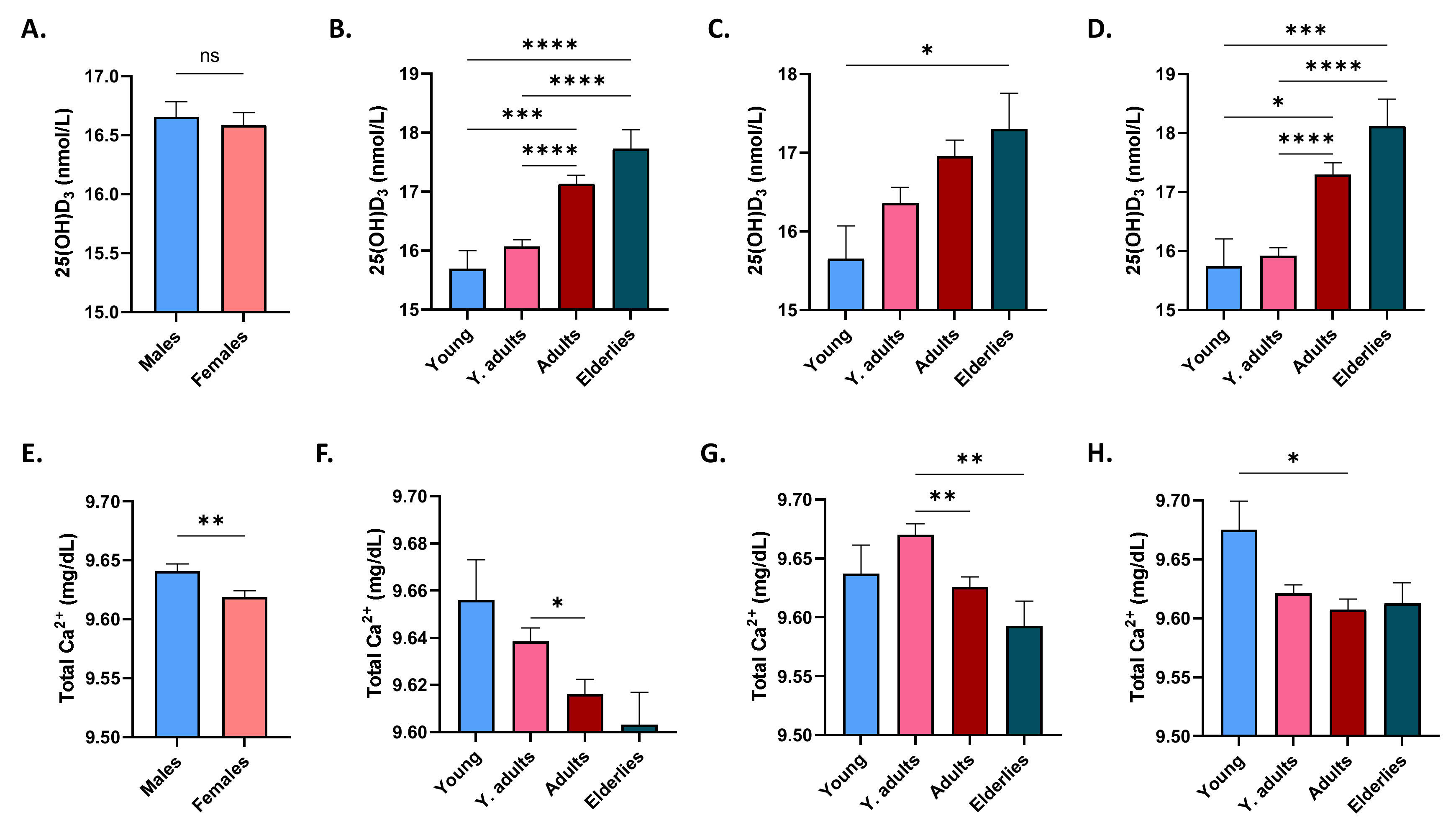
3.1. Effect of Gender and Age on 25(OH)D3 and Ca2+ Levels
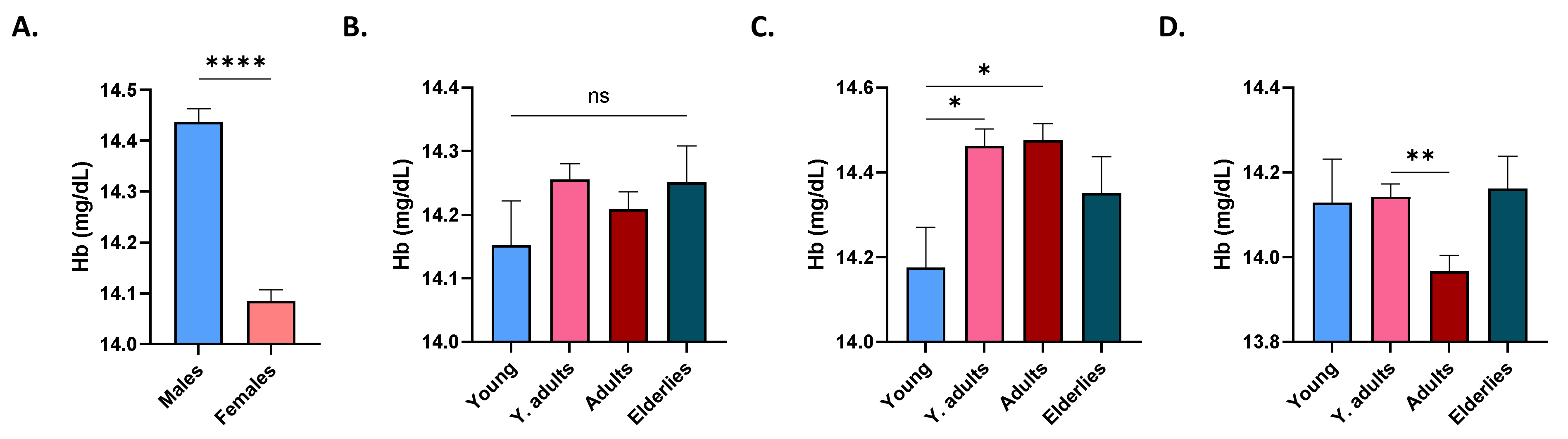
3.2. Effect of Gender and Age on Ferritin and Iron Levels
3.3. Distribution of Hb Level in Study Subjects
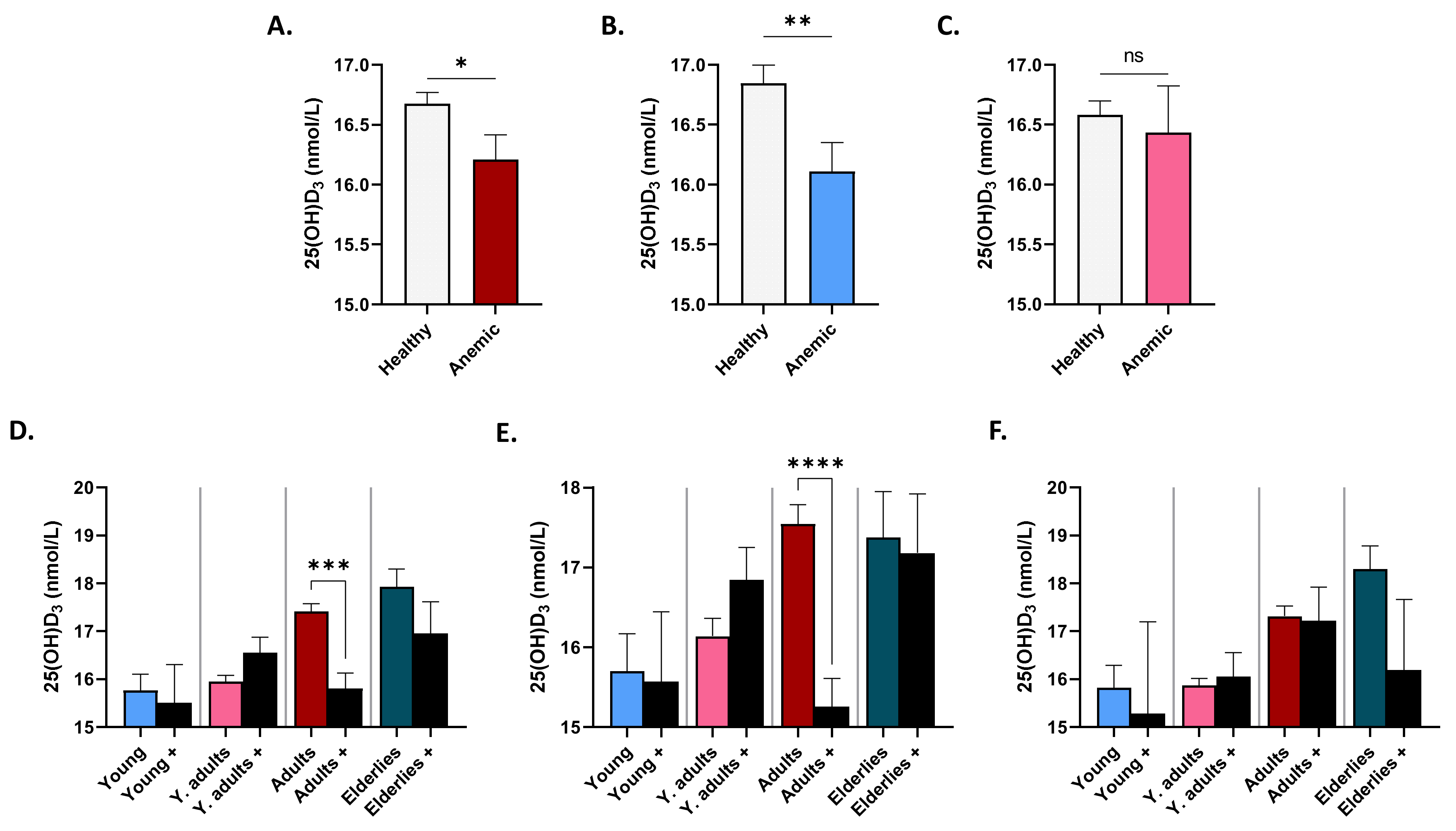
3.4. Patterns of Serum 25(OH)D3 in Anemic Subjects
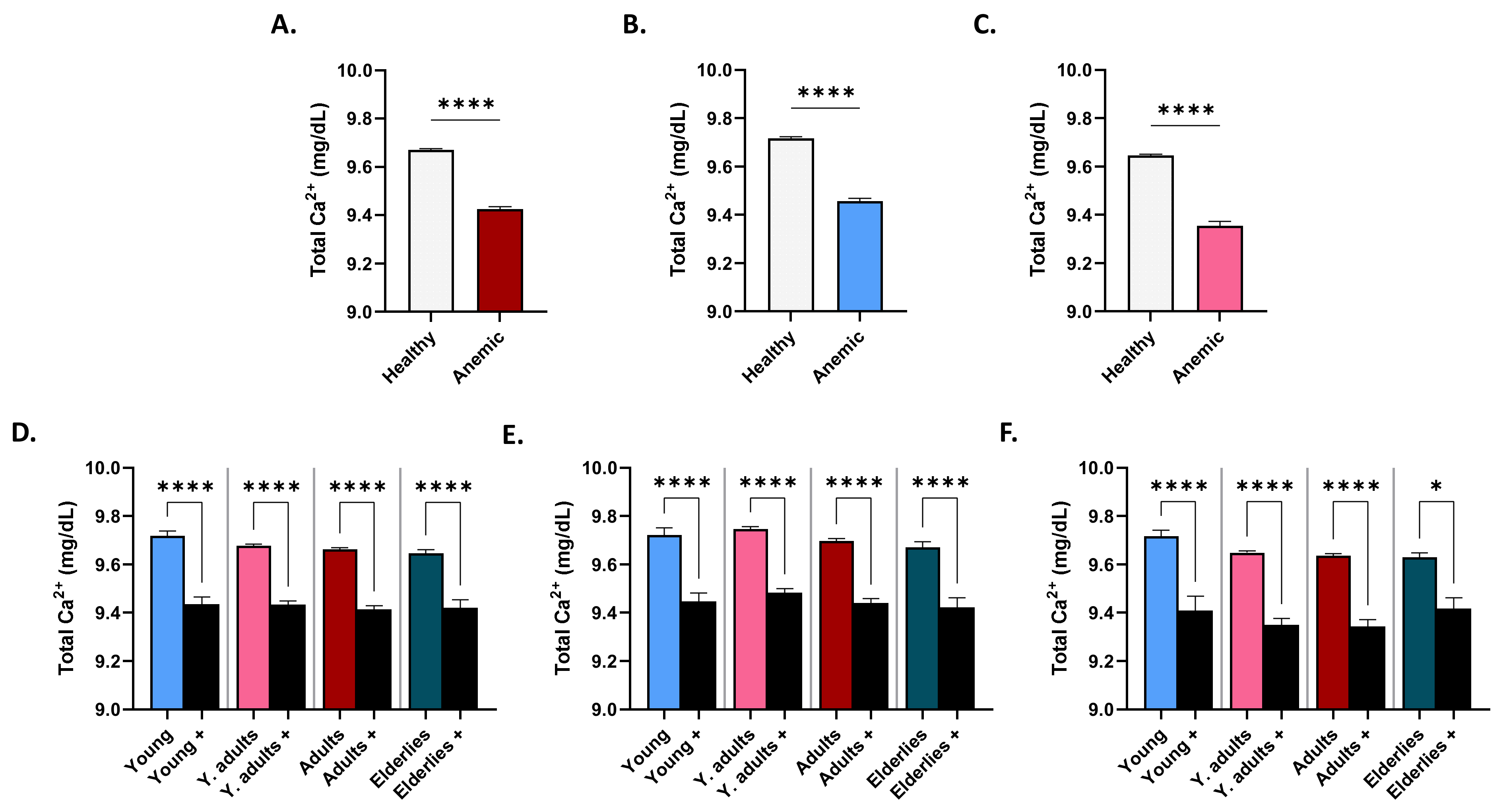
3.5. Patterns of Serum Ca2+ in Anemic Subjects
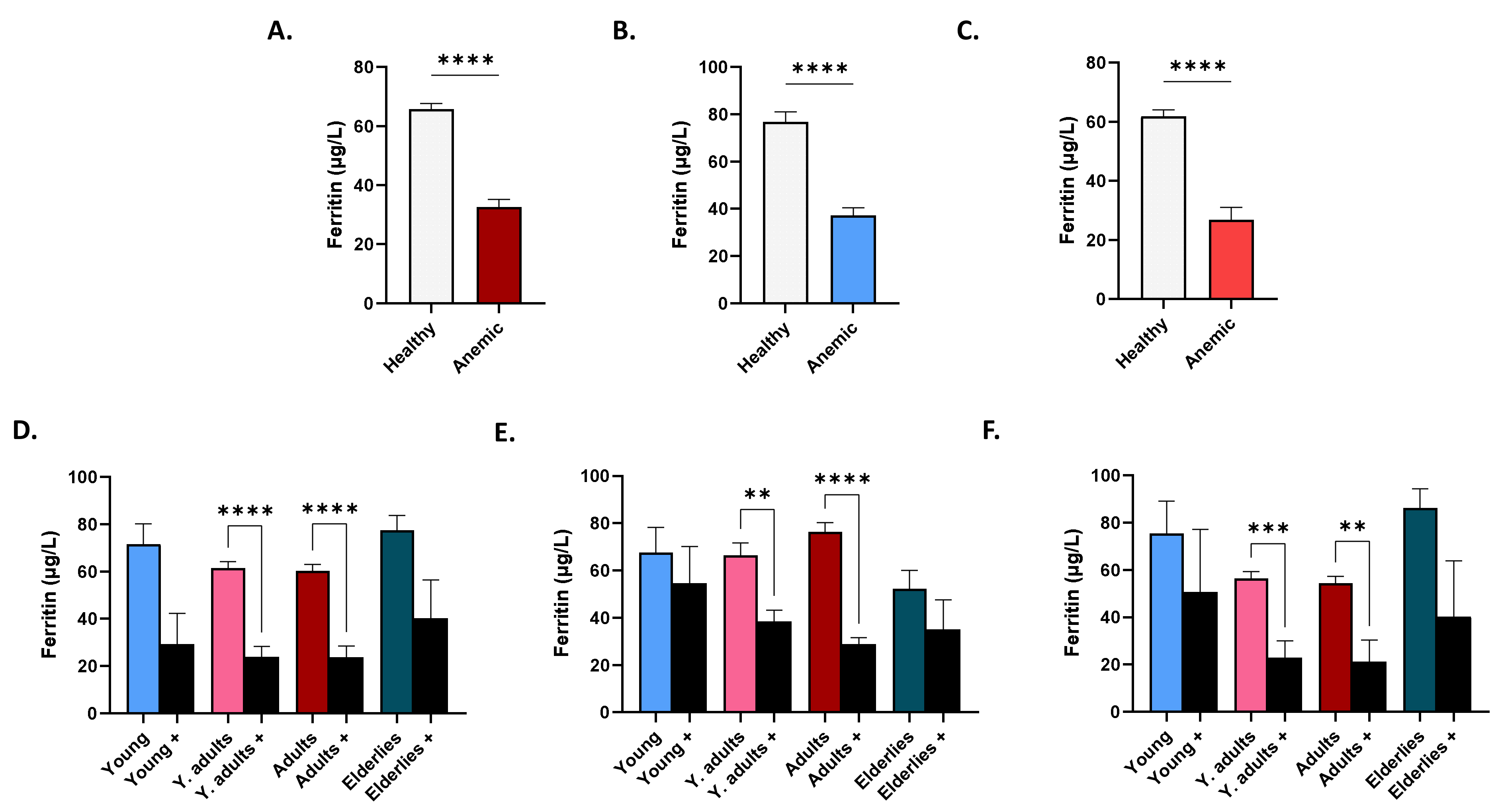
3.6. Patterns of Serum Ferritin in Anemic Subjects
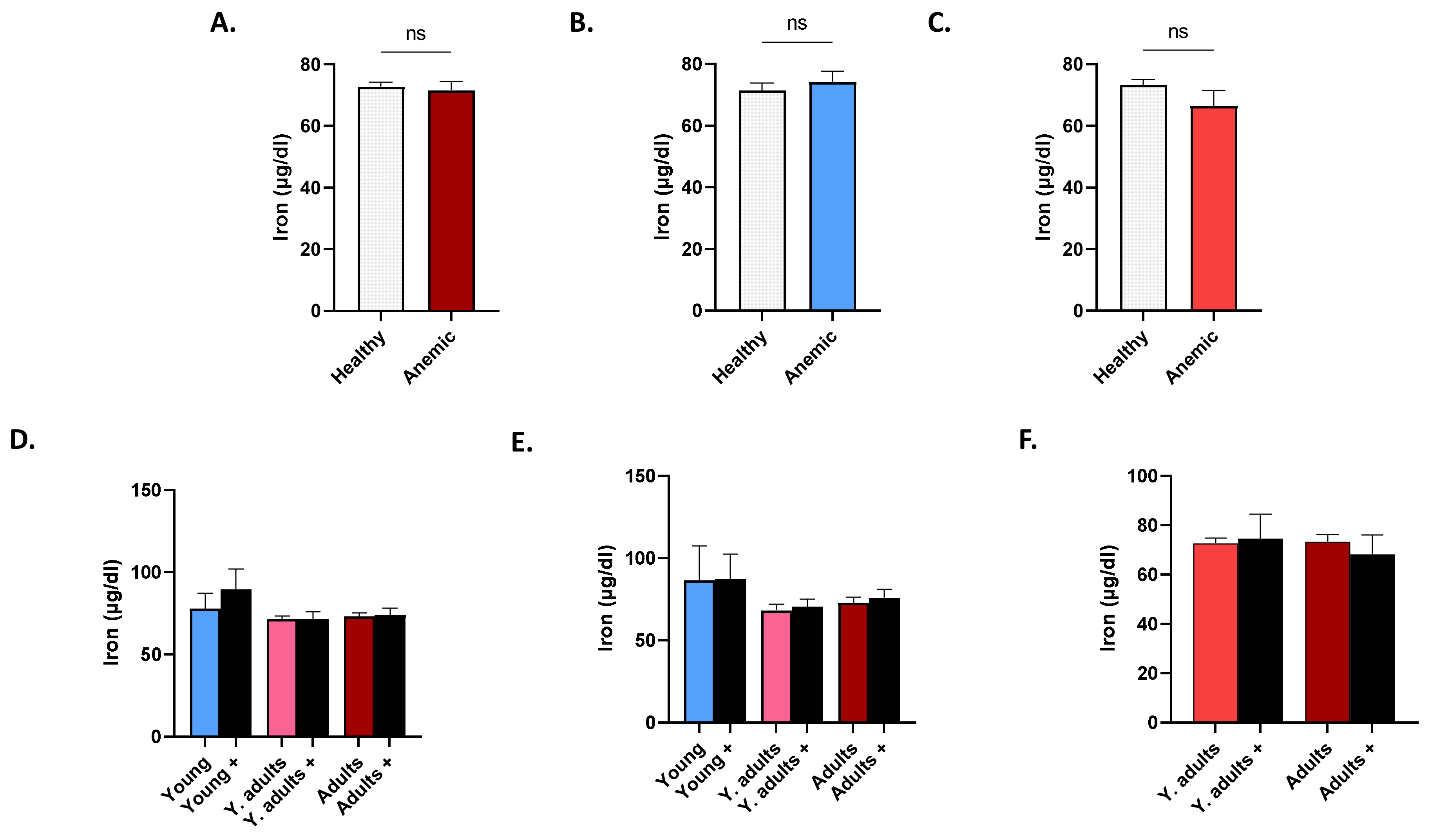
3.7. Patterns of Serum Iron in Anemic Subjects
3.8. Prevalence of Serum 25(OH)D3, Ca2+, Ferritin, and Iron Disturbances in Anemia
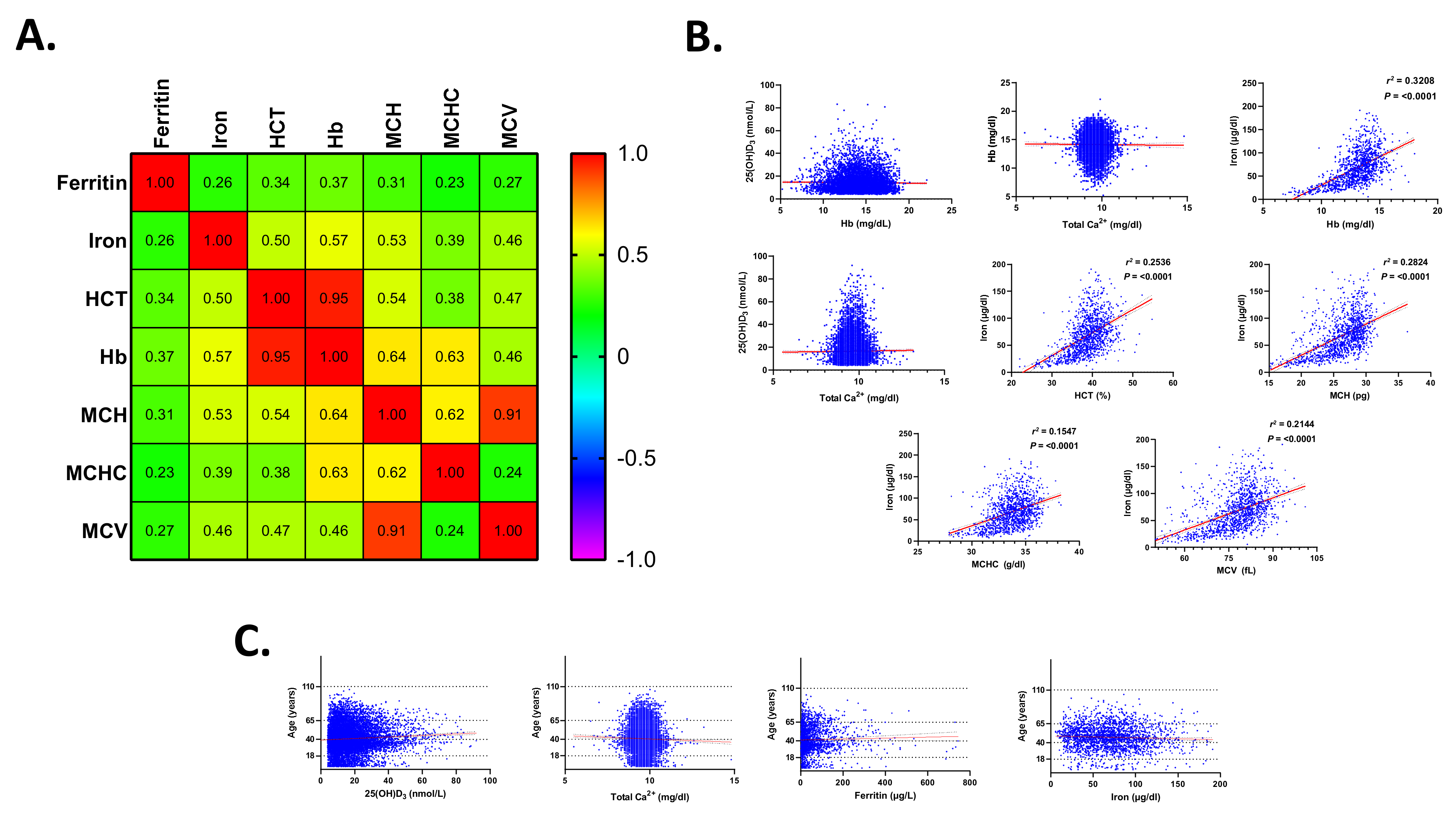
3.9. Association of Serum 25(OH)D3, Ca2+, Ferritin, and Iron Disturbances in Anemia
3.10. Risk Assessment of Serum 25(OH)D3, Total Ca2+, Ferritin, and Iron
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alsughayyir, J.; Alshaiddi, W.; Alsubki, R.; Alshammary, A.; Basudan, A.M.; Alfhili, M.A. Geraniin inhibits whole blood IFN-gamma and IL-6 and promotes IL-1beta and IL-8, and stimulates calcium-dependent and sucrose-sensitive erythrocyte death. Toxicol. Appl. Pharmacol. 2022, 436, 115881. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Anemia of inflammation. Hematol. Oncol. Clin. N. Am. 2014, 28, 671–681. [Google Scholar] [CrossRef] [PubMed]
- Al-Alyani, H.; Al-Turki, H.A.; Al-Essa, O.N.; Alani, F.M.; Sadat-Ali, M. Vitamin D deficiency in Saudi Arabians: A reality or simply hype: A meta-analysis (2008–2015). J. Fam. Community Med. 2018, 25, 1–4. [Google Scholar] [CrossRef]
- BinSaeed, A.A.; Torchyan, A.A.; AlOmair, B.N.; AlQadhib, N.S.; AlSuwayeh, F.M.; Monshi, F.M.; AlRumaih, F.I.; AlQahtani, S.A.; AlYousefi, N.; Al-Drees, A. Determinants of vitamin D deficiency among undergraduate medical students in Saudi Arabia. Eur. J. Clin. Nutr. 2015, 69, 1151–1155. [Google Scholar] [CrossRef] [PubMed]
- Tinawi, M. Disorders of Calcium Metabolism: Hypocalcemia and Hypercalcemia. Cureus 2021, 13, e12420. [Google Scholar] [CrossRef]
- Smith, E.M.; Tangpricha, V. Vitamin D and anemia: Insights into an emerging association. Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 432–438. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Alsughayyir, J.; Basudan, A.; Ghneim, H.K.; Aboul-Soud, M.A.M.; Marie, M.; Dera, A.; Alfaifi, M.; Alkhathami, A.G.; Awan, Z.A.; et al. Isolated and Combined Effect of Age and Gender on Neutrophil-Lymphocyte Ratio in the Hyperglycemic Saudi Population. Medicina 2022, 58, 1040. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Alsughayyir, J.; Basudan, A.M.; Alsubki, R.; Alqahtani, S.; Awan, Z.A.; Algethami, M.R.; Al-Sheikh, Y.A. Monocyte-Lymphocyte Ratio and Dysglycemia: A Retrospective, Cross-Sectional Study of the Saudi Population. Healthcare 2022, 10, 2289. [Google Scholar] [CrossRef]
- Almehmadi, M.; Salih, M.; Elmissbah, T.E.; Alsharif, A.; Alsiwiehri, N.; Alzahrani, K.; Shafie, A.; Dahlawi, H. Prevalence of anemia among Saudi patients with solid cancers at diagnosis in King Faisal Hospital, Taif Province, Kingdom of Saudi Arabia. PLoS ONE 2021, 16, e0246202. [Google Scholar] [CrossRef]
- Janus, J.; Moerschel, S.K. Evaluation of anemia in children. Am. Fam. Physician 2010, 81, 1462–1471. [Google Scholar]
- Goldstein, D.A. Clinical Methods: The History, Physical, and Laboratory Examinations. JAMA 1990, 264, 2808–2809. [Google Scholar]
- Briguglio, M.; Hrelia, S.; Malaguti, M.; Lombardi, G.; Riso, P.; Porrini, M.; Perazzo, P.; Banfi, G. The Central Role of Iron in Human Nutrition: From Folk to Contemporary Medicine. Nutrients 2020, 12, 1761. [Google Scholar] [CrossRef] [PubMed]
- Alfhili, M.A.; Alsughayyir, J.; Basudan, A.M.; Ghneim, H.K.; Alfaifi, M.; Alamri, H.S.; Awan, Z.A.; Algethami, M.R. Patterns of Dyslipidemia in the Anemic and Nonanemic Hypertensive Saudi Population: A Cross-Sectional Study. Int. J. Gen. Med. 2022, 15, 7895–7906. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, J.C. Vitamin D and aging. Endocrinol. Metab. Clin. N. Am. 2013, 42, 319–332. [Google Scholar] [CrossRef] [PubMed]
- Rock, C.L. Multivitamin-multimineral supplements: Who uses them? Am. J. Clin. Nutr. 2007, 85, 277S–279S. [Google Scholar] [CrossRef]
- Yin, K.; Agrawal, D.K. Vitamin D and inflammatory diseases. J. Inflamm. Res. 2014, 7, 69–87. [Google Scholar] [CrossRef]
- Boronat, M.; Santana, A.; Bosch, E.; Lorenzo, D.; Riano, M.; Garcia-Canton, C. Relationship between Anemia and Serum Concentrations of Calcium and Phosphorus in Advanced Non-Dialysis-Dependent Chronic Kidney Disease. Nephron 2017, 135, 97–104. [Google Scholar] [CrossRef]
- Holick, M.F. The vitamin D epidemic and its health consequences. J. Nutr. 2005, 135, 2739S–2748S. [Google Scholar] [CrossRef]
- Shirazi, L.; Almquist, M.; Malm, J.; Wirfalt, E.; Manjer, J. Determinants of serum levels of vitamin D: A study of life-style, menopausal status, dietary intake, serum calcium, and PTH. BMC Women’s Health 2013, 13, 33. [Google Scholar] [CrossRef]
- Lotito, A.; Teramoto, M.; Cheung, M.; Becker, K.; Sukumar, D. Serum Parathyroid Hormone Responses to Vitamin D Supplementation in Overweight/Obese Adults: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Nutrients 2017, 9, 241. [Google Scholar] [CrossRef]
- Frelut, M.L.; Girardet, J.P.; Bocquet, A.; Briend, A.; Chouraqui, J.P.; Darmaun, D.; Dupont, C.; Feillet, F.; Hankard, R.; Roze, J.C.; et al. Impact of obesity on biomarkers of iron and vitamin D status in children and adolescents: The risk of misinterpretation. Arch. Pediatr. 2018, 25, 3–5. [Google Scholar] [CrossRef] [PubMed]
- Moslehi, N.; Shab-Bidar, S.; Mirmiran, P.; Hosseinpanah, F.; Azizi, F. Determinants of parathyroid hormone response to vitamin D supplementation: A systematic review and meta-analysis of randomised controlled trials. Br. J. Nutr. 2015, 114, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Mesinovic, J.; Mousa, A.; Wilson, K.; Scragg, R.; Plebanski, M.; de Courten, M.; Scott, D.; Naderpoor, N.; de Courten, B. Effect of 16-weeks vitamin D replacement on calcium-phosphate homeostasis in overweight and obese adults. J. Steroid Biochem. Mol. Biol. 2019, 186, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Rolighed, L.; Rejnmark, L.; Sikjaer, T.; Heickendorff, L.; Vestergaard, P.; Mosekilde, L.; Christiansen, P. Vitamin D treatment in primary hyperparathyroidism: A randomized placebo controlled trial. J. Clin. Endocrinol. Metab. 2014, 99, 1072–1080. [Google Scholar] [CrossRef]
- Nur-Eke, R.; Ozen, M. The Relationship between Vitamin D Levels and Iron Deficiency and Anemia in Adults Applied for Periodic Medical Examination. Clin. Lab. 2020, 66, 1019–1026. [Google Scholar] [CrossRef]
- El-Adawy, E.H.; Zahran, F.E.; Shaker, G.A.; Seleem, A. Vitamin D Status in Egyptian Adolescent Females with Iron Deficiency Anemia and Its Correlation with Serum Iron Indices. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 519–525. [Google Scholar] [CrossRef]
- Malczewska-Lenczowska, J.; Sitkowski, D.; Surala, O.; Orysiak, J.; Szczepanska, B.; Witek, K. The Association between Iron and Vitamin D Status in Female Elite Athletes. Nutrients 2018, 10, 167. [Google Scholar] [CrossRef]
- Johnson, L.K.; Hofso, D.; Aasheim, E.T.; Tanbo, T.; Holven, K.B.; Andersen, L.F.; Roislien, J.; Hjelmesaeth, J. Impact of gender on vitamin D deficiency in morbidly obese patients: A cross-sectional study. Eur. J. Clin. Nutr. 2012, 66, 83–90. [Google Scholar] [CrossRef]
- Bolland, M.J.; Grey, A.B.; Ames, R.W.; Mason, B.H.; Horne, A.M.; Gamble, G.D.; Reid, I.R. The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am. J. Clin. Nutr. 2007, 86, 959–964. [Google Scholar] [CrossRef]
- Maeda, S.S.; Kunii, I.S.; Hayashi, L.F.; Lazaretti-Castro, M. Increases in summer serum 25-hydroxyvitamin D (25OHD) concentrations in elderly subjects in Sao Paulo, Brazil vary with age, gender and ethnicity. BMC Endocr. Disord. 2010, 10, 12. [Google Scholar] [CrossRef]
- Galan, P.; Yoon, H.C.; Preziosi, P.; Viteri, F.; Valeix, P.; Fieux, B.; Briancon, S.; Malvy, D.; Roussel, A.M.; Favier, A.; et al. Determining factors in the iron status of adult women in the SU.VI.MAX study. SUpplementation en VItamines et Mineraux AntioXydants. Eur. J. Clin. Nutr. 1998, 52, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Harris, S.S. The effect of calcium consumption on iron absorption and iron status. Nutr. Clin. Care 2002, 5, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Zitt, E.; Sprenger-Mahr, H.; Mundle, M.; Lhotta, K. Efficacy and safety of body weight-adapted oral cholecalciferol substitution in dialysis patients with vitamin D deficiency. BMC Nephrol. 2015, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Abbas, M.A. Physiological functions of Vitamin D in adipose tissue. J. Steroid Biochem. Mol. Biol. 2017, 165 Pt B, 369–381. [Google Scholar] [CrossRef]
- Pereira-Santos, M.; Costa, P.R.; Assis, A.M.; Santos, C.A.; Santos, D.B. Obesity and vitamin D deficiency: A systematic review and meta-analysis. Obes. Rev. 2015, 16, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Azizi-Soleiman, F.; Vafa, M.; Abiri, B.; Safavi, M. Effects of Iron on Vitamin D Metabolism: A Systematic Review. Int. J. Prev. Med. 2016, 7, 126. [Google Scholar] [CrossRef]
- Valtuena, J.; Gracia-Marco, L.; Huybrechts, I.; Breidenassel, C.; Ferrari, M.; Gottrand, F.; Dallongeville, J.; Sioen, I.; Gutierrez, A.; Kersting, M.; et al. Cardiorespiratory fitness in males, and upper limbs muscular strength in females, are positively related with 25-hydroxyvitamin D plasma concentrations in European adolescents: The HELENA study. QJM Int. J. Med. 2013, 106, 809–821. [Google Scholar] [CrossRef]
- Cediel, G.; Corvalan, C.; Aguirre, C.; de Romana, D.L.; Uauy, R. Serum 25-Hydroxyvitamin D associated with indicators of body fat and insulin resistance in prepubertal chilean children. Int. J. Obes. 2016, 40, 147–152. [Google Scholar] [CrossRef]
- Carson, E.L.; Pourshahidi, L.K.; Hill, T.R.; Cashman, K.D.; Strain, J.J.; Boreham, C.A.; Mulhern, M.S. Vitamin D, Muscle Function, and Cardiorespiratory Fitness in Adolescents From the Young Hearts Study. J. Clin. Endocrinol. Metab. 2015, 100, 4621–4628. [Google Scholar] [CrossRef]
- Saneei, P.; Salehi-Abargouei, A.; Esmaillzadeh, A. Serum 25-hydroxy vitamin D levels in relation to body mass index: A systematic review and meta-analysis. Obes. Rev. 2013, 14, 393–404. [Google Scholar] [CrossRef]
- Wortsman, J.; Matsuoka, L.Y.; Chen, T.C.; Lu, Z.; Holick, M.F. Decreased bioavailability of vitamin D in obesity. Am. J. Clin. Nutr. 2000, 72, 690–693. [Google Scholar] [CrossRef] [PubMed]
- Ekbom, K.; Marcus, C. Vitamin D deficiency is associated with prediabetes in obese Swedish children. Acta Paediatr. 2016, 105, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Manios, Y.; Moschonis, G.; Chrousos, G.P.; Lionis, C.; Mougios, V.; Kantilafti, M.; Tzotzola, V.; Skenderi, K.P.; Petridou, A.; Tsalis, G.; et al. The double burden of obesity and iron deficiency on children and adolescents in Greece: The Healthy Growth Study. J. Hum. Nutr. Diet. 2013, 26, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, C. A review of iron studies in overweight and obese children and adolescents: A double burden in the young? Eur. J. Nutr. 2016, 55, 2179–2197. [Google Scholar] [CrossRef] [PubMed]

| Age Group | Range (Years) | No. of Subjects |
|---|---|---|
| Males | ||
| Young | 0–17 | 435 |
| Young adults * | 18–39 | 2358 |
| Adults | 40–64 | 2599 |
| Elderlies | 65–110 | 563 |
| Females | ||
| Young | 0–17 | 430 |
| Young adults * | 18–39 | 4320 |
| Adults | 40–64 | 2870 |
| Elderlies | 65–110 | 629 |
| Condition | Prevalence (%) | ||
|---|---|---|---|
| All Subjects | Healthy | Anemic | |
| VDD | 98.47 | 98.49 | 98.34 |
| Hypocalcemia | 0.80 | 0.57 | 1.87 |
| Hypercalcemia | 2.40 | 2.68 | 1.06 |
| Ferritin deficiency | 51.11 | 40.32 | 90.70 |
| Iron deficiency | 36.36 | 35.67 | 40.74 |
| Condition | PR | 95% CI | p | OR | 95% CI | p |
|---|---|---|---|---|---|---|
| VDD | 0.92 | 0.69–1.22 | 0.5749 | 0.90 | 0.64–1.27 | 0.5782 |
| Hypocalcemia | 2.36 | 1.88–2.95 | <0.0001 | 3.32 | 2.27–4.86 | <0.0001 |
| Hypercalcemia | 0.42 | 0.10–1.70 | 0.2257 | 0.42 | 0.10–1.70 | 0.2240 |
| Ferritin deficiency | 9.33 | 6.27–13.87 | <0.0001 | 14.44 | 9.38–22.22 | <0.0001 |
| Iron deficiency | 1.20 | 0.59–2.44 | 0.6102 | 1.24 | 0.54–2.84 | 0.6114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alfhili, M.A.; Basudan, A.M.; Alfaifi, M.; Awan, Z.A.; Algethami, M.R.; Alsughayyir, J. Patterns of 25-Hydroxyvitamin D3, Calcium Status, and Anemia in the Saudi Population: A Cross-Sectional Study. Life 2022, 12, 2119. https://doi.org/10.3390/life12122119
Alfhili MA, Basudan AM, Alfaifi M, Awan ZA, Algethami MR, Alsughayyir J. Patterns of 25-Hydroxyvitamin D3, Calcium Status, and Anemia in the Saudi Population: A Cross-Sectional Study. Life. 2022; 12(12):2119. https://doi.org/10.3390/life12122119
Chicago/Turabian StyleAlfhili, Mohammad A., Ahmed M. Basudan, Mohammed Alfaifi, Zuhier A. Awan, Mohammed R. Algethami, and Jawaher Alsughayyir. 2022. "Patterns of 25-Hydroxyvitamin D3, Calcium Status, and Anemia in the Saudi Population: A Cross-Sectional Study" Life 12, no. 12: 2119. https://doi.org/10.3390/life12122119
APA StyleAlfhili, M. A., Basudan, A. M., Alfaifi, M., Awan, Z. A., Algethami, M. R., & Alsughayyir, J. (2022). Patterns of 25-Hydroxyvitamin D3, Calcium Status, and Anemia in the Saudi Population: A Cross-Sectional Study. Life, 12(12), 2119. https://doi.org/10.3390/life12122119







