Foliar Fertilizer Application Alters the Effect of Girdling on the Nutrient Contents and Yield of Camellia oleifera
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material and Study Site
2.2. Study Design
2.3. Experimental Method
2.3.1. Girdling Technique
2.3.2. Foliar Fertilizer Spray Technique
2.3.3. Sample Collection
2.3.4. Determination of Nutrient Content and Fruit Economic Characteristics
2.4. Data Analysis
3. Results
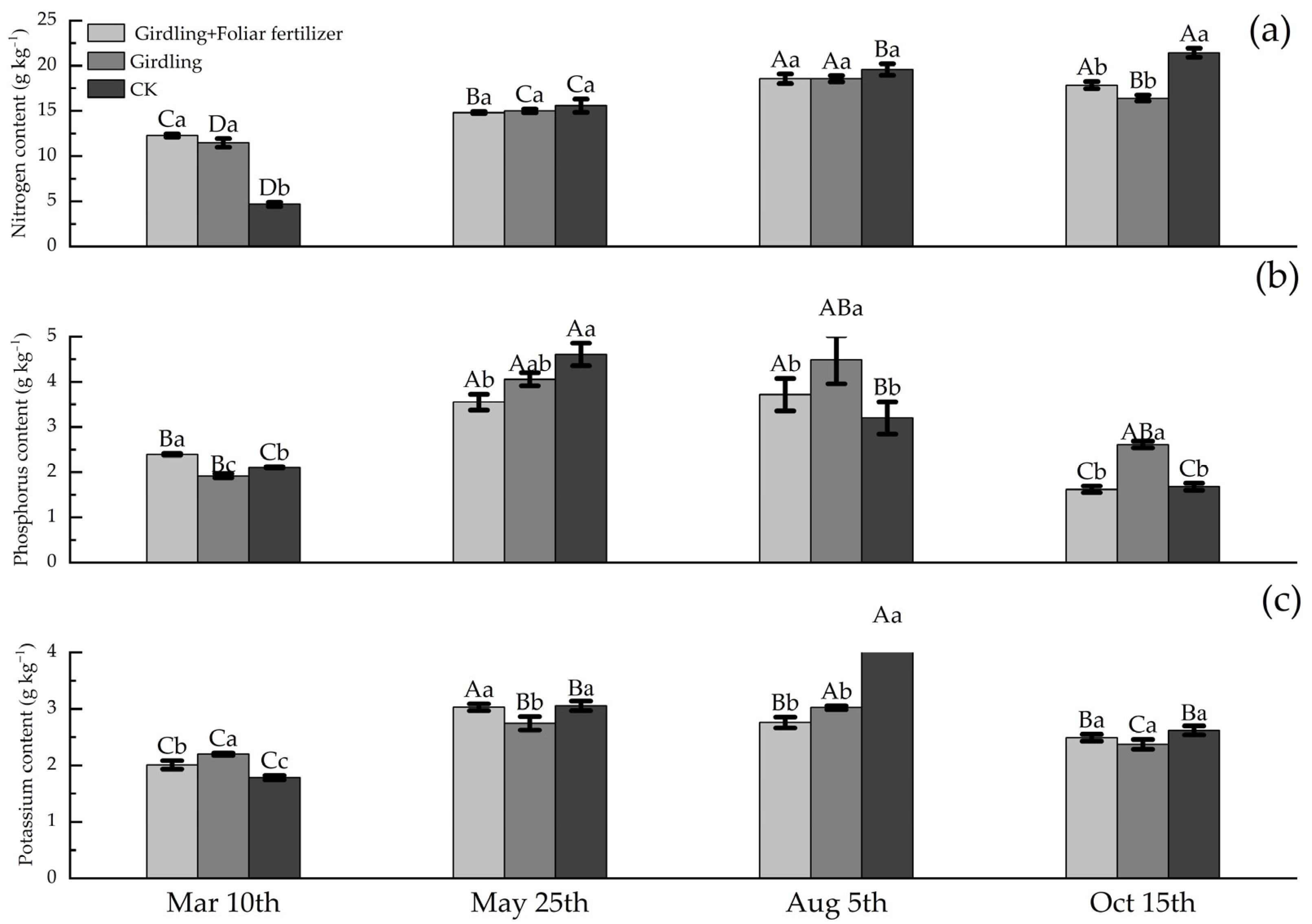
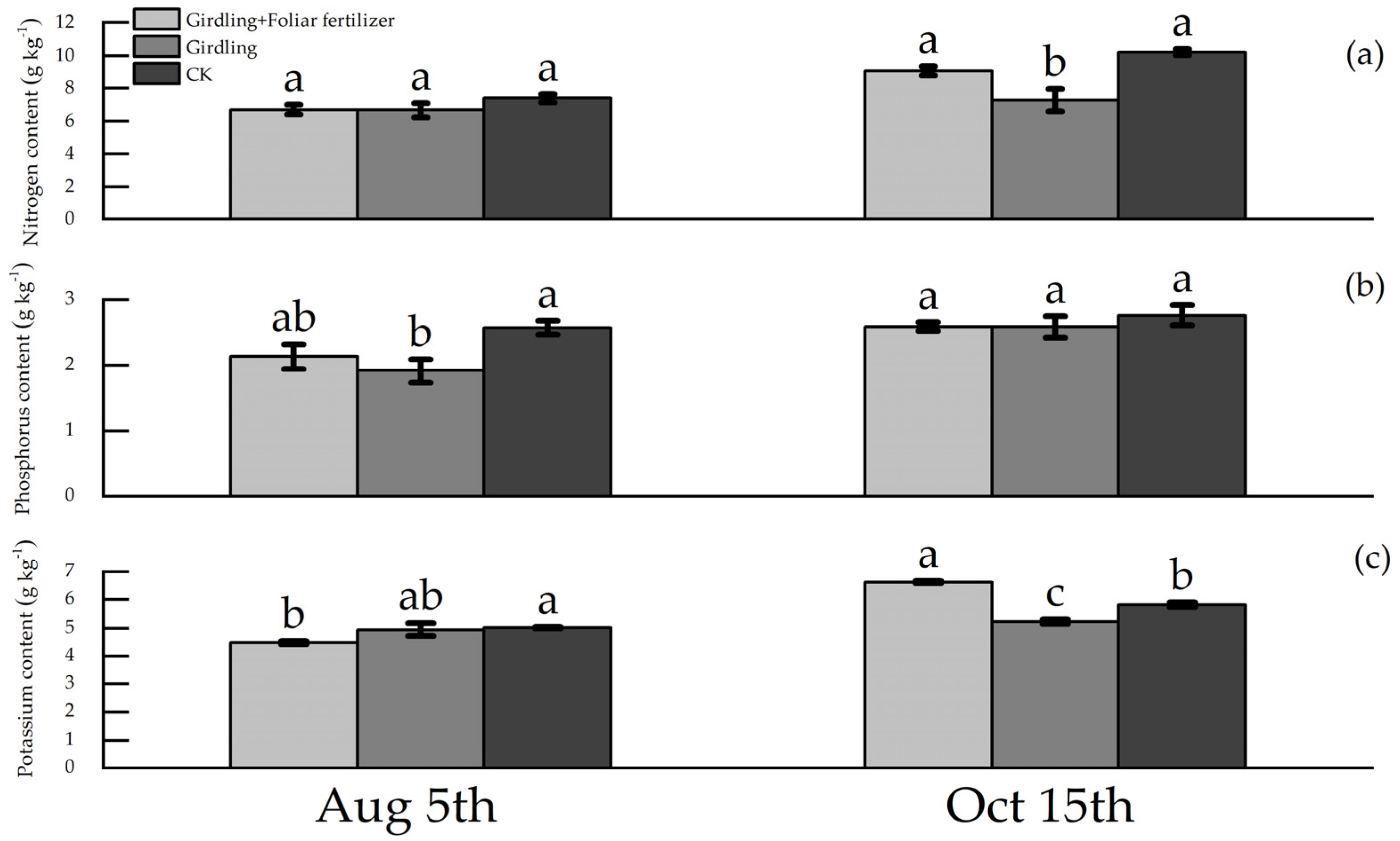
3.1. Effects of Girdling and Foliar Fertilizer Application on the Nutrient Contents of Various C. oleifera Organs
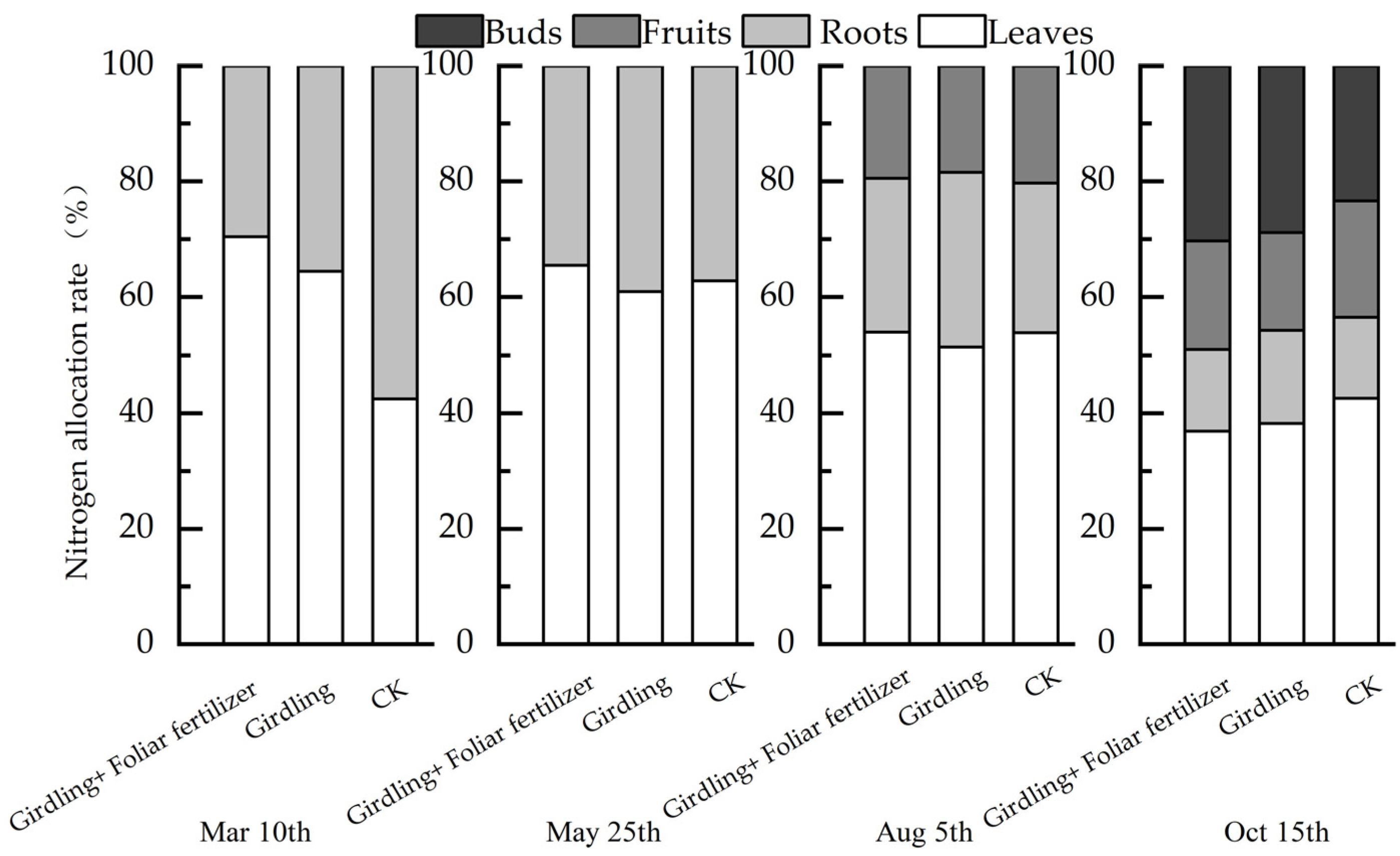
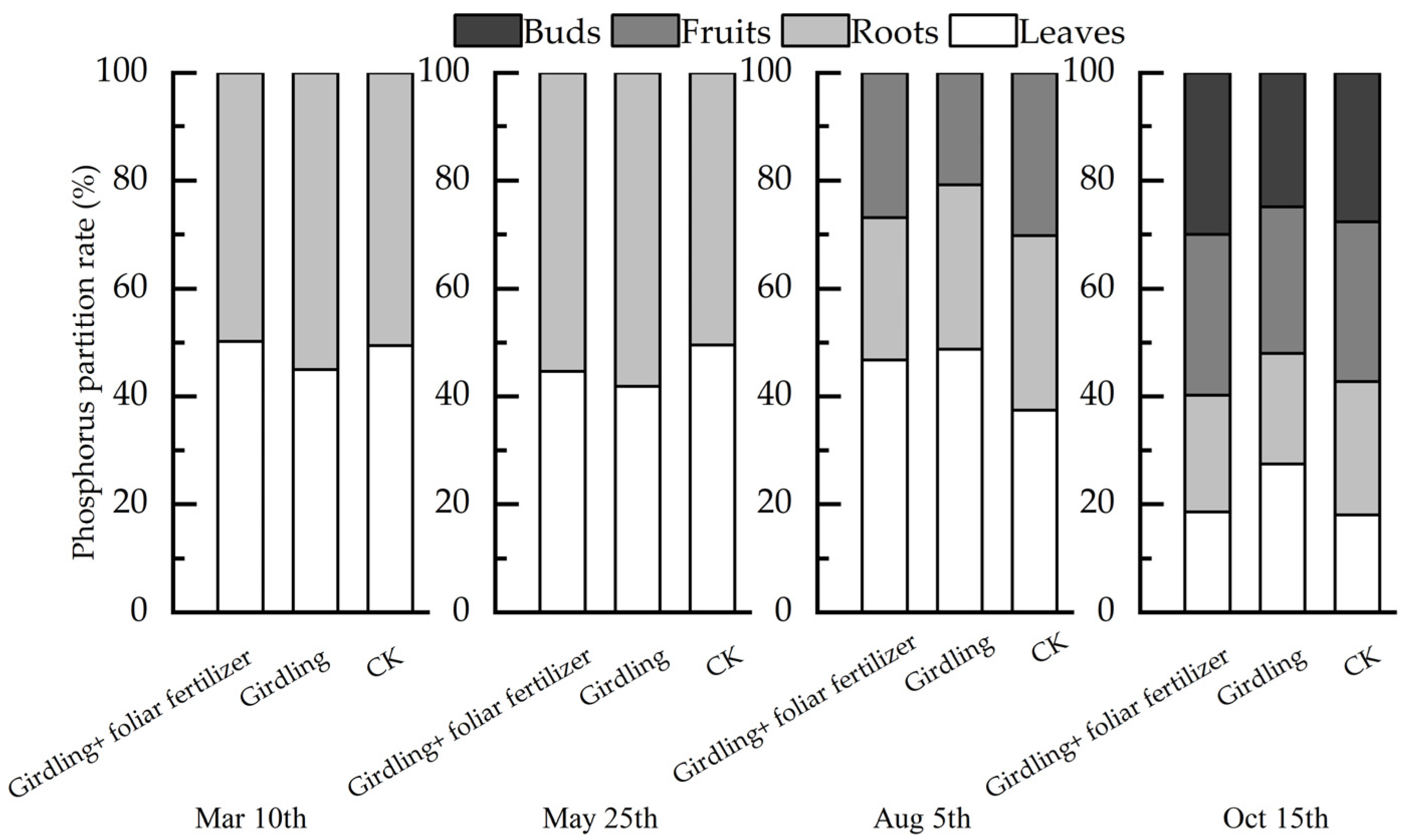
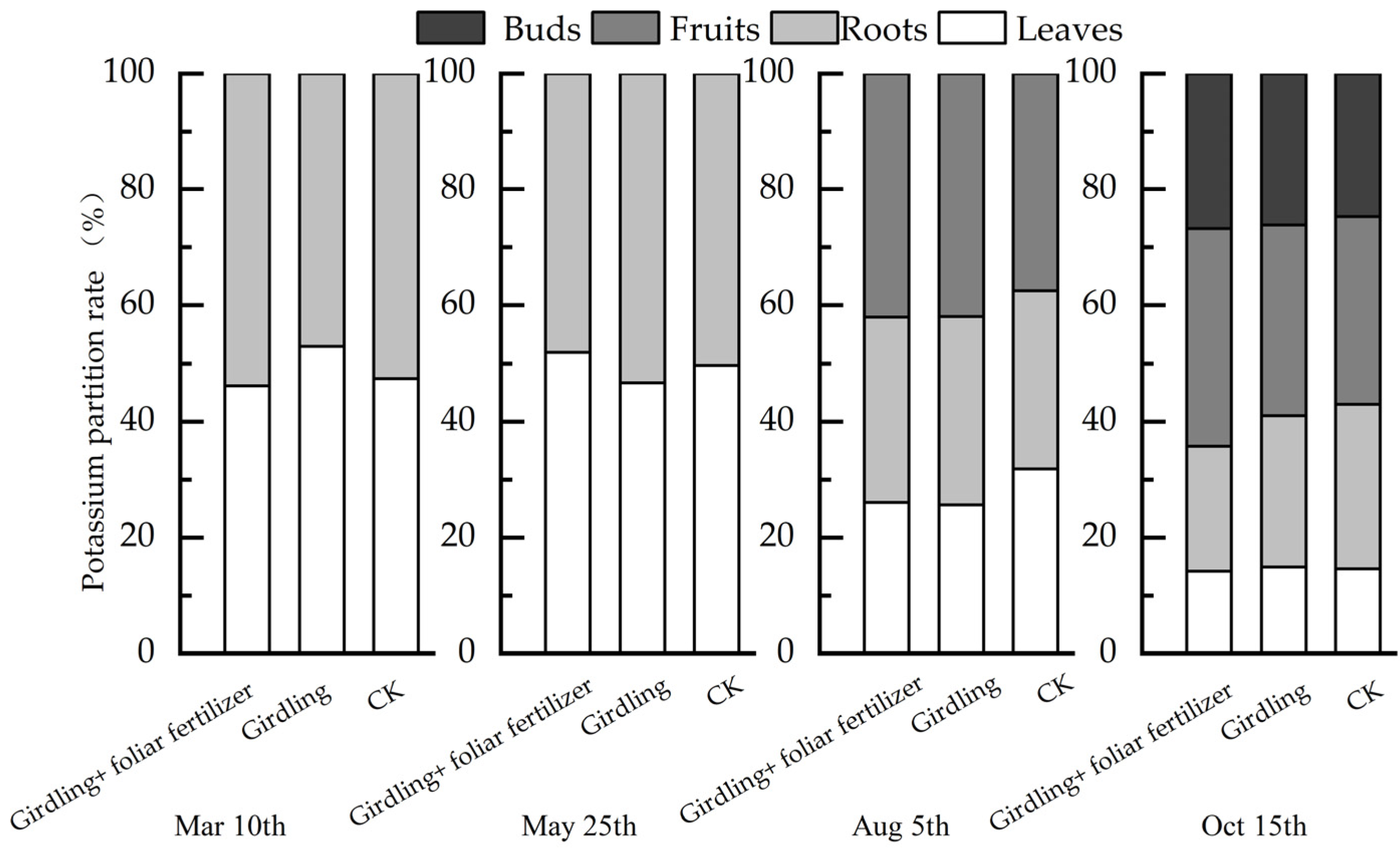
3.2. Effects of Girdling and Foliar Fertilizer on Nutrient Distribution in Different C. oleifera Organs
3.3. Effects of Girdling and Foliar Fertilizer Application on N:P and N:K Ratios in Different C. oleifera Organs
3.4. Influence of Girdling and Foliar Fertilizer Application on C. oleifera Yield
3.5. Path Analysis of Nutrient Content, Nutrient Stoichiometric Ratio, and Per-Plant Oil Yield
4. Discussion
4.1. Effect of Girdling on the Nutrient Contents of C. oleifera Plants
4.2. Influence of Ring Cutting on the Stoichiometric Ratios of N, P, and K in C. oleifera
4.3. Foliar Fertilizer-Induced Modification of the Effect of Girdling on the Nutrient Content of C. oleifera Organs
4.4. Key Nutrient Characteristics of Girdled C. oleifera Trees for Increasing Yield
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- You, L.; Yu, S.; Liu, H.; Wang, C.; Zhou, Z.; Zhang, L.; Hu, D. Effects of Biogas Slurry Fertilization on Fruit Economic Traits and Soil Nutrients of Camellia Oleifera Abel. PLoS ONE 2019, 14, e0208289. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Liu, C.; Zhang, Z.; Wang, R.; Chen, Y. Integration of Mrna and Mirna Analysis Reveals the Differentially Regulatory Network in Two Different Camellia Oleifera Cultivars under Drought Stress. Front. Plant Sci. 2022, 13, 1001357. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.-L.; Chen, Z.-G.; Jia, T.-T.; Su, Q.-W.; Su, S.-C. Response of Different Organic Mulch Treatments on Yield and Quality of Camellia Oleifera. Agric. Water Manag. 2021, 245, 1001357. [Google Scholar] [CrossRef]
- Yu, F.; Xin, M.; Yao, Y.; Wang, X.; Liu, K.; Li, Y. Manganese Accumulation and Plant Physiology Behaviour of Camellia oleifera in Response to Different Levels of Phosphate Fertilization. J. Soil Sci. Plant Nutr. 2021, 21, 2562–2572. [Google Scholar] [CrossRef]
- He, X. Effects of Foliar Fertilizers on Fruit Setting in Camellia Oleifera. Nonwood For. Res. 2015, 33, 18–24. [Google Scholar]
- Lu, Z.-J.; Yu, H.-Z.; Mi, L.-F.; Liu, Y.-X.; Huang, Y.-L.; Xie, Y.-X.; Li, N.-Y.; Zhong, B.-L. The Effects of Inarching Citrus reticulata Blanco Var. Tangerine on the Tree Vigor, Nutrient Status and Fruit Quality of Citrus sinensis Osbeck ‘Newhall’ Trees That Have Poncirus trifoliata (L.) Raf. As Rootstocks. Sci. Hortic. 2019, 256, 108600. [Google Scholar] [CrossRef]
- Tyagi, K.; Maoz, I.; Lewinsohn, E.; Lerno, L.; Ebeler, S.E.; Lichter, A. Girdling of Table Grapes at Fruit Set Can Divert the Phenylpropanoid Pathway Towards Accumulation of Proanthocyanidins and Change the Volatile Composition. Plant Sci. 2020, 296, 110495. [Google Scholar] [CrossRef]
- Baïram, E.; le Morvan, C.; Delaire, M.; Buck-Sorlin, G. Fruit and Leaf Response to Different Source-Sink Ratios in Apple, at the Scale of the Fruit-Bearing Branch. Front. Plant Sci. 2019, 10, 1039. [Google Scholar] [CrossRef]
- Figiel-Kroczyńska, M.; Ochmian, I.; Lachowicz, S.; Krupa-Małkiewicz, M.; Wróbel, J.; Gamrat, R. Actinidia (Mini Kiwi) Fruit Quality in Relation to Summer Cutting. Agronomy 2021, 11, 964. [Google Scholar] [CrossRef]
- Christopoulos, M.V.; Kafkaletou, M.; Karantzi, A.D.; Tsantili, E. Girdling Effects on Fruit Maturity, Kernel Quality, and Nutritional Value of Walnut (Juglans regia L.) Alongside the Effects on Leaf Physiological Characteristics. Agronomy 2021, 11, 200. [Google Scholar] [CrossRef]
- Singh, D.; Dhillon, W.S.; Singh, N.P.; Gill, P.P.S. Effect of Girdling on Leaf Nutrient Levels in Pear Cultivars Patharnakh and Punjab Beauty. Indian J. Hortic. 2015, 72, 319–324. [Google Scholar] [CrossRef]
- de Lange, J.H.; Skarup, O.; Vincent, A.P. The Influence of Cross-Pollination and Girdling on Fruit Set and Seed Content of Citrus ‘Ortanique’. Sci. Hortic. 1974, 2, 285–292. [Google Scholar] [CrossRef]
- Mann, M.S.; Josan, J.S.; Chohan, G.S.; Vij, V.K. Effect of Foliar Application of Micronutrients on Leaf Composition, Fruit Yield and Quality of Sweet Orange (Citrus sinensis Osbeck) Cv. Blood Red. Indian J. Hortic. 1985, 42, 45–49. [Google Scholar]
- Norozi, M.; ValizadehKaji, B.; Karimi, R.; Sedghi, M. Effects of Foliar Application of Potassium and Zinc on Pistachio (Pistacia vera L.) Fruit Yield. J. Hortic. Sci. 2019, 6, 113–123. [Google Scholar]
- Duan, W. Effect of Foliar Fertilizer on Leaf Anatomy Structure and Photosynthetic Characteristics of Camellia oleifera Container Seedlings. J. Northwest A F Univ. 2015, 43, 92–97. [Google Scholar]
- Yilmaz, B.; Çimen, B.; Incesu, M.; Yeşiloğlu, T.; Yilmaz, M.I. Influence of Girdling on the Seasonal Leaf Nutrition Status and Fruit Size of Robinson Mandarin (Citrus reticulata Blanco). Appl. Ecol. Environ. Res. 2018, 16, 6205–6218. [Google Scholar] [CrossRef]
- Li, Y.; Zou, N.; Liang, X.; Zhou, X.; Guo, S.; Wang, Y.; Qin, X.; Tian, Y.; Lin, J. Effects of Nitrogen Input on Soil Bacterial Community Structure and Soil Nitrogen Cycling in the Rhizosphere Soil of Lycium barbarum L. Front. Microbiol. 2022, 13, 1070817. [Google Scholar] [CrossRef]
- Meng, D.; Xu, P.; Dong, Q.; Wang, S.; Wang, Z. Comparison of Foliar and Root Application of Potassium Dihydrogen Phosphate in Regulating Cadmium Translocation and Accumulation in Tall Fescue (Festuca arundinacea). Water Air Soil Pollut. 2017, 228, 118. [Google Scholar] [CrossRef]
- Sánchez-Ramos, M.; Berman-Bahena, S.; Alvarez, L.; Sánchez-Carranza, J.N.; Bernabé-Antonio, A.; Román-Guerrero, A.; Marquina-Bahena, S.; Cruz-Sosa, F. Effect of Plant Growth Regulators on Different Explants of Artemisia ludoviciana under Photoperiod and Darkness Conditions and Their Influence on Achillin Production. Processes 2022, 10, 1439. [Google Scholar] [CrossRef]
- Thakur, P.; Kumar, P.; Shukla, A.K.; Butail, N.P.; Sharma, M.; Kumar, P.; Sharma, U. Quantitative, Qualitative, and Energy Assessment of Boron Fertilization on Maize Production in North-West Himalayan Region. Int. J. Plant Prod. 2023. [Google Scholar] [CrossRef]
- Zeng, J.; Liu, J.; Lian, L.; Xu, A.; Guo, X.; Zhang, L.; Zhang, W.; Hu, D. Effects of Scion Variety on the Phosphorus Efficiency of Grafted Camellia oleifera Seedlings. Forests 2022, 13, 203. [Google Scholar] [CrossRef]
- Levin, A.G.; Lavee, S. The Influence of Girdling on Flower Type, Number, Inflorescence Density, Fruit Set, and Yields in Three Different Olive Cultivars (Barnea, Picual, and Souri). Crop Pasture Sci. 2005, 56, 827–831. [Google Scholar] [CrossRef]
- Ma, J.; Zhang, M.; Liu, Z.; Wang, M.; Sun, Y.; Zheng, W.; Lu, H. Copper-Based-Zinc-Boron Foliar Fertilizer Improved Yield, Quality, Physiological Characteristics, and Microelement Concentration of Celery (Apium graveolens L.). Environ. Pollut. Bioavailab. 2019, 31, 261–271. [Google Scholar] [CrossRef]
- Wu, F.; Li, J.; Chen, Y.; Zhang, L.; Zhang, Y.; Wang, S.; Shi, X.; Li, L.; Liang, J. Effects of Phosphate Solubilizing Bacteria on the Growth, Photosynthesis, and Nutrient Uptake of Camellia Oleifera Abel. Forests 2019, 10, 348. [Google Scholar] [CrossRef]
- Berüter, J.; Feusi, M.E.S. The Effect of Girdling on Carbohydrate Partitioning in the Growing Apple Fruit. J. Plant Physiol. 1997, 151, 277–285. [Google Scholar] [CrossRef]
- Huang, D.; Liu, S.; Zhou, X.; Qian, D.; Wu, C.; Xu, Y. The Effect of Spiral Girdling on the Fruit-Setting and Development of Young Litchi Trees. Acta Hortic. 2012, 145–150. [Google Scholar] [CrossRef]
- Baninasab, B.; Rahemi, M.; Shariatmadari, H. Seasonal Changes in Mineral Content of Different Organs in the Alternate Bearing of Pistachio Trees. Commun. Soil Sci. Plant Anal. 2007, 38, 241–258. [Google Scholar] [CrossRef]
- Zhong, Z.; Zhang, X.; Wang, X.; Dai, Y.; Chen, Z.X.; Han, X.; Yang, G.; Ren, C.; Wang, X. C:N:P Stoichiometries Explain Soil Organic Carbon Accumulation During Afforestation. Nutr. Cycling Agroecosyst. 2020, 117, 243–259. [Google Scholar] [CrossRef]
- Yan, Z.; Kim, N.; Guo, Y.; Han, T.-S.; Du, E.Z.; Han, W.; Fang, J. Effects of Nitrogen and Phosphorus Fertilization on Leaf Carbon, Nitrogen and Phosphorus Stoichiometry of Arabidopsis thaliana. Chin. J. Plant Ecol. 2013, 37, 551–557. [Google Scholar] [CrossRef]
- Szczepanek, M.; Siwik-Ziomek, A. P and K Accumulation by Rapeseed as Affected by Biostimulant under Different Npk and S Fertilization Doses. Agronomy 2019, 9, 477. [Google Scholar] [CrossRef]
- González, C.E.A.; Hernández, M.M.; Fernández-Falcón, M. Nutrient Distribution and Stem Length in Flowering Stems of Protea Plants. J. Plant Nutr. 2008, 31, 1624–1641. [Google Scholar] [CrossRef]

| Treatment | Nutrient | ||
|---|---|---|---|
| N Content (g kg−1) | P Content (g kg−1) | K Content (g kg−1) | |
| Girdling+ foliar fertilizer | 14.70 ± 0.73a | 2.62 ± 0.14a | 4.73 ± 0.15a |
| Girdling | 12.42 ± 0.39b | 2.35 ± 0.03a | 4.15 ± 0.2a |
| CK | 11.74 ± 0.21b | 2.58 ± 0.17a | 4.44 ± 0.12a |
| p value | 0.013 * | 0.353 | 0.145 |
| Date | Treatment | N:P | N:K | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Roots | Fruits | Buds | Leaves | Roots | Fruits | Buds | ||
| 10 Mar | Girdling+ foliar fertilizer | 5.12 ± 0.07b | 2.19 ± 0.13b | – | – | 6.11 ± 0.12a | 2.19 ± 0.09b | – | – |
| Girdling | 5.98 ± 0.28a | 2.69 ± 0.10a | – | − | 5.21 ± 0.26b | 3.24 ± 0.07a | − | − | |
| CK | 2.22 ± 0.11c | 2.96 ± 0.06a | − | − | 2.63 ± 0.16c | 3.20 ± 0.06a | − | − | |
| 25 May | Girdling+ foliar fertilizer | 4.19 ± 0.17a | 1.52 ± 0.06b | − | − | 4.89 ± 0.13a | 5.76 ± 0.07ab | − | − |
| Girdling | 3.71 ± 0.16a | 1.28 ± 0.03c | − | − | 5.49 ± 0.24a | 5.14 ± 0.35b | − | − | |
| CK | 3.41 ± 0.31a | 1.79 ± 0.07a | − | − | 5.11 ± 0.24a | 5.85 ± 0.17a | − | − | |
| 5 Aug. | Girdling+ foliar fertilizer | 5.11 ± 0.59a | 5.75 ± 0.76a | 3.19 ± 0.35a | − | 6.74 ± 0.30a | 5.84 ± 0.36ab | 1.50 ± 0.06a | − |
| Girdling | 4.60 ± 0.91a | 6.45 ± 0.96a | 3.52 ± 0.36a | − | 6.14 ± 0.08a | 7.29 ± 1.75a | 1.35 ± 0.13a | − | |
| CK | 6.13 ± 0.27a | 4.37 ± 0.43a | 2.88 ± 0.18a | − | 4.59 ± 0.02b | 3.74 ± 0.36b | 1.47 ± 0.05a | − | |
| 15 Oct. | Girdling+ foliar fertilizer | 11.06 ± 0.61a | 5.78 ± 0.01a | 3.50 ± 0.14ab | 5.63 ± 0.20a | 7.17 ± 0.27b | 7.50 ± 0.38a | 1.37 ± 0.04a | 3.10 ± 0.09a |
| Girdling | 8.11 ± 2.30b | 5.82 ± 0.40a | 2.79 ± 0.14b | 5.27 ± 0.13b | 6.93 ± 0.13b | 6.54 ± 0.28a | 1.38 ± 0.11a | 3.01 ± 0.13ab | |
| CK | 12.79 ± 0.46a | 6.11 ± 0.26a | 3.71 ± 0.18a | 4.59 ± 0.25b | 8.18 ± 0.22a | 7.36 ± 0.18a | 1.75 ± 0.03a | 2.65 ± 0.03b | |
| Treatment | Yield per Plant (kg Plant−1) | Oil Content of Fresh Fruit (%) | Oil Production per Plant (g Plant−1) |
|---|---|---|---|
| Girdling+ foliar fertilizer | 9.58 ± 1.97ab | 4.71 ± 0.20b | 451.38 ± 85.56b |
| Girdling | 11.76 ± 1.61a | 5.12 ± 0.20b | 603.29 ± 82.59a |
| CK | 7.19 ± 1.67b | 5.89 ± 0.23a | 425.13 ± 99.18b |
| p value | 0.173 | 0.000 | 0.013 |
| Item | Major Affecting Factors | Regression Equation | R2 |
|---|---|---|---|
| Oil production per plant (g·plant−1) Y1 | Leaf of N:K ratio in March X1 | Y1 = 2164.853 + 175.036X1 − 206.702X2 − 213.266X3 − 320.795X4 | 0.992 |
| Root of K content in August X2 | |||
| Fruit of N:P ratio in August X3 | |||
| Fruit of N:K ratio in October X4 |
| Independent Variable | Direct Path Coefficient | Indirect Path Coefficient | Residual Path Coefficient | ||||
|---|---|---|---|---|---|---|---|
| X1 | X2 | X3 | X4 | Total | |||
| X1 | 1.065 | -- | −0.524 | 0.417 | −0.959 | −1.065 | 0.089 |
| X2 | −0.455 | 0.224 | -- | 0.122 | −0.281 | 0.065 | |
| X3 | −0.436 | −0.171 | 0.117 | -- | 0.262 | 0.208 | |
| X4 | −0.310 | 0.279 | −0.191 | 0.186 | -- | 0.274 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Li, D.; Liu, Z.; Wang, Y.; Ren, Z.; Li, C.; Cheng, Q.; Liu, J.; Zhang, L.; Zhang, L.; et al. Foliar Fertilizer Application Alters the Effect of Girdling on the Nutrient Contents and Yield of Camellia oleifera. Life 2023, 13, 591. https://doi.org/10.3390/life13020591
Xie S, Li D, Liu Z, Wang Y, Ren Z, Li C, Cheng Q, Liu J, Zhang L, Zhang L, et al. Foliar Fertilizer Application Alters the Effect of Girdling on the Nutrient Contents and Yield of Camellia oleifera. Life. 2023; 13(2):591. https://doi.org/10.3390/life13020591
Chicago/Turabian StyleXie, Shuangling, Dongmei Li, Zhouying Liu, Yuman Wang, Zhihua Ren, Cheng Li, Qinhua Cheng, Juan Liu, Ling Zhang, Linping Zhang, and et al. 2023. "Foliar Fertilizer Application Alters the Effect of Girdling on the Nutrient Contents and Yield of Camellia oleifera" Life 13, no. 2: 591. https://doi.org/10.3390/life13020591
APA StyleXie, S., Li, D., Liu, Z., Wang, Y., Ren, Z., Li, C., Cheng, Q., Liu, J., Zhang, L., Zhang, L., & Hu, D. (2023). Foliar Fertilizer Application Alters the Effect of Girdling on the Nutrient Contents and Yield of Camellia oleifera. Life, 13(2), 591. https://doi.org/10.3390/life13020591







