Left Atrial Remodeling in Response to Aortic Valve Replacement: Pathophysiology and Myocardial Strain Analysis
Abstract
1. Introduction
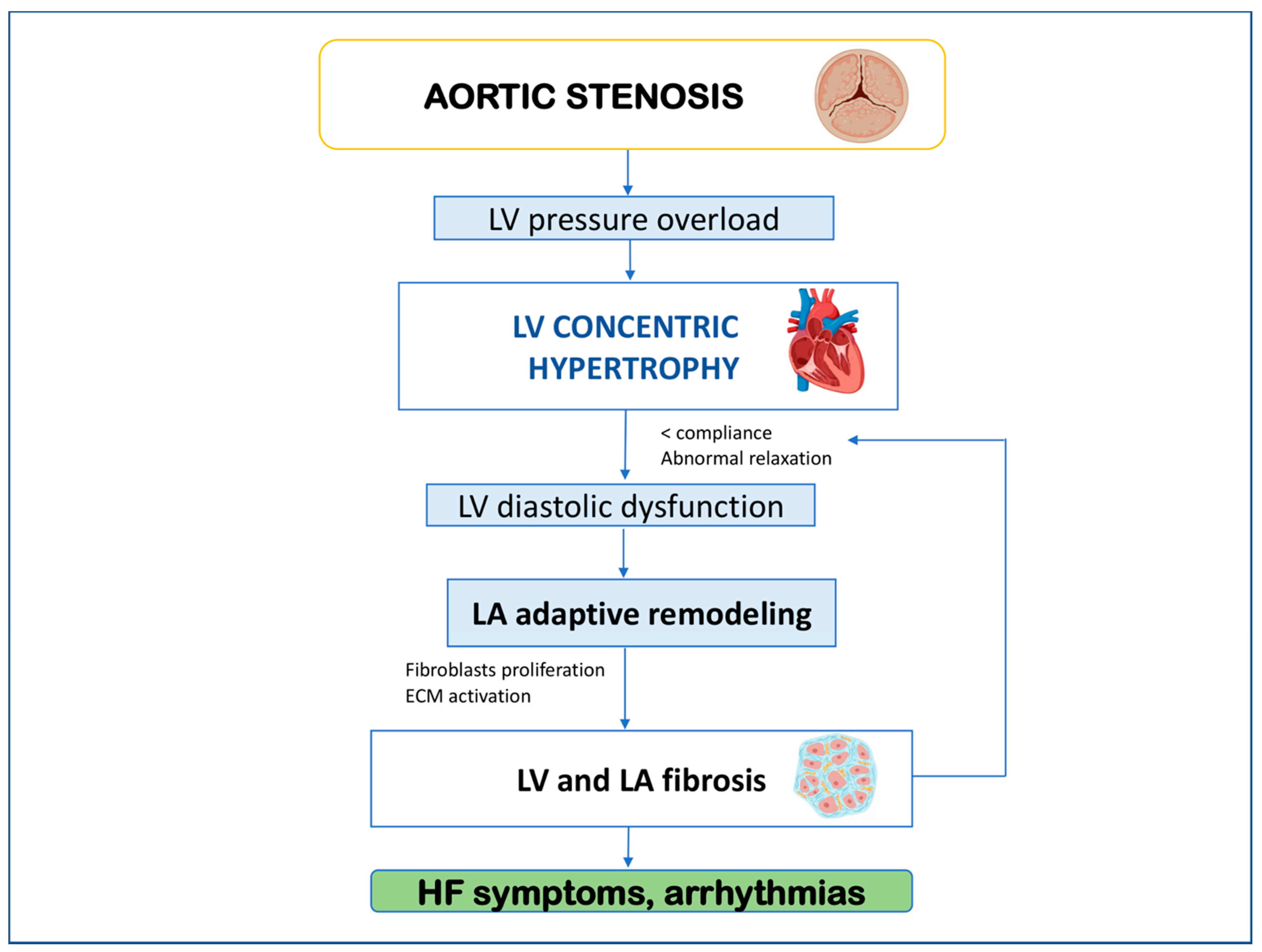
2. Left Atrial Remodeling and Myocardial Fibrosis in Aortic Stenosis
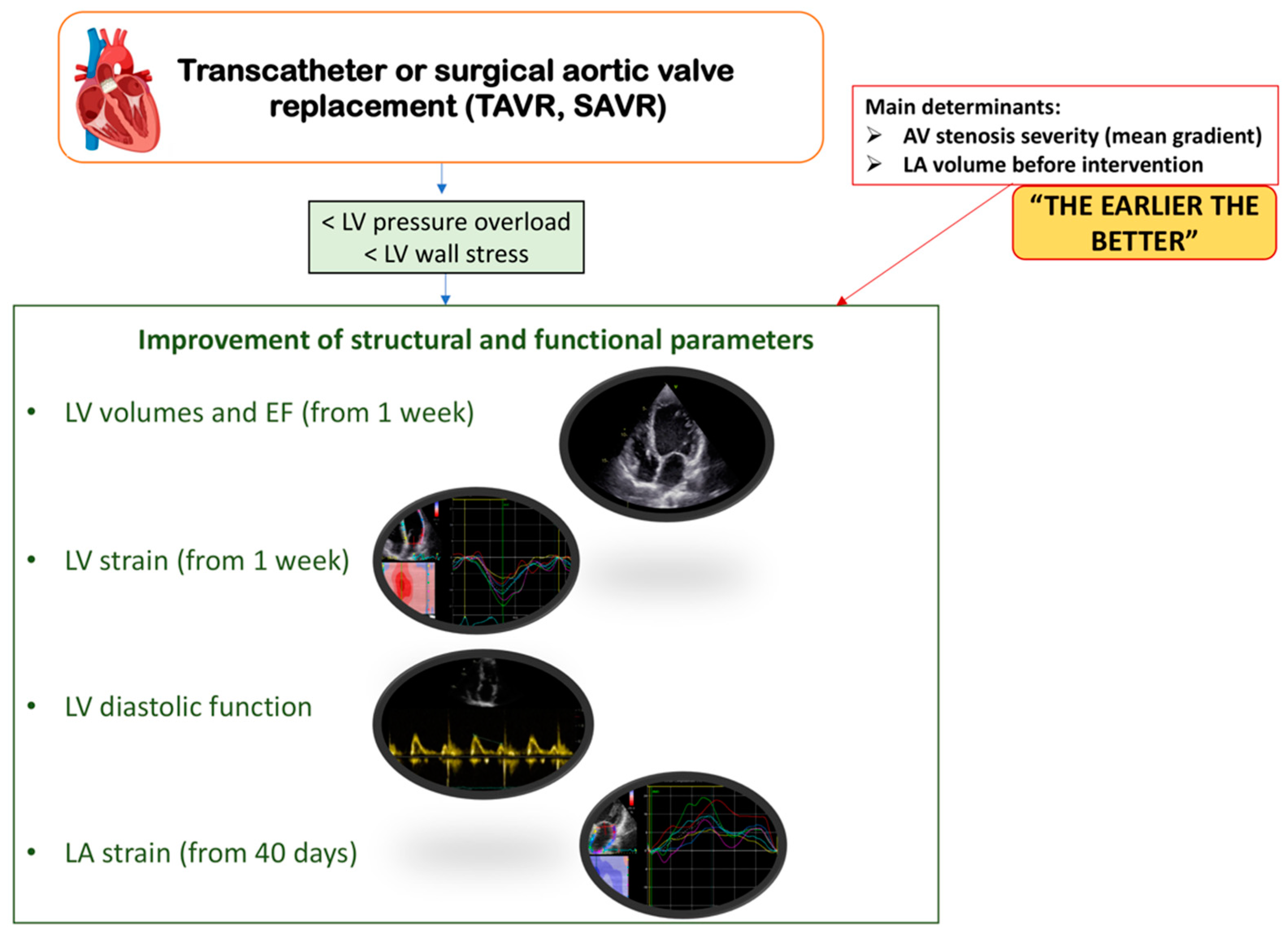
3. Left Ventricular and Left Atrial Remodeling after Aortic Valve Replacement
4. Clinical Implications
5. Speckle-Tracking Echocardiography (STE)—Technical Aspects
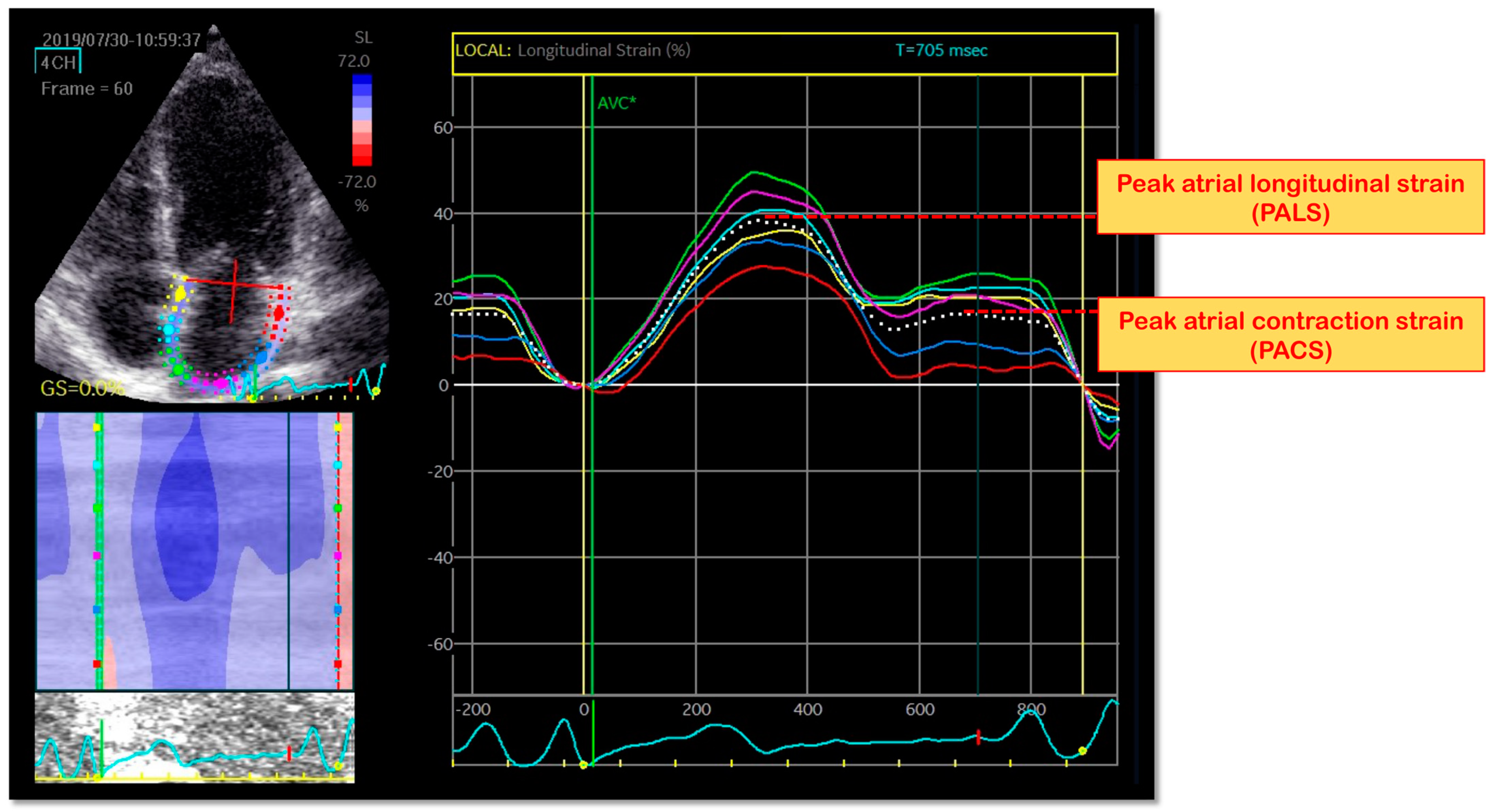
6. LA Longitudinal Myocardial Intrinsic Function by STE
7. Left Atrial Strain in Aortic Stenosis
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nkomo, V.T.; Gardin, J.M.; Skelton, T.N.; Gottdiener, J.S.; Scott, C.G.; Enriquez-Sarano, M. Burden of valvular heart diseases: A population-based study. Lancet 2006, 368, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M.; Lind, B.K.; Kitzman, D.W.; Gersh, B.J.; Siscovick, D.S. Association of aortic-valve sclerosis with cardiovascular mortality and morbidity in the elderly. N. Engl. J. Med. 1999, 341, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.M. Calcific aortic stenosis--time to look more closely at the valve. N. Engl. J. Med. 2008, 359, 1395–1398. [Google Scholar] [CrossRef] [PubMed]
- Iung, B.; Baron, G.; Butchart, E.G.; Delahaye, F.; Gohlke-Bärwolf, C.; Levang, O.W.; Tornos, P.; Vanoverschelde, J.L.; Vermeer, F.; Boersma, E.; et al. A prospective survey of patients with valvular heart disease in Europe: The Euro Heart Survey on Valvular Heart Disease. Eur. Heart J. 2003, 24, 1231–1243. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Edwardsen, T.; Goldstein, S.; Lancellotti, P.; LeFevre, M.; Miller, F., Jr.; Otto, C.M. Focus update on the echocardiographic assessment of aortic valve stenosis: EAE/ASE recommendations for clinical practice. Eur. J. Echocardiogr. 2017, 18, 254–275. [Google Scholar]
- Strauer, B.E. Ventricular function and coronary hemodynamics in hypertensive heart disease. Am. J. Cardiol. 1979, 44, 999–1006. [Google Scholar] [CrossRef]
- Henein, M.Y.; Holmgren, A.; Lindqvist, P. Left atrial function in volume versus pressure overloaded left atrium. Int. J. Cardiovasc. Imaging 2015, 31, 959–965. [Google Scholar] [CrossRef]
- Ross, J., Jr.; Braunwald, E. Aortic stenosis. Circulation 1968, 38 (Suppl. 1), 61–67. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Popma, J.J.; Deeb, G.M.; Yakubov, S.J.; Mumtaz, M.; Gada, H.; O’Hair, D.; Bajwa, T.; Heiser, J.C.; Merhi, W.; Kleiman, N.S.; et al. Transcatheter aortic-valve replacement with a selfexpanding valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1706–1715. [Google Scholar] [CrossRef]
- Thyregod, H.G.; Steinbrüchel, D.A.; Ihlemann, N.; Nissen, H.; Kjeldsen, B.J.; Petursson, P.; Chang, Y.; Franzen, O.W.; Engstrøm, T.; Clemmensen, P.; et al. Transcatheter versus surgical aortic valve replacement in patients with severe aortic valve stenosis: 1-Year results from the all-comers NOTION randomized clinical trial. J. Am. Coll. Cardiol. 2015, 65, 2184–2194. [Google Scholar] [CrossRef] [PubMed]
- Mack, M.J.; Leon, M.B.; Thourani, V.H.; Makkar, R.; Kodali, S.K.; Russo, M.; Kapadia, S.R.; Malaisrie, S.C.; Cohen, D.J.; Pibarot, P.; et al. Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N. Engl. J. Med. 2019, 380, 1695–1705. [Google Scholar] [CrossRef] [PubMed]
- Henein, M.Y.; Gibson, D.G. Normal long axis function. Heart 1999, 81, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Carabello, B.A.; Paulus, W.J. Aortic stenosis. Lancet 2009, 373, 956–966. [Google Scholar] [CrossRef] [PubMed]
- Kampaktsis, N.P.; Kokkinidis, D.G.; Wong, S.C.; Vavuranakis, M.; Skubas, N.J.; Devereux, R.B. The role and clinical implications of diastolic dysfunction in aortic stenosis. Heart 2017, 103, 1481–1487. [Google Scholar] [CrossRef]
- Gyongyosi, M.; Winkler, J.; Ramos, I.; Do, Q.T.; Firat, H.; McDonald, K.; Gonzalez, A.; Thum, T.; Diez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail 2017, 19, 177–191. [Google Scholar] [CrossRef]
- Lisi, M.; Cameli, M.; Mandoli, G.E.; Pastore, M.C.; Righini, F.M.; D’Ascenzi, F.; Focardi, M.; Rubboli, A.; Mondillo, S.; Henein, M.Y. Detection of myocardial fibrosis by speckle-tracking echocardiography: From prediction to clinical applications. Heart Fail. Rev. 2022, 27, 1857–1867. [Google Scholar] [CrossRef]
- Bing, R.; Dweck, M.R. Myocardial fibrosis: Why image, how to image and clinical implications. Heart 2019, 105, 1832–1840. [Google Scholar] [CrossRef]
- Bozkurt, B.; Colvin, M.; Cook, J.; Cooper, L.T.; Deswal, A.; Fonarow, G.C.; Francis, G.S.; Lenihan, D.; Lewis, E.F.; McNamara, D.M.; et al. Current Diagnostic and Treatment Strategies for Specific Dilated Cardiomyopathies: A Scientific Statement From the American Heart Association. Circulation 2016, 134, e579–e646. [Google Scholar] [CrossRef]
- Mondillo, S.; Cameli, M.; Caputo, M.L.; Lisi, M.; Palmerini, E.; Padeletti, M.; Ballo, P. Early detection of left atrial strain abnormalities by speckle-tracking in hypertensive and diabetic patients with normal left atrial size. J. Am. Soc. Echocardiogr. 2011, 24, 898–908. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur. J. Heart Fail 2019, 21, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Malagoli, A.; Rossi, L.; Bursi, F.; Zanni, A.; Sticozzi, C.; Piepoli, M.F.; Villani, G.Q. Left atrial function predicts cardiovascular events in patients with chronic heart failure with reduced ejection fraction. J. Am. Soc. Echocardiogr. 2019, 32, 248–256. [Google Scholar] [CrossRef] [PubMed]
- Park, J.J.; Park, J.H.; Hwang, I.C.; Park, J.B.; Cho, G.Y.; Marwick, T.H. Left atrial strain as a predictor of new-onset atrial fibrillation in patients with heart failure. JACC Cardiovasc. Imaging 2020, 13, 2071–2081. [Google Scholar] [CrossRef] [PubMed]
- Pavasini, R.; Fabbri, G.; Fiorio, A.; Campana, R.; Passarini, G.; Verardi, F.M.; Contoli, M.; Campo, G. Peak atrial longitudinal strain is predictive of atrial fibrillation in patients with chronic obstructive pulmonary disease and coronary artery disease. Echocardiography 2021, 38, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Lisi, M.; Righini, F.M.; Massoni, A.; Natali, B.M.; Focardi, M.; Tacchini, D.; Geyer, A.; Curci, V.; Di Tommaso, C.; et al. Usefulness of atrial deformation analysis to predict left atrial fibrosis and endocardial thickness in patients undergoing mitral valve operations for severe mitral regurgitation secondary to mitral valve prolapse. Am. J. Cardiol. 2013, 111, 595–601. [Google Scholar] [CrossRef]
- Lisi, M.; Mandoli, G.E.; Cameli, M.; Pastore, M.C.; Righini, F.M.; Benfari, G.; Rubboli, A.; D’Ascenzi, F.; Focardi, M.; Tsioulpas, C.; et al. Left atrial strain by speckle tracking predicts atrial fibrosis in patients undergoing heart transplantation. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 829–835. [Google Scholar] [CrossRef]
- Garg, V.; Ho, J.K.; Vorobiof, G. Changes in myocardial deformation after transcatheter and surgical aortic valve replacement. Echocardiography 2017, 34, 603–613. [Google Scholar] [CrossRef]
- Lindqvist, P.; Bajraktari, G.; Molle, R.; Palmerini, E.; Holmgren, A.; Mondillo, S.; Henein, M.Y. Valve replacement for aortic stenosis normalizes subendocardial function in patients with normal ejection fraction. Eur. J. Echocardiogr. 2010, 11, 608–613. [Google Scholar] [CrossRef][Green Version]
- Becker, M.; Kramann, R.; Dohmen, G.; Lückhoff, A.; Autschbach, R.; Kelm, M.; Hoffmann, R. Impact of left ventricular loading conditions on myocardial deformation parameters: Analysis of early and late changes of myocardial deformation parameters after aortic valve replacement. J. Am. Soc. Echocardiogr. 2007, 20, 681–689. [Google Scholar] [CrossRef]
- Carasso, S.; Cohen, O.; Mutlak, D.; Adler, Z.; Lessick, J.; Aronson, D.; Reisner, S.A.; Rakowski, H.; Bolotin, G.; Agmon, Y. Relation of myocardial mechanics in severe aortic stenosis to left ventricular ejection fraction and response to aortic valve replacement. Am J Cardiol. 2011, 107, 1052–1057. [Google Scholar] [CrossRef]
- Carasso, S.; Cohen, O.; Mutlak, D.; Adler, Z.; Lessick, J.; Reisner, S.A.; Rakowski, H.; Bolotin, G.; Agmon, Y. Differential effects of afterload on left ventricular long- and short-axis function: Insights from a clinical model of patients with aortic valve stenosis undergoing aortic valve replacement. Am. Heart J. 2009, 158, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Delgado, M.; Ruiz, M.; Mesa, D.; de Lezo Cruz Conde, J.S.; Pan, M.; López, J.; Villanueva, E.; Cejudo, L. Early improvement of the regional and global ventricle function estimated by two-dimensional speckle tracking echocardiography after percutaneous aortic valve implantation speckle tracking after CoreValve implantation. Echocardiography 2013, 30, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Schattke, S.; Baldenhofer, G.; Prauka, I.; Zhang, K.; Laule, M.; Stangl, V.; Sanad, W.; Spethmann, S.; Borges, A.C.; Baumann, G.; et al. Acute regional improvement of myocardial function after interventional transfemoral aortic valve replacement in aortic stenosis: A speckle tracking echocardiography study. Cardiovasc. Ultrasound 2012, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Løgstrup, B.B.; Andersen, H.R.; Thuesen, L.; Christiansen, E.H.; Terp, K.; Klaaborg, K.E.; Poulsen, S.H. Left ventricular global systolic longitudinal deformation and prognosis 1 year after femoral and apical transcatheter aortic valve implantation. J. Am. Soc. Echocardiogr. 2013, 26, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Spethmann, S.; Baldenhofer, G.; Dreger, H.; Stüer, K.; Sanad, W.; Saghabalyan, D.; Müller, E.; Stangl, V.; Baumann, G.; Stangl, K.; et al. Recovery of left ventricular and left atrial mechanics in various entities of aortic stenosis 12 months after TAVI. Eur. Heart J. Cardiovasc. Imaging 2014, 15, 389–398. [Google Scholar] [CrossRef]
- Delgado, V.; Tops, L.F.; van Bommel, R.J.; van der Kley, F.; Marsan, N.A.; Klautz, R.J.; Versteegh, M.I.; Holman, E.R.; Schalij, M.J.; Bax, J.J. Strain analysis in patients with severe aortic stenosis and preserved left ventricular ejection fraction undergoing surgical valve replacement. Eur. Heart J. 2009, 30, 3037–3047. [Google Scholar] [CrossRef]
- Clavel, M.A.; Webb, J.G.; Rodés-Cabau, J.; Masson, J.B.; Dumont, E.; De Larochellière, R.; Doyle, D.; Bergeron, S.; Baumgartner, H.; Burwash, I.G.; et al. Comparison between transcatheter and surgical prosthetic valve implantation in patients with severe aortic stenosis and reduced left ventricular ejection fraction. Circulation 2010, 122, 1928–1936. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cameli, M.; Iadanza, A.; Lisi, M.; Zacà, V.; Reccia, R.; Curci, V.; Torrisi, A.; Sinicropi, G.; Pierli, C.; et al. Improvement of left ventricular longitudinal systolic function after transcatheter aortic valve implantation: A speckle-tracking prospective study. Int. J. Cardiovasc. Imaging 2013, 29, 1007–1015. [Google Scholar] [CrossRef]
- Lisi, M.; Henein, M.Y.; Cameli, M.; Ballo, P.; Reccia, R.; Bennati, E.; Chiavarelli, M.; Maccherini, M.; Mondillo, S. Severity of aortic stenosis predicts early post-operative normalization of left atrial size and function detected by myocardial strain. Int. J. Cardiol. 2013, 167, 1450–1455. [Google Scholar] [CrossRef]
- D’Ascenzi, F.; Cameli, M.; Henein, M.; Iadanza, A.; Reccia, R.; Lisi, M.; Curci, V.; Sinicropi, G.; Torrisi, A.; Pierli, C.; et al. Left atrial remodelling in patients undergoing transcatheter aortic valve implantation: A speckle-tracking prospective, longitudinal study. Int J Cardiovasc. Imaging 2013, 29, 1717–1724. [Google Scholar] [CrossRef]
- Galli, E.; Fournet, M.; Chabanne, C.; Lelong, B.; Leguerrier, A.; Flecher, E.; Mabo, P.; Donal, E. Prognostic value of left atrial reservoir function in patients with severe aortic stenosis: A 2D speckle-tracking echocardiographic study. Eur. Heart J. Cardiovasc. Imaging 2016, 17, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.; Bond, K.; Flanagan, J.; Passick, M.; Petillo, F.; Pollack, S.; Robinson, N.; Petrossian, G.; Cao, J.J.; Barasch, E. The Prognostic Value of Left Atrial Global Longitudinal Strain and Left Atrial Phasic Volumes in Patients Undergoing Transcatheter Valve Implantation for Severe Aortic Stenosis. Cardiology 2021, 146, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Rusinaru, D.; Bohbot, Y.; Kowalski, C.; Ringle, A.; Maréchaux, S.; Tribouilloy, C. Left Atrial Volume and Mortality in Patients with Aortic Stenosis. J. Am. Heart Assoc. 2017, 6, e006615. [Google Scholar] [CrossRef] [PubMed]
- Cameli, M.; Lisi, M.; Reccia, R.; Bennati, E.; Malandrino, A.; Solari, M.; Bigio, E.; Biagioli, B.; Righini, F.M.; Maccherini, M.; et al. Pre-operative left atrial strain predicts post-operative atrial fibrillation in patients undergoing aortic valve replacement for aortic stenosis. Int. J. Cardiovasc. Imaging 2014, 30, 279–286. [Google Scholar] [CrossRef] [PubMed]
- Kawczynski, M.J.; Gilbers, M.; Van De Walle, S.; Schalla, S.; Crijns, H.J.; Maessen, J.G.; Schotten, U.; Maesen, B.; Bidar, E. Role of pre-operative transthoracic echocardiography in predicting post-operative atrial fibrillation after cardiac surgery: A systematic review of the literature and meta-analysis. Europace 2021, 23, 1731–1743. [Google Scholar] [CrossRef]
- Salas-Pacheco, J.L.; Avila-Vanzzini, N.; Eugenia, R.M.; Arias-Godinez, J.A. Left atrium function by 2D speckle tracking in aortic valve disease. Echocardiography 2016, 33, 1828–1834. [Google Scholar] [CrossRef]
- Calin, A.; Mateescu, A.D.; Rosca, M.; Beladan, C.C.; Enache, R.; Botezatu, S.; Cosei, I.; Calin, C.; Simion, M.; Ginghina, C.; et al. Left atrial dysfunction as a determinant of pulmonary hypertension in patients with severe aortic stenosis and preserved left ventricular ejection fraction. Int. J. Cardiovasc. Imaging 2017, 33, 1939–1947. [Google Scholar] [CrossRef]
- Leitman, M.; Lysyansky, P.; Sidenko, S.; Shir, V.; Peleg, E.; Binenbaum, M.; Kaluski, E.; Krakover, R.; Vered, Z. Two-dimensional strain-a novel software for real-time quantitative echocardiographic assessment of myocardial function. J. Am. Soc. Echocardiogr. 2004, 17, 1021–1029. [Google Scholar] [CrossRef]
- Cameli, M.; Lisi, M.; Righini, F.M.; Mondillo, S. Novel echocardiographic techniques to assess left atrial size, anatomy and function. Cardiovasc. Ultrasound 2012, 10, 4. [Google Scholar] [CrossRef]
- Cameli, M.; Caputo, M.; Mondillo, S.; Ballo, P.; Palmerini, E.; Lisi, M.; Marino, E.; Galderisi, M. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc. Ultrasound 2009, 7, 6. [Google Scholar] [CrossRef]
- Meimoun, P.; Djebali, M.; Botoro, T.; Djou, U.; Bidounga, H.; Elmkies, F.; Martis, S.; Clerc, J. Left atrial strain and distensibility in relation to left ventricular dysfunction and prognosis in aortic stenosis. Echocardiography 2019, 36, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Pernigo, M.; Benfari, G.; Geremia, G.; Noni, M.; Borio, G.; Mazzali, G.; Zamboni, M.; Onorati, F.; Faggian, G.; Vassanelli, C.; et al. Atrial function as an independent predictor of postoperative atrial fibrillation in patients undergoing aortic valve surgery for severe aortic stenosis. J. Am. Soc. Echocardiogr. 2017, 30, 956–965. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lisi, M.; Pastore, M.C.; Fiorio, A.; Cameli, M.; Mandoli, G.E.; Righini, F.M.; Cavigli, L.; D’Ascenzi, F.; Focardi, M.; Rubboli, A.; et al. Left Atrial Remodeling in Response to Aortic Valve Replacement: Pathophysiology and Myocardial Strain Analysis. Life 2022, 12, 2074. https://doi.org/10.3390/life12122074
Lisi M, Pastore MC, Fiorio A, Cameli M, Mandoli GE, Righini FM, Cavigli L, D’Ascenzi F, Focardi M, Rubboli A, et al. Left Atrial Remodeling in Response to Aortic Valve Replacement: Pathophysiology and Myocardial Strain Analysis. Life. 2022; 12(12):2074. https://doi.org/10.3390/life12122074
Chicago/Turabian StyleLisi, Matteo, Maria Concetta Pastore, Alessio Fiorio, Matteo Cameli, Giulia Elena Mandoli, Francesca Maria Righini, Luna Cavigli, Flavio D’Ascenzi, Marta Focardi, Andrea Rubboli, and et al. 2022. "Left Atrial Remodeling in Response to Aortic Valve Replacement: Pathophysiology and Myocardial Strain Analysis" Life 12, no. 12: 2074. https://doi.org/10.3390/life12122074
APA StyleLisi, M., Pastore, M. C., Fiorio, A., Cameli, M., Mandoli, G. E., Righini, F. M., Cavigli, L., D’Ascenzi, F., Focardi, M., Rubboli, A., Campo, G., Mondillo, S., & Henein, M. Y. (2022). Left Atrial Remodeling in Response to Aortic Valve Replacement: Pathophysiology and Myocardial Strain Analysis. Life, 12(12), 2074. https://doi.org/10.3390/life12122074








