The Role of Stem Cell Factor, Epidermal Growth Factor and Angiopoietin-2 in HBV, HCV, HCC and NAFLD
Abstract
1. Introduction
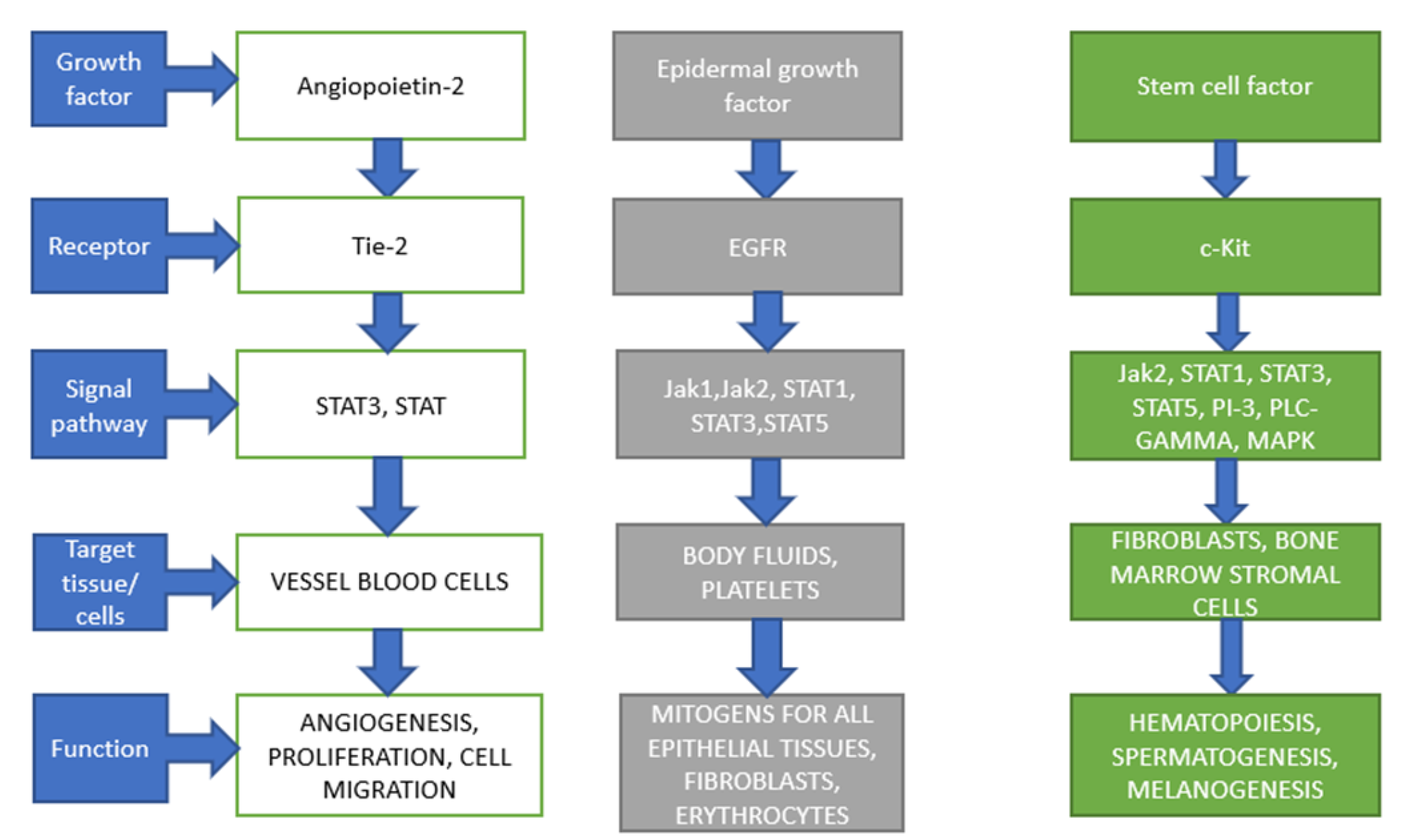
2. Stem Cell Factor (SCF)
3. Epidermal Growth Factor (EGF)
4. Angiopietin-2
5. Molecular and Cellular Mechanisms in the Immunopathogenesis of Liver Fibrosis
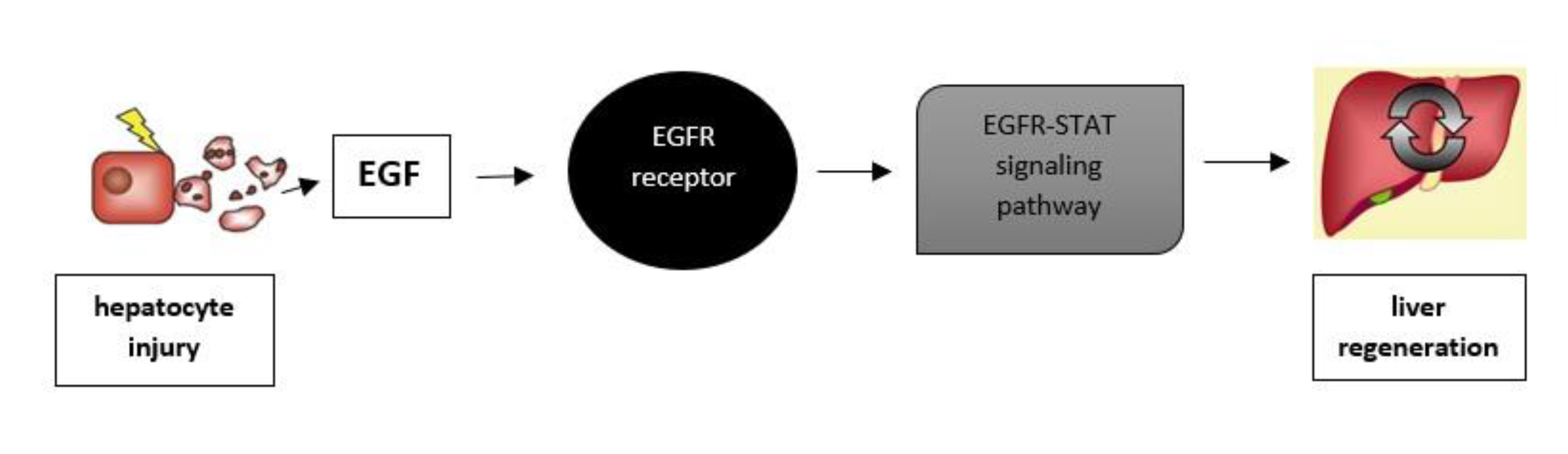
Liver Regeneration
6. SCF in Liver Disease
7. EGF in Liver Disease
8. Angiopoietin-2 in Liver Disease
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.C.; Zhang, Q.B.; Qiao, L. Pathogenesis of liver cirrhosis. World J. Gastroenterol. 2014, 20, 7312–7324. [Google Scholar] [CrossRef] [PubMed]
- Berasain, C.; Avila, M.A. The EGFR signalling system in the liver: From hepatoprotection to hepatocarcinogenesis. J. Gastroenterol. 2014, 49, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Global Burden of Disease Cancer Collaboration; Fitzmaurice, C.; Allen, C.; Barber, R.M.; Barregard, L.; Bhutta, Z.A.; Brenner, H.; Dicker, D.J.; Chimed-Orchir, O.; Dandona, R.; et al. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2017, 3, 524–548. [Google Scholar] [CrossRef] [PubMed]
- Konyn, P.; Ahmed, A.; Kim, D. Current epidemiology in hepatocellular carcinoma. Expert Rev. Gastroenterol. Hepatol. 2021, 15, 1295–1307. [Google Scholar] [CrossRef] [PubMed]
- Younossi, Z.M. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019, 70, 531–544. [Google Scholar] [CrossRef]
- Nobili, V.; Alisi, A.; Valenti, L.; Miele, L.; Feldstein, A.E.; Alkhouri, N. NAFLD in children: New genes, new diagnostic modalities and new drugs. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 517–530. [Google Scholar] [CrossRef]
- Estes, C.; Razavi, H.; Loomba, R.; Younossi, Z.; Sanyal, A.J. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018, 67, 123–133. [Google Scholar] [CrossRef]
- Ballestri, S.; Zona, S.; Targher, G.; Romagnoli, D.; Baldelli, E.; Nascimbeni, F.; Roverato, A.; Guaraldi, G.; Lonardo, A. Nonalcoholic fatty liver disease is associated with an almost twofold increased risk of incident type 2 diabetes and metabolic syndrome. Evidence from a systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2016, 31, 936–944. [Google Scholar] [CrossRef]
- Marra, F.; Svegliati-Baroni, G. Lipotoxicity and the gut-liver axis in NASH pathogenesis. J. Hepatol. 2018, 68, 280–295. [Google Scholar] [CrossRef]
- Wang, W.; Shui, L.; Liu, Y.; Zheng, M. C-Kit, a Double-Edged Sword in Liver Regeneration and Diseases. Front. Genet. 2021, 12, 598855. [Google Scholar] [CrossRef]
- Foster, B.M.; Langsten, K.L.; Mansour, A.; Shi, L.; Kerr, B.A. Tissue distribution of stem cell factor in adults. Exp. Mol. Pathol. 2021, 122, 104678. [Google Scholar] [CrossRef]
- Lennartsson, J.; Rönnstrand, L. Stem cell factor receptor/c-Kit: From basic science to clinical implications. Physiol. Rev. 2012, 92, 1619–1649. [Google Scholar] [CrossRef]
- Takematsu, E.; Massidda, M.; Auster, J.; Chen, P.C.; Im, B.; Srinath, S.; Canga, S.; Singh, A.; Majid, M.; Sherman, M.; et al. Transmembrane stem cell factor protein therapeutics enhance revascularization in ischemia without mast cell activation. Nat. Commun. 2022, 13, 2497. [Google Scholar] [CrossRef]
- Sheikh, E.; Tran, T.; Vranic, S.; Levy, A.; Bonfil, R.D. Role and significance of c-KIT receptor tyrosine kinase in cancer: A review. Bosn. J. Basic Med. Sci. 2022, 22, 683–698. [Google Scholar] [CrossRef]
- Feuser, K.; Feilhauer, K.; Staib, L.; Bischoff, S.C.; Lorentz, A. Akt cross-links IL-4 priming, stem cell factor signaling, and IgE-dependent activation in mature human mast cells. Mol. Immunol. 2011, 48, 546–552. [Google Scholar] [CrossRef]
- Berlanga-Acosta, J.; Gavilondo-Cowley, J.; López-Saura, P.; González-López, T.; Castro-Santana, M.D.; López-Mola, E.; Guillén-Nieto, G.; Herrera-Martinez, L. Epidermal growth factor in clinical practice—A review of its biological actions, clinical indications and safety implications. Int. Wound J. 2009, 6, 331–346. [Google Scholar] [CrossRef]
- Chen, J.; Zeng, F.; Forrester, S.J.; Eguchi, S.; Zhang, M.Z.; Harris, R.C. Expression and Function of the Epidermal Growth Factor Receptor in Physiology and Disease. Physiol. Rev. 2016, 96, 1025–1069. [Google Scholar] [CrossRef]
- Konturek, J.W.; Brzozowski, T.; Konturek, S.J. Epidermal growth factor in protection, repair, and healing of gastroduodenal mucosa. J. Clin. Gastroenterol. 1991, 13, S88–S97. [Google Scholar] [CrossRef]
- Warzecha, Z.; Kownacki, P.; Ceranowicz, P.; Dembinski, M.; Cieszkowski, J.; Dembinski, A. Ghrelin accelerates the healing of oral ulcers in non-sialoadenectomized and sialoadenectomized rats. J. Physiol. Pharmacol. 2013, 64, 657–668. [Google Scholar]
- Girdler, N.M.; McGurk, M.; Aqual, S.; Prince, M. The effect of epidermal growth factor mouthwash on cytotoxic-induced oral ulceration. A phase I clinical trial. Am. J. Clin. Oncol. 1995, 18, 403–406. [Google Scholar] [CrossRef]
- Konturek, S.J.; Dembinski, A.; Warzecha, Z.; Brzozowski, T.; Gregory, H. Role of epidermal growth factor in healing of chronic gastroduodenal ulcers in rats. Gastroenterology 1988, 94, 1300–1307. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.C.; Liu, K.Y.; Chen, S.H.; Fang, C.L.; Tsao, C.W. Effect of oral epidermal growth factor on mucosal healing in rats with duodenal ulcer. World J. Gastroenterol. 2003, 9, 2261–2265. [Google Scholar] [CrossRef]
- Lai, H.S.; Chung, Y.C.; Chen, W.J.; Chen, K.M. Rat liver regeneration after partial hepatectomy: Effects of insulin, glucagon and epidermal growth factor. J. Formos. Med. Assoc. 1992, 91, 685–690. [Google Scholar] [PubMed]
- Dembiński, A.; Warzecha, Z.; Konturek, P.C.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.J. Epidermal growth factor accelerates pancreatic recovery after caerulein-induced pancreatitis. Eur. J. Pharmacol. 2000, 398, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Orofiamma, L.A.; Vural, D.; Antonescu, C.N. Control of cell metabolism by the epidermal growth factor receptor. Biochim. Et Biophys. Acta Mol. Cell Res. 2022, 1869, 119359. [Google Scholar] [CrossRef]
- Cao, S.; Pan, Y.; Tang, J.; Terker, A.S.; Arroyo Ornelas, J.P.; Jin, G.N.; Wang, Y.; Niu, A.; Fan, X.; Wang, S.; et al. EGFR-mediated activation of adipose tissue macrophages promotes obesity and insulin resistance. Nat. Commun. 2022, 13, 4684. [Google Scholar] [CrossRef]
- Du, X.; Yang, B.; An, Q.; Assaraf, Y.G.; Cao, X.; Xia, J. Acquired resistance to third-generation EGFR-TKIs and emerging next-generation EGFR inhibitors. Innovation 2021, 2, 100103. [Google Scholar] [CrossRef]
- Janžič, U.; Turnšek, N.; Dediu, M.; Donev, I.S.; Lupu, R.; Teodorescu, G.; Ciuleanu, T.E.; Pluzanski, A. Real-World Testing Practices, Treatment Patterns and Clinical Outcomes in Patients from Central Eastern Europe with EGFR-Mutated Advanced Non-Small Cell Lung Cancer: A Retrospective Chart Review Study (REFLECT). Curr. Oncol. 2022, 29, 5833–5845. [Google Scholar] [CrossRef]
- Leong, A.; Kim, M. The Angiopoietin-2 and TIE Pathway as a Therapeutic Target for Enhancing Antiangiogenic Therapy and Immunotherapy in Patients with Advanced Cancer. Int. J. Mol. Sci. 2020, 21, 8689. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, T.; Li, Y.; You, J.; Deng, X.; Chen, N.; Li, T.; Zheng, Y.; Li, R.; Luo, M.; et al. Glycation of Tie-2 Inhibits Angiopoietin-1 Signaling Activation and Angiopoietin-1-Induced Angiogenesis. Int. J. Mol. Sci. 2022, 23, 7137. [Google Scholar] [CrossRef]
- Liu, N.; Liu, M.; Fu, S.; Wang, J.; Tang, H.; Isah, A.D.; Chen, D.; Wang, X. Ang2-Targeted Combination Therapy for Cancer Treatment. Front. Immunol. 2022, 13, 949553. [Google Scholar] [CrossRef]
- Liu, L.; Li, X.; Zhao, Q.; Yang, L.; Jiang, X. Pathogenesis of Port-Wine Stains: Directions for Future Therapies. Int. J. Mol. Sci. 2022, 23, 12139. [Google Scholar] [CrossRef]
- Burks, K.H.; Basu, D.; Goldberg, I.J.; Stitziel, N.O. Angiopoietin-like 3: An important protein in regulating lipoprotein levels. Best Pract. Res. Clin. Endocrinol. Metab. 2022, 1, 101688. [Google Scholar] [CrossRef]
- Shirley, M. Faricimab: First Approval. Drugs 2022, 82, 825–830. [Google Scholar] [CrossRef]
- Acharya, P.; Chouhan, K.; Weiskirchen, S.; Weiskirchen, R. Cellular Mechanisms of Liver Fibrosis. Front. Pharmacol. 2021, 12, 671640. [Google Scholar] [CrossRef]
- Tanaka, S.; Hikita, H.; Tatsumi, T.; Sakamori, R.; Nozaki, Y.; Sakane, S.; Shiode, Y.; Nakabori, T.; Saito, Y.; Hiramatsu, N.; et al. Rubicon inhibits autophagy and accelerates hepatocyte apoptosis and lipid accumulation in nonalcoholic fatty liver disease in mice. Hepatology 2016, 64, 1994–2014. [Google Scholar] [CrossRef]
- Zhou, Z.; Xu, M.J.; Gao, B. Hepatocytes: A key cell type for innate immunity. Cell. Mol. Immunol. 2016, 13, 301–315. [Google Scholar] [CrossRef]
- Shetty, S.; Lalor, P.F.; Adams, D.H. Liver sinusoidal endothelial cells—Gatekeepers of hepatic immunity. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 555–567. [Google Scholar] [CrossRef]
- Kisseleva, T.; Brenner, D. Molecular and cellular mechanisms of liver fibrosis and its regression. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 151–166. [Google Scholar] [CrossRef]
- Schuppan, D.; Ashfaq-Khan, M.; Yang, A.T.; Kim, Y.O. Liver fibrosis: Direct antifibrotic agents and targeted therapies. Matrix Biol. 2018, 68–69, 435–451. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Yuan, W.G.; He, P.; Lei, J.H.; Wang, C.X. Liver fibrosis and hepatic stellate cells: Etiology, pathological hallmarks and therapeutic targets. World J. Gastroenterol. 2016, 22, 10512–10522. [Google Scholar] [CrossRef] [PubMed]
- Mederacke, I. Liver fibrosis—Mouse models and relevance in human liver diseases. Z. Gastroenterol. 2013, 51, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Krenke, K.; Szczałuba, K.; Bielecka, T.; Rydzanicz, M.; Lange, J.; Koppolu, A.; Płoski, R. FARSA mutations mimic phenylalanyl-tRNA synthetase deficiency caused by FARSB defects. Clin. Genet. 2019, 96, 468–472. [Google Scholar] [CrossRef] [PubMed]
- Higashi, T.; Friedman, S.L.; Hoshida, Y. Hepatic stellate cells as key target in liver fibrosis. Adv. Drug Deliv. Rev. 2017, 121, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Iwaisako, K.; Jiang, C.; Zhang, M.; Cong, M.; Moore-Morris, T.J.; Park, T.J.; Liu, X.; Xu, J.; Wang, P.; Paik, Y.H.; et al. Origin of myofibroblasts in the fibrotic liver in mice. Proc. Natl. Acad. Sci. USA 2014, 111, E3297–E3305. [Google Scholar] [CrossRef]
- He, Z.; Yang, D.; Fan, X.; Zhang, M.; Li, Y.; Gu, X.; Yang, M. The Roles and Mechanisms of lncRNAs in Liver Fibrosis. Int. J. Mol. Sci. 2020, 21, 1482. [Google Scholar] [CrossRef]
- Li, W.; Li, L.; Hui, L. Cell Plasticity in Liver Regeneration. Trends Cell Biol. 2020, 30, 329–338. [Google Scholar] [CrossRef]
- Michalopoulos, G.K.; Bhushan, B. Liver regeneration: Biological and pathological mechanisms and implications. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 40–55. [Google Scholar] [CrossRef]
- Xia, H.Z.; Du, Z.; Craig, S.; Klisch, G.; Noben-Trauth, N.; Kochan, J.P.; Huff, T.H.; Irani, A.M.; Schwartz, L.B. Effect of recombinant human IL-4 on tryptase, chymase, and Fc epsilon receptor type I expression in recombinant human stem cell factor-dependent fetal liver-derived human mast cells. J. Immunol. 1997, 159, 2911–2921. [Google Scholar]
- Meng, F.; Francis, H.; Glaser, S.; Han, Y.; DeMorrow, S.; Stokes, A.; Staloch, D.; Venter, J.; White, M.; Ueno, Y.; et al. Role of stem cell factor and granulocyte colony-stimulating factor in remodeling during liver regeneration. Hepatology 2012, 55, 209–221. [Google Scholar] [CrossRef]
- Gaça, M.D.; Pickering, J.A.; Arthur, M.J.; Benyon, R.C. Human and rat hepatic stellate cells produce stem cell factor: A possible mechanism for mast cell recruitment in liver fibrosis. J. Hepatol. 1999, 30, 850–858. [Google Scholar] [CrossRef]
- Meadows, V.; Kennedy, L.; Hargrove, L.; Demieville, J.; Meng, F.; Virani, S.; Reinhart, E.; Kyritsi, K.; Invernizzi, P.; Yang, Z.; et al. Downregulation of hepatic stem cell factor by Vivo-Morpholino treatment inhibits mast cell migration and decreases biliary damage/senescence and liver fibrosis in Mdr2−/− mice. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 165557. [Google Scholar] [CrossRef]
- Radmanić, L.; Bodulić, K.; Šimičić, P.; Vince, A.; Lepej, S.Ž. The Effect of Treatment-Induced Viral Eradication on Cytokine and Growth Factor Expression in Chronic Hepatitis C. Viruses 2022, 14, 1613. [Google Scholar] [CrossRef]
- Khodadi, E.; Shahrabi, S.; Shahjahani, M.; Azandeh, S.; Saki, N. Role of stem cell factor in the placental niche. Cell Tissue Res. 2016, 366, 523–531. [Google Scholar] [CrossRef]
- Rybtsov, S.; Batsivari, A.; Bilotkach, K.; Paruzina, D.; Senserrich, J.; Nerushev, O.; Medvinsky, A. Tracing the origin of the HSC hierarchy reveals an SCF-dependent, IL-3-independent CD43(-) embryonic precursor. Stem Cell Rep. 2014, 3, 489–501. [Google Scholar] [CrossRef]
- Pocino, K.; Napodano, C.; Marino, M.; Di Santo, R.; Miele, L.; De Matthaeis, N.; Gulli, F.; Saporito, R.; Rapaccini, G.L.; Ciasca, G.; et al. A Comparative Study of Serum Angiogenic Biomarkers in Cirrhosis and Hepatocellular Carcinoma. Cancers 2021, 14, 11. [Google Scholar] [CrossRef]
- Tarantino, G.; Balsano, C.; Santini, S.J.; Brienza, G.; Clemente, I.; Cosimini, B.; Sinatti, G. It Is High Time Physicians Thought of Natural Products for Alleviating NAFLD. Is There Sufficient Evidence to Use Them? Int. J. Mol. Sci. 2021, 22, 13424. [Google Scholar] [CrossRef]
- Tarantino, G.; Citro, V.; Balsano, C.; Capone, D. Could SCGF-Beta Levels Be Associated with Inflammation Markers and Insulin Resistance in Male Patients Suffering from Obesity-Related NAFLD? Diagnostics 2020, 10, 395. [Google Scholar] [CrossRef]
- Aydın, M.M.; Akçalı, K.C. Liver fibrosis. Turk. J. Gastroenterol. 2018, 29, 14–21. [Google Scholar] [CrossRef]
- Shehata, F.; Abdel Monem, N.; Sakr, M.; Kasem, S.; Balbaa, M. Epidermal growth factor, its receptor and transforming growth factor-β1 in the diagnosis of HCV-induced hepatocellular carcinoma. Med. Oncol. 2013, 30, 673. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Y.L.; Sun, D.; Zhu, X.L.; Li, Z.; Ni, C.F. Increased expression of epidermal growth factor-like domain-containing protein 7 is predictive of poor prognosis in patients with hepatocellular carcinoma. J. Cancer Res. Ther. 2018, 14, 867–872. [Google Scholar] [PubMed]
- Li, Y.; Xie, Q.; Lu, F.; Zhao, J.; Mao, P.; Li, Z.; Liu, S.; Zhuang, H. Association between epidermal growth factor 61A/G polymorphism and hepatocellular carcinoma susceptibility in Chinese patients. Liver Int. 2010, 30, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Carver, R.S.; Stevenson, M.C.; Scheving, L.A.; Russell, W.E. Diverse expression of ErbB receptor proteins during rat liver development and regeneration. Gastroenterology 2002, 123, 2017–2127. [Google Scholar] [CrossRef] [PubMed]
- El Taghdouini, A.; Najimi, M.; Sancho-Bru, P.; Sokal, E.; van Grunsven, L.A. In vitro reversion of activated primary human hepatic stellate cells. Fibrogenesis Tissue Repair 2015, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Bartolomé, Á.; López-Rodríguez, R.; Borque, M.J.; González-Moreno, L.; Real-Martínez, Y.; García-Buey, L.; Moreno-Otero, R.; Sanz-Cameno, P. Angiopoietin-2/angiopoietin-1 as non-invasive biomarker of cirrhosis in chronic hepatitis C. World J. Gastroenterol. 2016, 22, 9744–9751. [Google Scholar] [CrossRef] [PubMed]
- Lefere, S.; Van de Velde, F.; Hoorens, A.; Raevens, S.; Van Campenhout, S.; Vandierendonck, A.; Neyt, S.; Vandeghinste, B.; Vanhove, C.; Debbaut, C.; et al. Angiopoietin-2 Promotes Pathological Angiogenesis and Is a Therapeutic Target in Murine Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 1087–1104. [Google Scholar] [CrossRef]
- Manco, M.; Panera, N.; Crudele, A.; Braghini, M.R.; Bianchi, M.; Comparcola, D.; De Vito, R.; Maggiore, G.; Alisi, A. Angiopoietin-2 levels correlates with disease activity in children with nonalcoholic fatty liver disease. Pediatr. Res. 2022, 91, 1781–1786. [Google Scholar] [CrossRef]
- Akwii, R.G.; Sajib, M.S.; Zahra, F.T.; Mikelis, C.M. Role of Angiopoietin-2 in Vascular Physiology and Pathophysiology. Cells 2019, 8, 471. [Google Scholar] [CrossRef]
- Wang, Q.; Lash, G.E. Angiopoietin 2 in placentation and tumor biology: The yin and yang of vascular biology. Placenta 2017, 56, 73–78. [Google Scholar] [CrossRef]
- Gehrke, N.; Schattenberg, J.M. Metabolic Inflammation-A Role for Hepatic Inflammatory Pathways as Drivers of Comorbidities in Nonalcoholic Fatty Liver Disease? Gastroenterology 2020, 158, 1929–1947. [Google Scholar] [CrossRef]
- Hammoutene, A.; Rautou, P.E. Role of liver sinusoidal endothelial cells in non-alcoholic fatty liver disease. J. Hepatol. 2019, 70, 1278–1291. [Google Scholar] [CrossRef]
- Kimura, H.; Mochida, S.; Inao, M.; Matsui, A.; Fujiwara, K. Angiopoietin/tie receptors system may play a role during reconstruction and capillarization of the hepatic sinusoids after partial hepatectomy and liver necrosis in rats. Hepatol. Res. 2004, 29, 51–59. [Google Scholar] [CrossRef]


| Study | Design | Main Outcomes | Conclusions and Implications | |
|---|---|---|---|---|
| HEPATITIS B/C and/or hepatocellular carcinoma | Radmanić et al., 2022 [53] | Retrospective study, 56 patients with chronic hepatitis C and 15 controls | Significantly higher levels in hepatitis C patients after reaching SVR when compared to healthy individuals were showm in Ang-2 (medians 2180.4 and 1206.6 pg/mL, p = 0.027), EGF (medians 73.4 and 30.8 pg/mL, p = 0.037) and SCF (medians 86.5 and 28.6 pg/mL, p < 0.001). | Tje results suggested promotion of liver regeneration in chronic hepatitis C patients during direct acting antiviral treatment. |
| Shehata et al., 2013 [60] | Retrospective study, 20 healthy volunteers, patients are subdivided into 20 patients with chronic hepatitis C infection and 30 patients with hepatocellular carcinoma | Significantly higher serum levels of EGF in patients with HCC (1476 ± 970 pg/mL), compared to the level in patients with chronic hepatitis C infection (747 ± 296 pg/mL) and control subjects (625 ± 175 pg/mL). | EGF can be used as a sensitive biomarker in the diagnosis, prognosis, metastasis and recurrence of hepatocellular carcinoma patients and in the management of hepatocellular carcinoma. | |
| Yang et al., 2018 [61] | Retrospective study, 182 cases of hepatocellular carcinoma formalin-fixed and paraffin-embedded tissues and 110 cases of hepatocellular carcinoma/chronic hepatitis C serum samples | The correlations between serum EGF and vascular invasion (32 pg/mL) and extrahepatic metastasis (31.7 pg/mL) were statistically significant (p < 0.0001). | EGF is a potential indicator of the survival of patients with hepatocellular carcinoma and can be a biomarker and therapeutic target structure in hepatocellular carcinoma. | |
| Li et al., 2010 [62] | Retrospective study,186 hepatitis C/hepatitis B patients, 152 cirrhotic patients with hepatitis B and 186 healthy individuals | Mean level of EGF protein (61 A/G polymorphism) in the hepatocellular carcinoma cell lysate with the GG genotype was higher than that with the AA genotype (47 vs. 32 pg/mL). | EGF gene diversity is associated with susceptibility to several types of cancer. | |
| Hernández-Bartolomé et al., 2016 [65] | Prospective study, 179 cirrhotic and non-cirrhotic chronic hepatitis C patients | Ang-2 was significantly increased as chronic hepatitis C progressed to the end stage of liver disease (6000 pg/mL, p < 0.001). | Ang-2/Ang-1 ratio might be useful for monitoring the progression of chronic liver disease and plays an important role as a therapeutic target. | |
| Pocino et al., 2021 [56] | Non-profit interventional study, 62 patients, out of whom 33 were diagnosed with hepatocellular carcinoma and 29 with liver cirrhosis | A reduction of Ang-2 (0.047 pg/mL) levels and the Ang-2/Ang-1 ratio (0.031 pg/mL) was observed before and after the treatment of patients diagnosed with viral hepatitis with the required antiviral treatment. | Serum angiogenic markers, with emphasis on Ang-1/2, can contribute to the development of quantitative tools for liver disease staging and therapy monitoring. | |
| NAFLD | Lefere et al., 2019 [66] | Prospective study, 13 patients with no nonalcoholic fatty liver disease, 41 patients with nonalcoholic fatty liver, 50 patients with nonalcoholic steatohepatitis | Serum Ang-2 levels were increased in patients with histological nonalcoholic steatohepatitis (400.4 pg/mL (279.2–630.2), compared with patients with simple steatosis (249.8 pg/mL (182.4–317-9). | Ang-2 inhibition could be a therapeutic strategy to target pathological angiogenesis in nonalcoholic steatohepatitis. |
| Manco et al., 2022 [67] | Observational study, children with diagnosis of nonalcoholic fatty liver disease (N = 76), control group (N = 28) included children negative to steatosis | The mean plasma level of Ang-2 was higher in children with nonalcoholic fatty liver disease than in age-matched controls (Ang-2 155.4 ± 72.5 vs. 7.5 ± 2.3 ng/mL, p < 0.001). | Ang-2 could be a suitable biomarker of nonalcoholic steatohepatitis in the pediatric population. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radmanić, L.; Zidovec-Lepej, S. The Role of Stem Cell Factor, Epidermal Growth Factor and Angiopoietin-2 in HBV, HCV, HCC and NAFLD. Life 2022, 12, 2072. https://doi.org/10.3390/life12122072
Radmanić L, Zidovec-Lepej S. The Role of Stem Cell Factor, Epidermal Growth Factor and Angiopoietin-2 in HBV, HCV, HCC and NAFLD. Life. 2022; 12(12):2072. https://doi.org/10.3390/life12122072
Chicago/Turabian StyleRadmanić, Leona, and Snježana Zidovec-Lepej. 2022. "The Role of Stem Cell Factor, Epidermal Growth Factor and Angiopoietin-2 in HBV, HCV, HCC and NAFLD" Life 12, no. 12: 2072. https://doi.org/10.3390/life12122072
APA StyleRadmanić, L., & Zidovec-Lepej, S. (2022). The Role of Stem Cell Factor, Epidermal Growth Factor and Angiopoietin-2 in HBV, HCV, HCC and NAFLD. Life, 12(12), 2072. https://doi.org/10.3390/life12122072






