Naringin Attenuates the Diabetic Neuropathy in STZ-Induced Type 2 Diabetic Wistar Rats
Abstract
1. Introduction
2. Materials and Methods
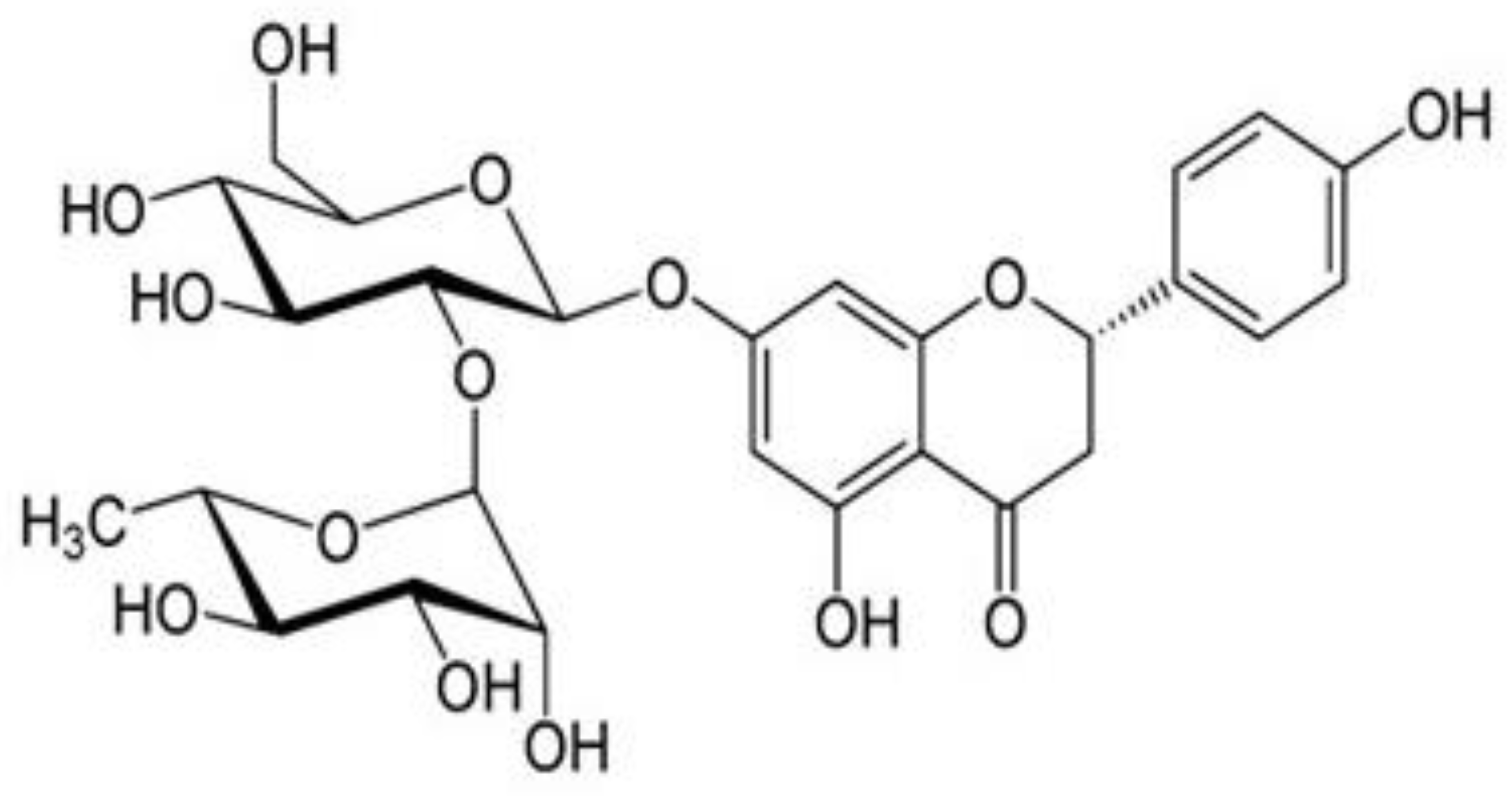
2.1. Materials
2.2. Animals
2.3. Experimental Design
2.4. Induction of Type 2 Diabetes Mellitus
2.5. Validation of Diabetic Neuropathy Rats
2.6. Sample Collection and Tissue Preparation
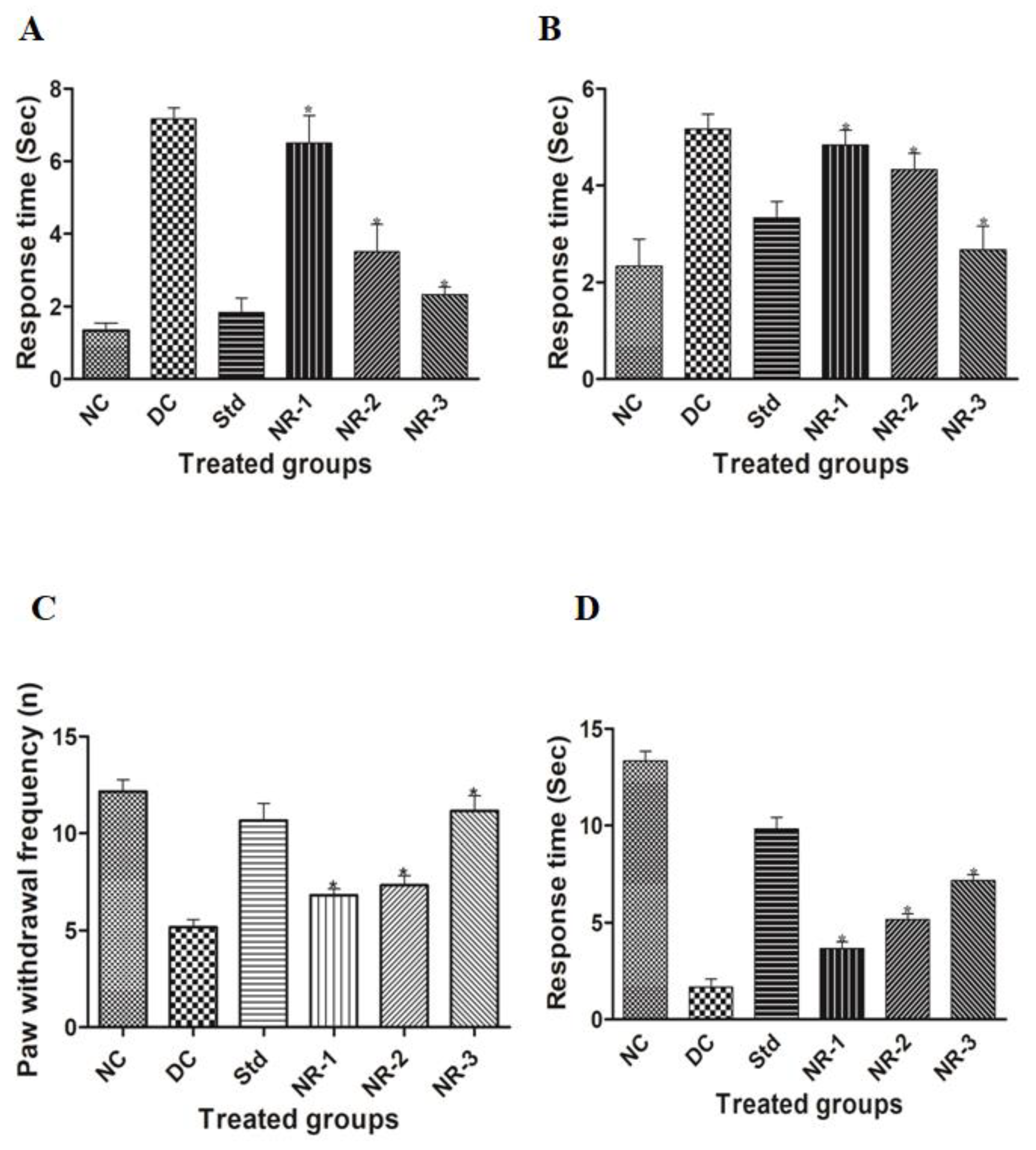
2.7. Assessment of Neuropathic Pain
2.7.1. Acetone Drop Test
2.7.2. Plantar Test
2.7.3. Hot Plate Test
2.7.4. Tail Flick Test
2.8. Serum Biochemical Estimation
2.8.1. Fasting Blood Glucose Measurement
2.8.2. Estimation of Serum Insulin Level
2.8.3. Determination of Percent Glycated Hemoglobin (%HbA1c) Level
2.9. Lipid Profiling
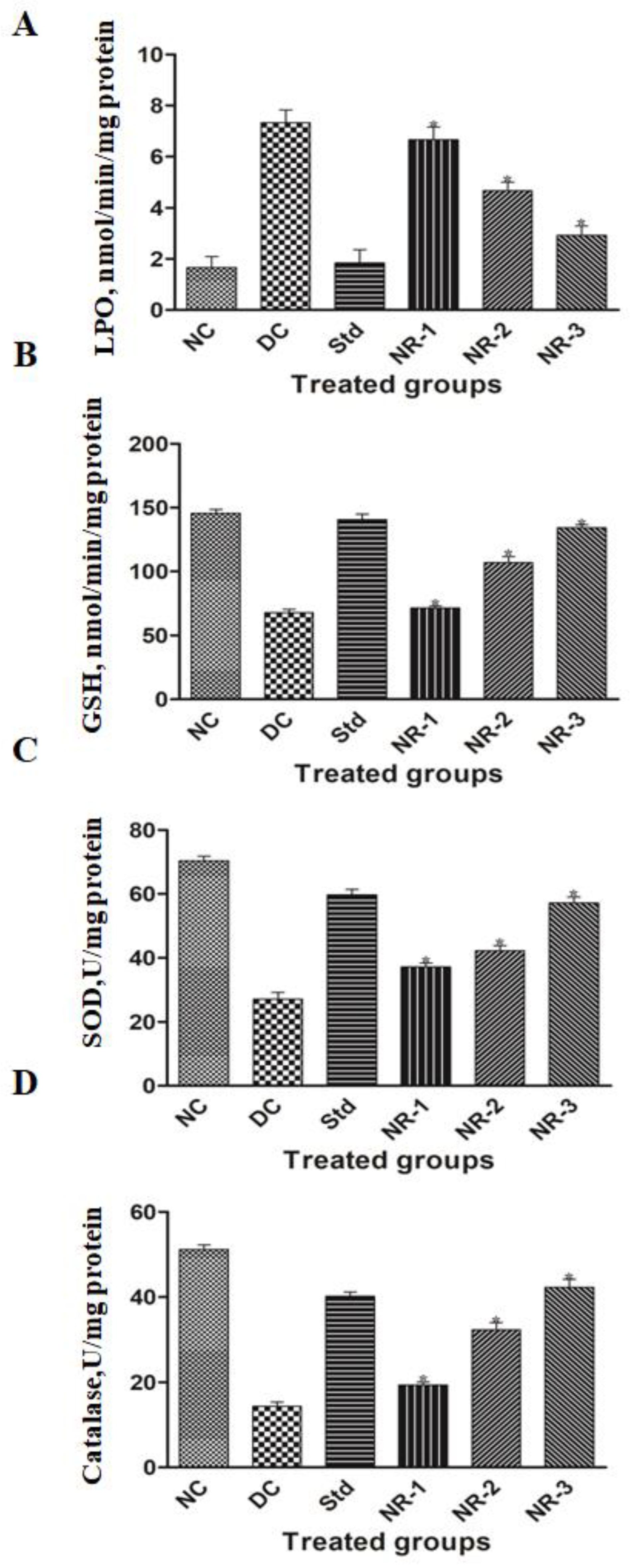
2.10. Assessment of Oxidative Stress in Brain Tissue
2.10.1. Estimation of Lipid Peroxidation Level
2.10.2. Glutathione S-Transferase Activity Assay
2.10.3. Superoxide Dismutase (SOD) Activity Assay
2.10.4. Catalase (CAT) Activity Assay
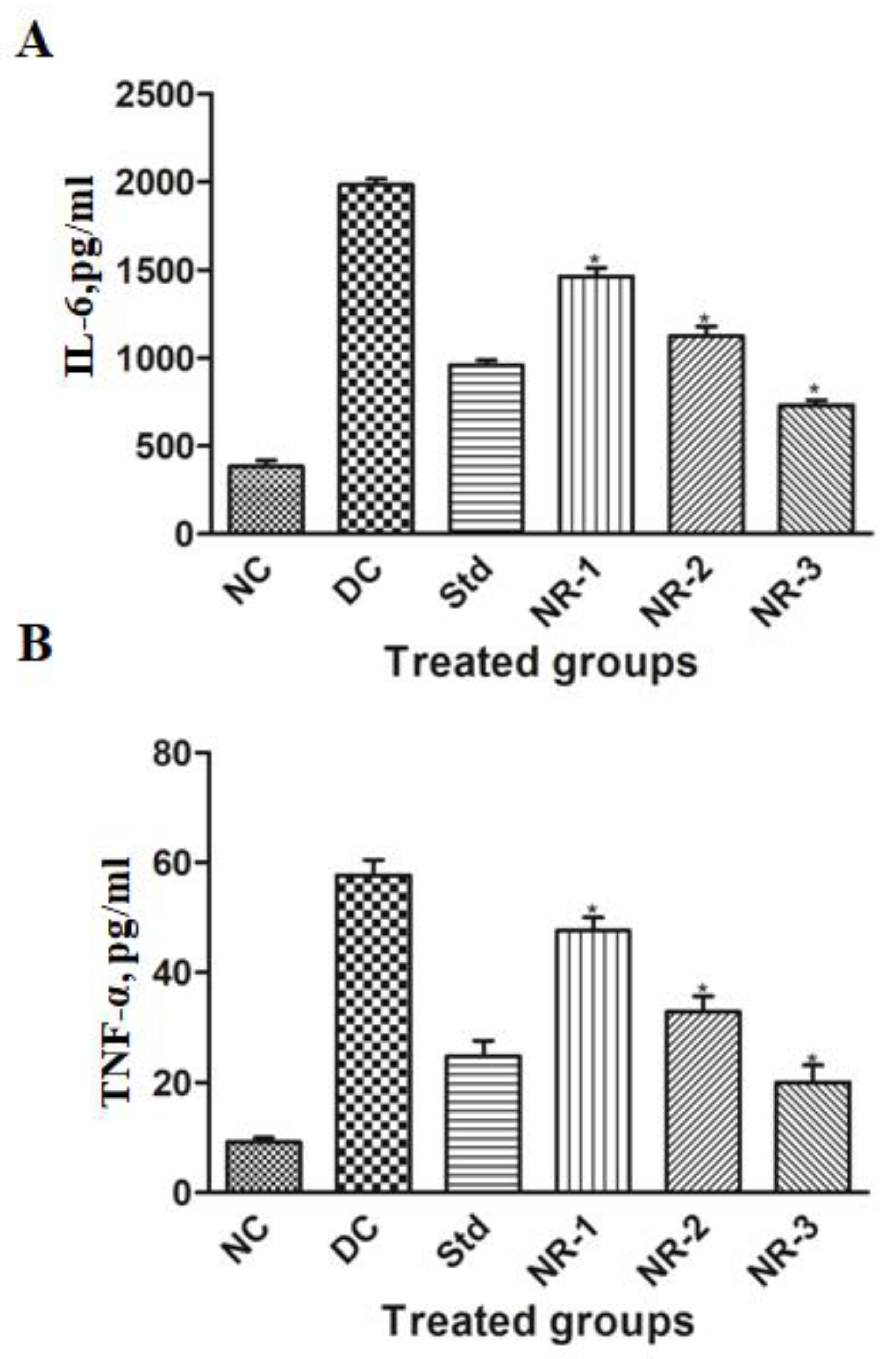
2.11. Proinflammatory Cytokine Analysis
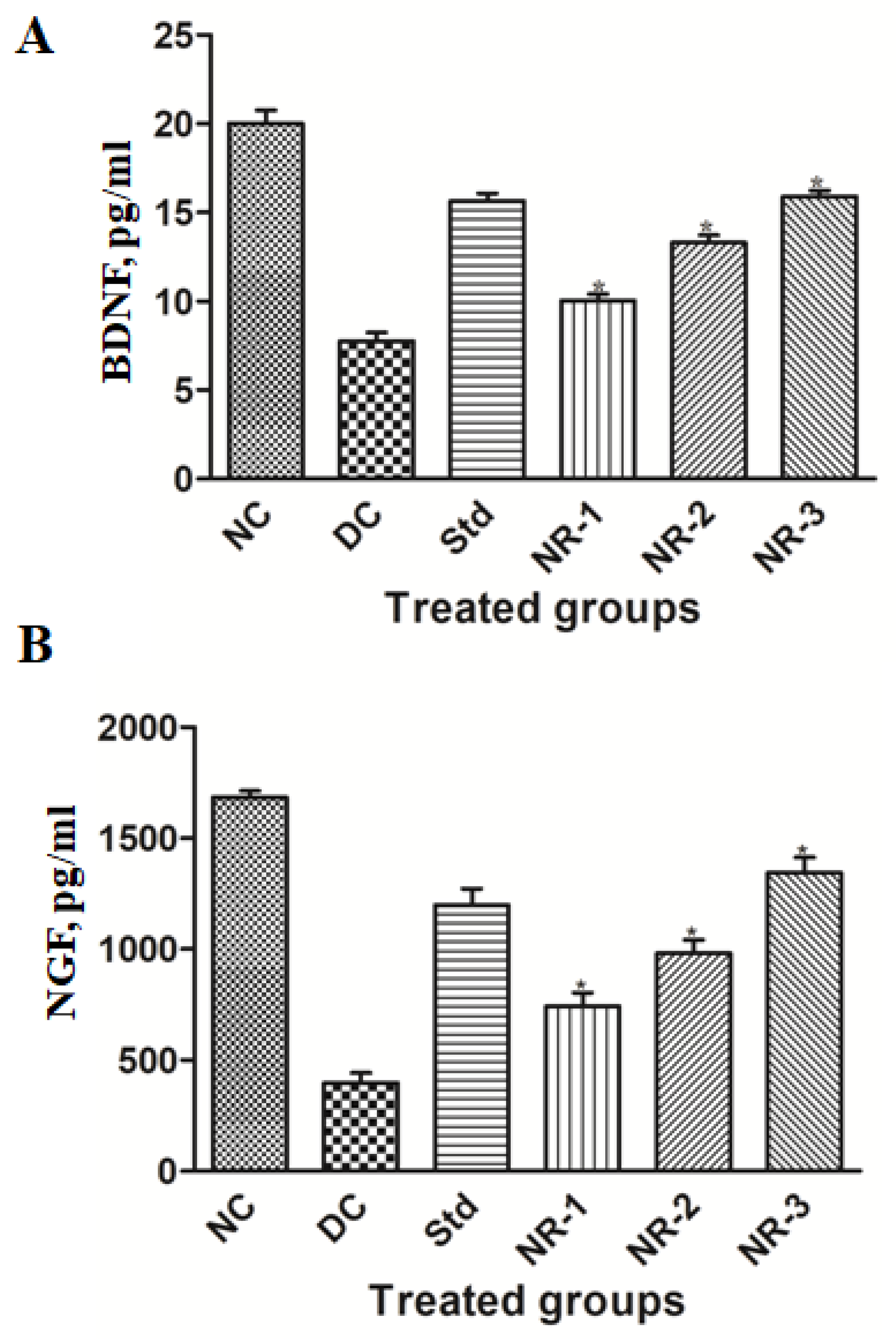
2.12. Neuron-Specific Markers Analysis
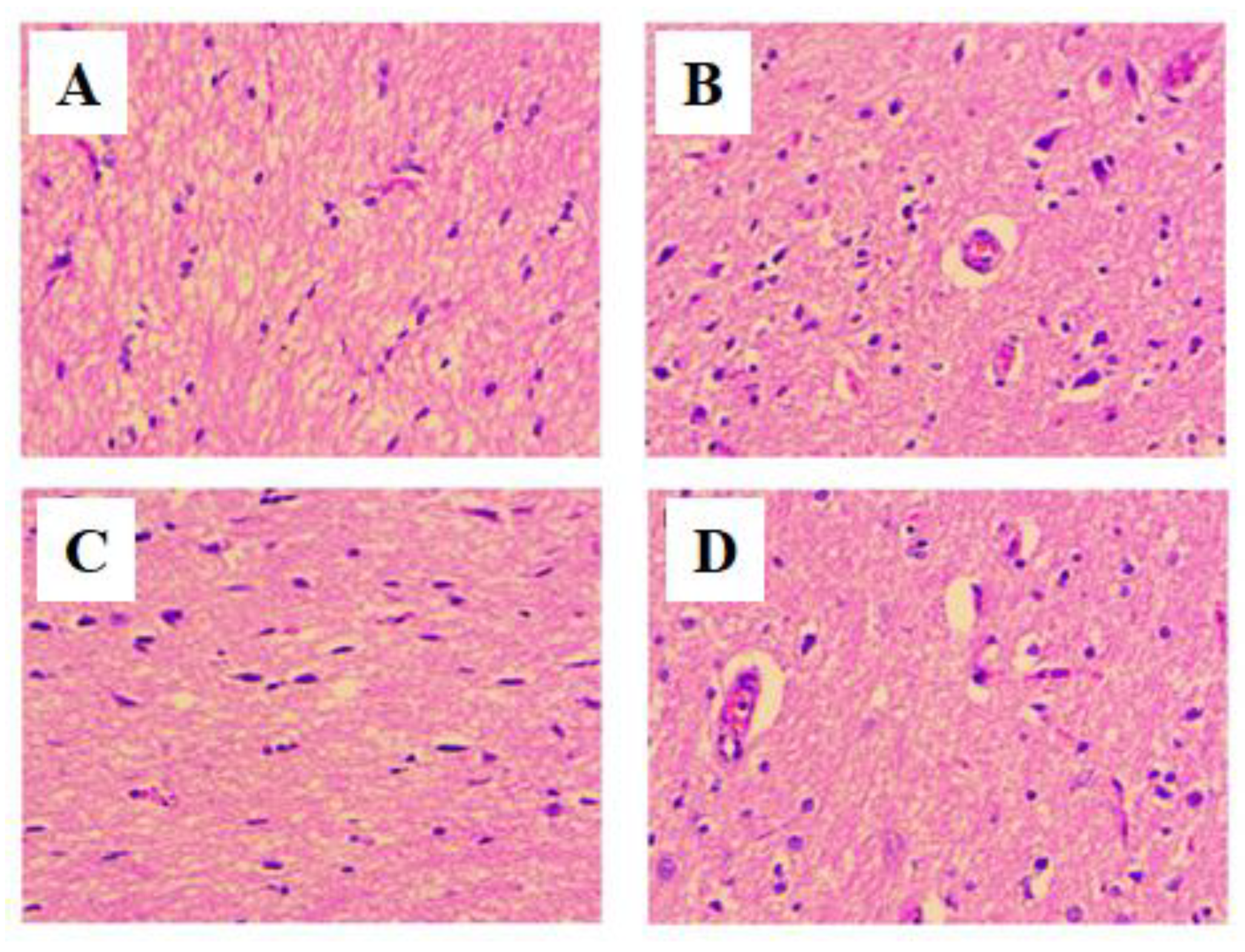
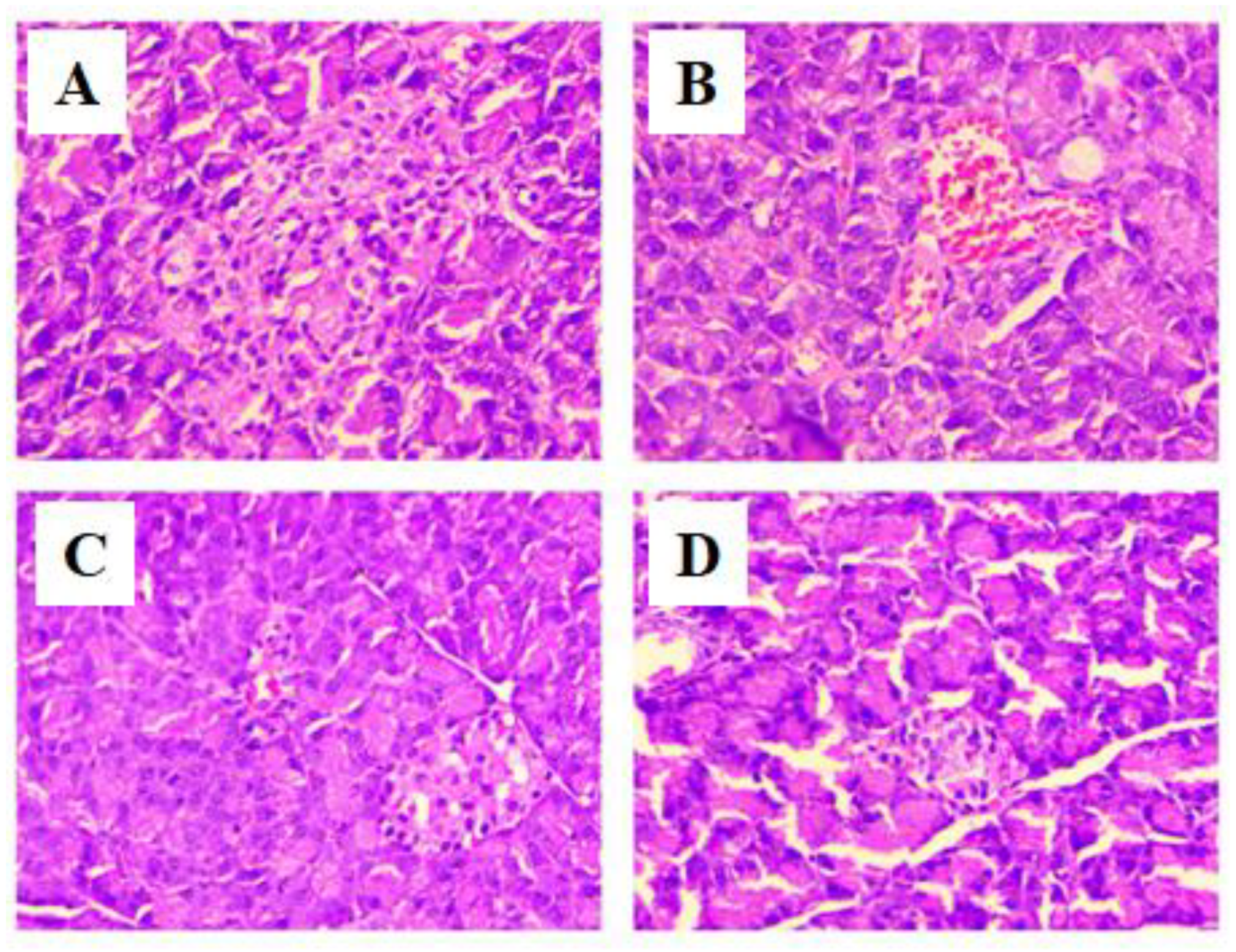
2.13. Histopathological Examination of Brain and Pancreas
2.14. Statistical Analysis
3. Results
3.1. Induction of Diabetes
3.2. Serum Biochemical Estimation
3.3. Lipid Profile
3.4. Oxidative Stress
3.5. Proinflammatory Cytokine Analysis
3.6. Histopathology
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Vinik, A.I.; Nevoret, M.L.; Casellini, C.; Parson, H. Diabetic neuropathy. Endocrinol. Metab. Clin. N. Am. 2013, 42, 747–787. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.E.; Cotter, M.A. Effects of antioxidants on nerve and vascular dysfunction in experimental diabetes. Diabetes Res. Clin. Pract. 1999, 45, 137–146. [Google Scholar] [CrossRef]
- Calcutt, N.A. Modeling diabetic sensory neuropathy in rats. Methods Mol. Med. 2004, 99, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sima, A.A.; Zhang, W.; Xu, G.; Sugimoto, K.; Guberski, D.; Yorek, M.A. A comparison of diabetic polyneuropathy in type II diabetic BBZDR/Wor rats and in type I diabetic BB/Wor rats. Diabetologia 2000, 43, 786–793. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.R.; Peterson, K.D.; Mu, Y.; Banerjee, S.; Allen-Bridson, K.; Morrell, G.; Dudeck, M.A.; Pollock, D.A.; Horan, T.C. National Healthcare Safety Network (NHSN) report: Data summary for 2006 through 2008, issued December 2009. Am. J. Infect. Control 2009, 37, 783–805. [Google Scholar] [CrossRef] [PubMed]
- Brands, A.M.; Kessels, R.P.; de Haan, E.H.; Kappelle, L.J.; Biessels, G.J. Cerebral dysfunction in type 1 diabetes: Effects of insulin, vascular risk factors and blood-glucose levels. Eur. J. Pharmacol. 2004, 490, 159–168. [Google Scholar] [CrossRef]
- Nishikawa, T.; Edelstein, D.; Du, X.L.; Yamagishi, S.; Matsumura, T.; Kaneda, Y.; Yorek, M.A.; Beebe, D.; Oates, P.J.; Hammes, H.P.; et al. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycaemic damage. Nature 2000, 404, 787–790. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Boulton, A.J.; Feldman, E.L.; Bril, V.; Freeman, R.; Malik, R.A.; Sosenko, J.M.; Ziegler, D. Diabetic Neuropathy: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 136–154. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxid. Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef]
- Shyh-Chang, N.; Daley, G.Q.; Cantley, L.C. Stem cell metabolism in tissue development and aging. Development 2013, 140, 2535–2547. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, W.; Liu, Z.; Zhang, Z.; Liu, C. Systematic Analysis of Main Constituents in Rat Biological Samples after Oral Administration of the Methanol Extract of Fructus Aurantii by HPLC-ESI-MS/MS. Iran. J. Pharm. Res. 2014, 13, 493–503. [Google Scholar] [PubMed]
- Yin, L.; Cheng, W.; Qin, Z.; Yu, H.; Yu, Z.; Zhong, M.; Sun, K.; Zhang, W. Effects of Naringin on Proliferation and Osteogenic Differentiation of Human Periodontal Ligament Stem Cells In Vitro and In Vivo. Stem. Cells Int. 2015, 2015, 758706. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.C.; Pang, W.Y.; Wang, X.L.; Mok, S.K.; Lai, W.P.; Chow, H.K.; Leung, P.C.; Yao, X.S.; Wong, M.S. Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen-like protective effects in bone. Br. J. Nutr. 2013, 110, 475–485. [Google Scholar] [CrossRef]
- Chtourou, Y.; Gargouri, B.; Kebieche, M.; Fetoui, H. Naringin Abrogates Cisplatin-Induced Cognitive Deficits and Cholinergic Dysfunction Through the Down-Regulation of AChE Expression and iNOS Signaling Pathways in Hippocampus of Aged Rats. J. Mol. Neurosci. 2015, 56, 349–362. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z. Profiling of phenolic compounds and their antioxidant and anticancer activities in pandan (Pandanus amaryllifolius Roxb.) extracts from different locations of Malaysia. BMC Complement. Altern Med. 2013, 13, 341. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.M.; Yang, Y.J.; Zhang, L.; Zhang, X.; Guan, F.F.; Zhang, L.F. Naringin Enhances CaMKII Activity and Improves Long-Term Memory in a Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2013, 14, 5576–5586. [Google Scholar] [CrossRef]
- Elsawy, H.; Alzahrani, A.M.; Alfwuaires, M.; Abdel-Moneim, A.M.; Khalil, M. Beneficial role of naringin against methotrexate-induced injury to rat testes: Biochemical and ultrastructural analyses. Redox Rep. 2022, 27, 158–166. [Google Scholar] [CrossRef]
- Elsawy, H.; Alzahrani, A.M.; Alfwuaires, M.; Abdel-Moneim, A.M.; Khalil, M. Nephroprotective effect of naringin in methotrexate induced renal toxicity in male rats. Biomed. Pharmacother. 2021, 143, 112180. [Google Scholar] [CrossRef] [PubMed]
- Elsawy, H.; Algefare, A.I.; Alfwuaires, M.; Khalil, M.; Elmenshawy, O.M.; Sedky, A.; Abdel-Moneim, A.M. Naringin alleviates methotrexate-induced liver injury in male albino rats and enhances its antitumor efficacy in HepG2 cells. Biosci. Rep. 2020, 40, BSR20193686. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, I.; Jang, B.H.; Youn, D.Y.; Ryu, W.H.; Park, C.O.; Kim, I.D. Selective diagnosis of diabetes using Pt-functionalized WO3 hemitube networks as a sensing layer of acetone in exhaled breath. Anal. Chem. 2013, 85, 1792–1796. [Google Scholar] [CrossRef]
- Erichsen, H.K.; Blackburn-Munro, G. Pharmacological characterisation of the spared nerve injury model of neuropathic pain. Pain 2002, 98, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Hargreaves, K.; Dubner, R.; Brown, F.; Flores, C.; Joris, J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain 1988, 32, 77–88. [Google Scholar] [CrossRef] [PubMed]
- D’Amour, F.E.; Smith, D.L. A method for determining loss of pain sensation. J. Pharmacol. Exp. Ther. 1941, 72, 74–79. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Habig, W.H.; Jakoby, W.B. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981, 77, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef] [PubMed]
- Claiborne, A.K.; Martin, C.M.; McAlister, W.H.; Gast, M.J. Antenatal diagnosis of cystic adenomatoid malformation: Effect on patient management. Pediatr. Radiol. 1985, 15, 337–339. [Google Scholar] [CrossRef]
- Kros, J.M.; Mustafa, D.M.; Dekker, L.J.; Sillevis Smitt, P.A.; Luider, T.M.; Zheng, P.P. Circulating glioma biomarkers. Neuro Oncol. 2015, 17, 343–360. [Google Scholar] [CrossRef]
- Kawamura, T.; Morioka, T.; Nishio, S.; Fukui, K.; Fukui, M. Temporal lobe epilepsy and corpora amylacea in the hippocampus: Clinicopathologic correlation. Neurol. Res. 2002, 24, 563–569. [Google Scholar] [CrossRef]
- Gul, H.; Yildiz, O.; Dogrul, A.; Yesilyurt, O.; Isimer, A. The interaction between IL-1beta and morphine: Possible mechanism of the deficiency of morphine-induced analgesia in diabetic mice. Pain 2000, 89, 39–45. [Google Scholar] [CrossRef]
- Courteix, C.; Eschalier, A.; Lavarenne, J. Streptozocin-induced diabetic rats: Behavioural evidence for a model of chronic pain. Pain 1993, 53, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Kuhad, A.; Chopra, K. Tocotrienol attenuates oxidative-nitrosative stress and inflammatory cascade in experimental model of diabetic neuropathy. Neuropharmacology 2009, 57, 456–462. [Google Scholar] [CrossRef]
- Anjaneyulu, M.; Chopra, K. Quercetin attenuates thermal hyperalgesia and cold allodynia in STZ-induced diabetic rats. Indian J. Exp. Biol. 2004, 42, 766–769. [Google Scholar]
- Kandhare, A.D.; Raygude, K.S.; Ghosh, P.; Ghule, A.E.; Bodhankar, S.L. Neuroprotective effect of naringin by modulation of endogenous biomarkers in streptozotocin induced painful diabetic neuropathy. Fitoterapia 2012, 83, 650–659. [Google Scholar] [CrossRef] [PubMed]
- Vij, N.; Mazur, S.; Zeitlin, P.L. CFTR is a negative regulator of NFkappaB mediated innate immune response. PLoS ONE 2009, 4, e4664. [Google Scholar] [CrossRef] [PubMed]
- Di Girolamo, N.; Underwood, A.; McCluskey, P.J.; Wakefield, D. Functional activity of plasma fibronectin in patients with diabetes mellitus. Diabetes 1993, 42, 1606–1613. [Google Scholar] [CrossRef]
- Baskaran, G.; Salvamani, S.; Ahmad, S.A.; Shaharuddin, N.A.; Pattiram, P.D.; Shukor, M.Y. HMG-CoA reductase inhibitory activity and phytocomponent investigation of Basella alba leaf extract as a treatment for hypercholesterolemia. Drug Des. Dev. Ther. 2015, 9, 509–517. [Google Scholar] [CrossRef]
- Johnson, M.K.; Loo, G. Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat. Res. 2000, 459, 211–218. [Google Scholar] [CrossRef]
- Li, S.G.; Ding, Y.S.; Niu, Q.; Xu, S.Z.; Pang, L.J.; Ma, R.L.; Jing, M.X.; Feng, G.L.; Liu, J.M.; Guo, S.X. Grape Seed Proanthocyanidin Extract Alleviates Arsenic-induced Oxidative Reproductive Toxicity in Male Mice. Biomed. Environ. Sci. 2015, 28, 272–280. [Google Scholar] [CrossRef]
- Bakheet, S.A.; Attia, S.M. Evaluation of chromosomal instability in diabetic rats treated with naringin. Oxid. Med. Cell. Longev. 2011, 2011, 365292. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; Ashour, M.B.; Abdel-Moneim, A.; Ahmed, O.M. Hesperidin and naringin attenuate hyperglycemia-mediated oxidative stress and proinflammatory cytokine production in high fat fed/streptozotocin-induced type 2 diabetic rats. J. Diabetes Complicat. 2012, 26, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Cameron, N.E.; Cotter, M.A. Potential therapeutic approaches to the treatment or prevention of diabetic neuropathy: Evidence from experimental studies. Diabet. Med. 1993, 10, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Chertoff, M.; Di Paolo, N.; Schoeneberg, A.; Depino, A.; Ferrari, C.; Wurst, W.; Pfizenmaier, K.; Eisel, U.; Pitossi, F. Neuroprotective and neurodegenerative effects of the chronic expression of tumor necrosis factor alpha in the nigrostriatal dopaminergic circuit of adult mice. Exp. Neurol. 2011, 227, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Dubovy, P. Wallerian degeneration and peripheral nerve conditions for both axonal regeneration and neuropathic pain induction. Ann. Anat. 2011, 193, 267–275. [Google Scholar] [CrossRef]
- Watkins, L.R.; Maier, S.F. Beyond neurons: Evidence that immune and glial cells contribute to pathological pain states. Physiol. Rev. 2002, 82, 981–1011. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Pop-Busui, R.; Ang, L.; Holmes, C.; Gallagher, K.; Feldman, E.L. Inflammation as a Therapeutic Target for Diabetic Neuropathies. Curr. Diab. Rep. 2016, 16, 29. [Google Scholar] [CrossRef]
- Ganesh Yerra, V.; Negi, G.; Sharma, S.S.; Kumar, A. Potential therapeutic effects of the simultaneous targeting of the Nrf2 and NF-kappaB pathways in diabetic neuropathy. Redox Biol. 2013, 1, 394–397. [Google Scholar] [CrossRef]
- Ito, T.; Ohtori, S.; Hata, K.; Inoue, G.; Moriya, H.; Takahashi, K.; Yamashita, T. Rho kinase inhibitor improves motor dysfunction and hypoalgesia in a rat model of lumbar spinal canal stenosis. Spine 2007, 32, 2070–2075. [Google Scholar] [CrossRef]
- Kandhare, A.D.; Raygude, K.S.; Ghosh, P.; Bodhankar, S.L. The ameliorative effect of fisetin, a bioflavonoid, on ethanol-induced and pylorus ligation-induced gastric ulcer in rats. Int. J. Green Pharm. 2011, 5, 236–243. [Google Scholar]
- Cameron, N.E.; Cotter, M.A. Metabolic and vascular factors in the pathogenesis of diabetic neuropathy. Diabetes 1997, 46 (Suppl. S2), S31–S37. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.A. BDNF: No gain without pain? Neuroscience 2014, 283, 107–123. [Google Scholar] [CrossRef] [PubMed]
- Trang, T.; Beggs, S.; Wan, X.; Salter, M.W. P2X4-receptor-mediated synthesis and release of brain-derived neurotrophic factor in microglia is dependent on calcium and p38-mitogen-activated protein kinase activation. J. Neurosci. 2009, 29, 3518–3528. [Google Scholar] [CrossRef] [PubMed]
- Hildebrand, M.E.; Xu, J.; Dedek, A.; Li, Y.; Sengar, A.S.; Beggs, S.; Lombroso, P.J.; Salter, M.W. Potentiation of Synaptic GluN2B NMDAR Currents by Fyn Kinase Is Gated through BDNF-Mediated Disinhibition in Spinal Pain Processing. Cell Rep. 2016, 17, 2753–2765. [Google Scholar] [CrossRef]
- Tsuda, M. Microglia in the spinal cord and neuropathic pain. J. Diabetes Investig. 2016, 7, 17–26. [Google Scholar] [CrossRef]
- Alles, S.R.A.; Smith, P.A. Etiology and Pharmacology of Neuropathic Pain. Pharmacol. Rev. 2018, 70, 315–347. [Google Scholar] [CrossRef]
- Hulse, R.P.; Beazley-Long, N.; Ved, N.; Bestall, S.M.; Riaz, H.; Singhal, P.; Ballmer Hofer, K.; Harper, S.J.; Bates, D.O.; Donaldson, L.F. Vascular endothelial growth factor-A165b prevents diabetic neuropathic pain and sensory neuronal degeneration. Clin. Sci. 2015, 129, 741–756. [Google Scholar] [CrossRef]
- Simmons, Z.; Feldman, E.L. Update on diabetic neuropathy. Curr. Opin. Neurol. 2002, 15, 595–603. [Google Scholar] [CrossRef]
- Hilz, M.J.; Marthol, H.; Neundorfer, B. Diabetic somatic polyneuropathy. Pathogenesis, clinical manifestations and therapeutic concepts. Fortschr. Neurol. Psychiatr. 2000, 68, 278–288. [Google Scholar] [CrossRef]
- Steinbacher, B.C., Jr.; Nadelhaft, I. Increased levels of nerve growth factor in the urinary bladder and hypertrophy of dorsal root ganglion neurons in the diabetic rat. Brain Res. 1998, 782, 255–260. [Google Scholar] [CrossRef]
- Anand, P.; Terenghi, G.; Warner, G.; Kopelman, P.; Williams-Chestnut, R.E.; Sinicropi, D.V. The role of endogenous nerve growth factor in human diabetic neuropathy. Nat. Med. 1996, 2, 703–707. [Google Scholar] [CrossRef] [PubMed]
- Yiangou, Y.; Facer, P.; Sinicropi, D.V.; Boucher, T.J.; Bennett, D.L.; McMahon, S.B.; Anand, P. Molecular forms of NGF in human and rat neuropathic tissues: Decreased NGF precursor-like immunoreactivity in human diabetic skin. J. Peripher. Nerv. Syst. 2002, 7, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Kanbayashi, H.; Itoh, H.; Kashiwaya, T.; Atoh, K.; Makino, I. Spatial distribution of nociceptive neuropeptide and nerve growth factor depletion in experimental diabetic peripheral nervous system. J. Int. Med. Res. 2002, 30, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Dey, I.; Midha, N.; Singh, G.; Forsyth, A.; Walsh, S.K.; Singh, B.; Kumar, R.; Toth, C.; Midha, R. Diabetic Schwann cells suffer from nerve growth factor and neurotrophin-3 underproduction and poor associability with axons. Glia 2013, 61, 1990–1999. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, K.; Katoh, M.; Someno, K.; Fujimoto, Y. Apoptosis and mitochondrial damage in INS-1 cells treated with alloxan. Biol. Pharm. Bull. 2001, 24, 876–882. [Google Scholar] [CrossRef] [PubMed]
- Kaneto, H.; Matsuoka, T.A.; Miyatsuka, T.; Kawamori, D.; Katakami, N.; Yamasaki, Y.; Matsuhisa, M. PDX-1 functions as a master factor in the pancreas. Front. Biosci. 2008, 13, 6406–6420. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahmad, M.F.; Naseem, N.; Rahman, I.; Imam, N.; Younus, H.; Pandey, S.K.; Siddiqui, W.A. Naringin Attenuates the Diabetic Neuropathy in STZ-Induced Type 2 Diabetic Wistar Rats. Life 2022, 12, 2111. https://doi.org/10.3390/life12122111
Ahmad MF, Naseem N, Rahman I, Imam N, Younus H, Pandey SK, Siddiqui WA. Naringin Attenuates the Diabetic Neuropathy in STZ-Induced Type 2 Diabetic Wistar Rats. Life. 2022; 12(12):2111. https://doi.org/10.3390/life12122111
Chicago/Turabian StyleAhmad, Md Fahim, Nida Naseem, Inamur Rahman, Nazia Imam, Hina Younus, Swaroop Kumar Pandey, and Waseem A. Siddiqui. 2022. "Naringin Attenuates the Diabetic Neuropathy in STZ-Induced Type 2 Diabetic Wistar Rats" Life 12, no. 12: 2111. https://doi.org/10.3390/life12122111
APA StyleAhmad, M. F., Naseem, N., Rahman, I., Imam, N., Younus, H., Pandey, S. K., & Siddiqui, W. A. (2022). Naringin Attenuates the Diabetic Neuropathy in STZ-Induced Type 2 Diabetic Wistar Rats. Life, 12(12), 2111. https://doi.org/10.3390/life12122111








