Diabetes Patients’ Acceptance of Injectable Treatment, a Scientometric Analysis
Abstract
1. Introduction
2. Materials and Methods
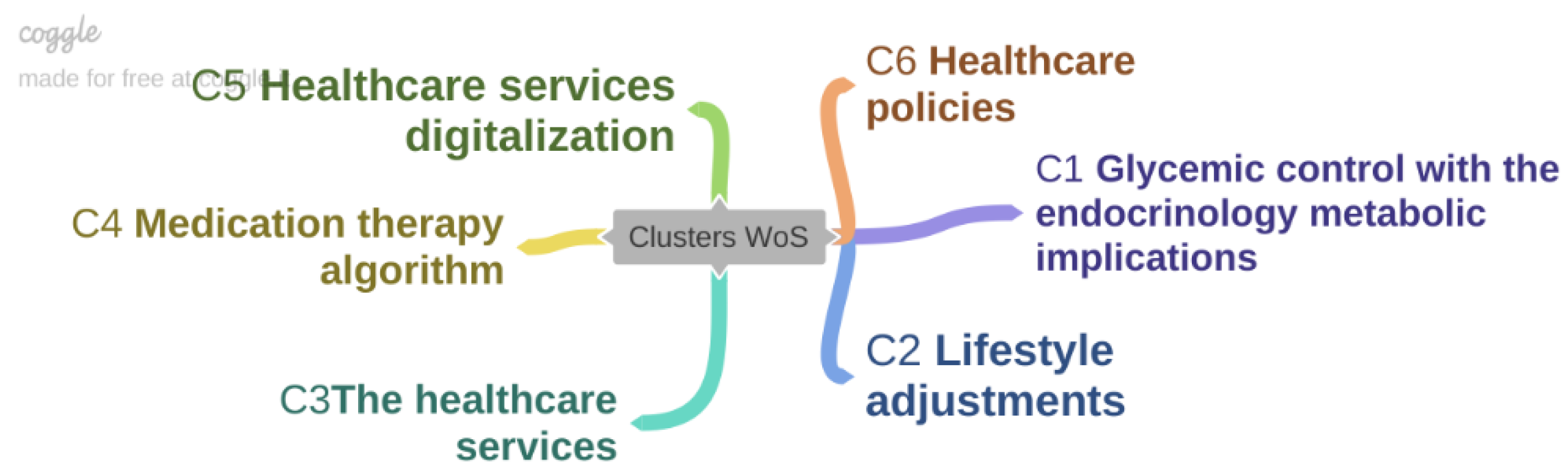
3. Results
3.1. Cluster 1—Glycemic Control with the Endocrinology Metabolic Implications
- (1)
- Sapkota et al. realized one analysis of 52 studies carried out between 2000–2013 suggested that diabetic patients’ medication adherence had beneficial effects on their health status. Still, a small number of patients identified the beneficial intervention for glycemic control [24].
- (2)
- Personalized diabetes management therapy combined with patient preferences suppose the administration of a reduced number of doses, minimal requirements for the preparation of injectable products, good glycemic control, and minimal side effects [25].
- (3)
- The follow-up of diabetic patients with religious habits (such as Ramadan) is mandatory to prevent complications. The follow-up could be facilitated by the devices that can record and send data related to blood glucose levels and insulin doses from the patient to the specialist doctor and vice versa [26].
- (4)
- Treatment options must aim at avoiding hypoglycemia and major vascular complications [27].
3.2. Cluster 2—Lifestyle Adjustments
- (1)
- Studies show that 40% of diabetic patients consider diet and lifestyle changes important in managing the disease, and 60% consider self-monitoring of blood sugar levels and check-ups with a specialist doctor to be determining factors in the evolution of the disease [26].
- (2)
- The management of the diabetic patient involves: educational self-management, lifestyle modification, and encouraging the patient in making decisions [28].
- (3)
- The personalization of diabetic patient management is a widely debated problem currently within multidisciplinary teams. The main objectives in personalized management are: (a) the patient’s lack of motivation, which leads to a lack of therapeutic compliance and adherence; (b) lack of information regarding patients’ preferences; and (c) the main target is represented by the decrease in glycosylated hemoglobin and the risk of myocardial infarction after 10 years of development of diabetes [29].
3.3. Cluster 3—The Healthcare Services
- (1)
- Hypoglycemia is a dreaded complication for any diabetic patient. Avoiding the hypoglycemic episodes can be achieved by training the patient’s caregivers in quick decision-making, to correct the critical situations that affect the state of health. Additionally, the patient’s own previous experiences can be of great help in solving the hypoglycemic occurrence [30].
- (2)
- Diabetics with cognitive impairments, by proving difficulties in adjusting the treatment doses and the occurrence of hypoglycemia episodes, represent essential risk factors in optimizing the therapeutic scheme and keeping the condition under control [31].
- (3)
- Deficiencies in doctor–patient communication are followed by clinical inertia in managing the proposed objectives. The effectiveness of this communication requires: establishing the objectives to be achieved; identifying the most important complications for the patient; identifying the reasons for non-adherence to the treatment; and granting acceptable compromises to the patient in achieving the objectives. Several other factors can improve communication. These involve prolonging visits to the doctor, avoiding the predominant presentation of the adverse effects of the treatment (weight gain, hypoglycemia), and not presenting insulin as a punishment applied to the patient [32].
- (4)
- Doctor–patient communication can help to eliminate many patients’ dilemmas regarding fear of injections and disease self-management [28].
3.4. Cluster 4—Medication Therapy Algorithm
- (1)
- The titration algorithm of daily insulin doses seems to be a solid reason for adhering to the treatment. Patients prefer simple algorithms differently from those doctors who prefer algorithms recommended by clinical guidelines. Treatment adherence is strongly influenced by the patient’s preference [9].
- (2)
- Telephone conversations between patients and medical staff are essential for identifying the factors that determine suboptimal treatment adherence of diabetic patients. Through these types of discussions, the lack of disease control due to adequate nutrition and physical effort was identified in 25% of patients who do not want to change these factors [33].
- (3)
- Diabetic patients’ conversations with pharmacists led to the improvement of HbA1c values in elderly diabetic patients [34].
- (4)
- The ideal treatment with insulin must ensure optimal glycemic control, have minimal adverse reactions, be administered orally, and have a low cost [35].
- (5)
- Adherence to insulin treatment seems to be preferential for certain types of insulin: insulin analogs and premixed insulins seem to be preferred by patients [36].
- (6)
- For diabetic patients with mental illnesses, it should be considered that the medication administration shall not cause weight gain and prove a reduced risk of hypoglycemia. Considering these aspects, the first option is suggested to be metformin, whereas the second line of treatment could include GLP1 agonists or DPP4 inhibitors, sulfonylureas, and ultimately insulin. The administration of injectable products with weekly administration seems to be the ideal solution for these patients [37].
- (7)
- Adherence to the treatment is influenced by several factors: weekly administration (GLP1 agonists), presence of injection systems (pens) prefilled with a single dose; important side effects are weight loss and reduced risk of hypoglycemia [38].
- (8)
- Self-interruption of insulin treatment occurs in 20% of patients. Non-adherence to treatment is due to forgetting the daily injections, especially when more doses are needed, and the short duration of the disease [39].
- (9)
- The administration of various new antidiabetic therapies (DPP4, SGLT2 inhibitors) shall be conducted with caution and not on a large scale, due to the short- and long-term effectiveness uncertainty and due to possibly severe side effects [40].
- (10)
- Adherence to antidiabetic medication can be improved if it is cautiously patient tailored and targets all the key factors specific to each individual [41].
3.5. Cluster 5—Healthcare Services Digitalization
- (1)
- The use of digital applications for the control of various biological parameters proved to be a useful method in strengthening glycemic control in diabetic patients [42].
- (2)
- Real-time blood glucose monitoring (RT-CGM) ensures a significant reduction of HbA1c compared to self-monitoring of blood glucose (SMGB) in diabetic patients. For severe hypoglycemia, the two monitoring systems did not show significant differences; instead, the costs of the care systems and the health benefits of RT-CGM are much higher [43]. The continuous monitoring of blood sugars demonstrated a decrease in HbA1c between 0.53–1% and hypoglycemia episodes by 38% [44].
- (3)
- For patients with type 1 diabetes, the development of a mobile application such as SOINS DM type would bring significant benefits in the diabetic patient’s life. SOINS DM is a new application with interactive visualization between type 1 diabetes, patients, and the doctor, which facilitates blood sugar monitoring, adjusting insulin doses, physical activity, and diet. This way, a type of personalized feedback could be achieved for this specific type of patient [45].
- (4)
- The presence of a mobile application, using a mobile health technology, with several components such as mobile phones and different sensors, can be beneficial by offering practical advice on monitoring treatment and adjusting insulin doses and optimal blood sugar levels [46].
- (5)
- An algorithm for type 2 diabetes management was developed to provide practical evidence-based guidance for treatment. Additionally, the American Association of Endocrinology has developed a guide with practical recommendations regarding the use of advanced technology in monitoring diabetic patients, allowing nursing staff to make appropriate adjustments to treatment, further enhancing the diabetes technology usability to improve the efficiency and effectiveness of clinical decision-making [47].
3.6. Cluster 6—Healthcare Policies
- (1)
- Health insurance systems through the imposed regulations are a determining factor in the inertia of insulin therapy initiation. The pharmaceutical industry is frequently a central source of information regarding new products, therapies, and treatment options. Research suggests that physicians are susceptible to the pharmaceutical industry and interactions with pharmaceutical sales representatives and that these influence prescribing practices. Thus, in the countries of Central and SE Europe, these barriers appear between doctors and their patients and the health insurance systems [48,49].
- (2)
- The implementation of a special patient decision aid (PDA) program, together with physicians, especially concerning poorly controlled diabetic patients, by favoring the decision-making process to enhance treatment and to avoid complications, seemed to be beneficial. This dedicated program includes information about new therapies, their beneficial and adverse effects, doses, and ways of administration [50].
- (3)
- The multidisciplinary team that cares for diabetic patients should address several lines of action: the promotion of supportive relationships, strong commitment, leadership capacity, and diversity in expertise. The establishment of a team consisting of physiotherapists, dieticians, psychologists, and primary care givers seems to be a successful solution in diabetic patients’ management [51].
- (4)
- The high cost of managing episodes of mild hypoglycemia for health systems represents a significant impediment; therefore, avoiding such situations would bring important economic benefits [52].
- (5)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Farthing, P.; Bally, J.; Rennie, D.C.; Leurer, M.D.; Holtslander, L.; Nour, M.A. Type 1 diabetes management responsibilities between adolescents with T1D and their parents: An integrative review. J. Spec. Pediatr. Nurs. 2022, 27, e12395. [Google Scholar] [CrossRef] [PubMed]
- Romero-Castillo, R.; Pabón-Carrasco, M.; Jiménez-Picón, N.; Ponce-Blandón, J.A. Effects of Nursing Diabetes Self-Management Education on Glycemic Control and Self-Care in Type 1 Diabetes: Study Protocol. Int. J. Environ. Res. Public Health 2022, 19, 5079. [Google Scholar] [CrossRef] [PubMed]
- Togo, A.; Minkailou, M.; Trebuchet, P.; Sylla, D.; Sidibé, A.T.; Besançon, S.; Nguemeni, M.; Signe, T.; Lab, B.; Castellsague, M. Éducation thérapeutique au Mali: Un atelier de formation pour soignants et jeunes diabétiques de type 1. Méd. Trop. St. Int. 2021, 1, 136. [Google Scholar] [CrossRef]
- Peña, A.; Olson, M.L.; Hooker, E.; Ayers, S.L.; Castro, F.G.; Patrick, D.L.; Corral, L. Effects of a Diabetes Prevention Program on Type 2 Diabetes Risk Factors and Quality of Life Among Latino Youths with Prediabetes A Randomized Clinical Trial. JAMA Netw. Open 2022, 5, 1–13. [Google Scholar] [CrossRef]
- Song, Y.; Wu, Z.; Zhao, P. The effects of metformin in the treatment of osteoarthritis: Current perspectives. Front. Pharmacol. 2022, 13, 952560. [Google Scholar] [CrossRef]
- Newby, D.; Linden, A.B.; Fernandes, M.; Molero, Y.; Winchester, L.; Sproviero, W.; Ghose, U.; Li, Q.S.; Launer, L.J.; van Duijn, C.M.; et al. Comparative effect of metformin versus sulfonylureas with dementia and Parkinson’s disease risk in US patients over 50 with type 2 diabetes mellitus. BMJ Open Diabetes Res. Care 2022, 10, e003036. [Google Scholar] [CrossRef]
- Vargas-Uricoechea, H.; Nuñez, J.L.B.; Guzmán, J.R.; Gomez, L.S.; Beltran, S.; Sañudo-Maury, M.E. Real-World Effectiveness and Safety of Insulin Glargine 300 U/mL in Insulin-Naïve People with Type 2 Diabetes in the Latin America Region: A Subgroup Analysis of the ATOS Study. Diabetes Obes. Metab. 2022, in press. [Google Scholar] [CrossRef]
- Riddle, M.C.; Herman, W.H. The cost of diabetes cared an elephant in the room. Diabetes Care 2018, 41, 929–932. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, Y.; Du, T.; Zhang, X.; Li, X.; Liu, R.; Wang, Y.; Chen, B.; He, L.; Li, W. Lack of coordination between partners: Investigation of physician-preferred and patient-preferred (4P) basal insulin titration algorithms in the real world. Patient Prefer. Adherence 2018, 12, 1253–1259. [Google Scholar] [CrossRef]
- Kang, H.; Lobo, J.M.; Kim, S.; Sohn, M.W. Cost-related medication non-adherence among U.S. adults with diabetes. Diabetes Res. Clin. Pract. 2018, 143, 24–33. [Google Scholar] [CrossRef]
- Patel, M.R.; Piette, J.D.; Resnicow, K.; Kowalski-Dobson, T.; Heisler, M. Social Determinants of Health, Cost-related Nonadherence, and Cost-reducing Behaviors among Adults with Diabetes: Findings from the National Health Interview Survey. Med. Care 2016, 54, 796–803. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study Group. Erratum: U.K. prospective diabetes study group: U.K. prospective diabetes study 16: Overview of 6 years’ therapy of type II diabetes: A progressive disease (Diabetes (1995) 44 (1249-1258)). Diabetes 1996, 45, 1655. [Google Scholar] [CrossRef]
- Koro, C.E.; Bowlin, S.J.; Bourgeois, N.; Fedder, D.O. Glycemic Control From 1988 to 2000. Diabetes Care 2004, 27, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Brod, M.; Cobden, D.; Lammert, M.; Bushnell, D.; Raskin, P. Examining correlates of treatment satisfaction for injectable insulin in type 2 diabetes: Lessons learned from a clinical trial comparing biphasic and basal analogues. Health Qual. Life Outcomes 2007, 5, 1–10. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larkin, M.E.; Capasso, V.A.; Chen, C.L.; Mahoney, E.K.; Hazard, B.; Cagliero, E.; Nathan, D.M. Measuring psychological insulin resistance: Barriers to insulin use. Diabetes Educ. 2008, 34, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Woudenberg, Y.J.C.; Lucas, C.; Latour, C.; Reimer, W.J.M.S.O. Acceptance of insulin therapy: A long shot? Psychological insulin resistance in primary care. Diabet. Med. 2012, 29, 796–802. [Google Scholar] [CrossRef]
- Polonsky, W.H.; Fisher, L.; Guzman, S.; Villa-Caballero, L.; Edelman, S.V. Psychological insulin resistance in patients with type 2 diabetes: The scope of the problem. Diabetes Care 2005, 28, 2543–2545. [Google Scholar] [CrossRef]
- Peyrot, M.; Rubin, R.R.; Lauritzen, T.; Skovlund, S.E.; Snoek, F.J.; Matthews, D.R.; Landgraf, R.; Kleinebreil, L. Resistance to insulin therapy among patients and providers: Results of the cross-national Diabetes Attitudes, Wishes, and Needs (DAWN) study. Diabetes Care 2005, 28, 2673–2679. [Google Scholar] [CrossRef]
- Pantea, I.; Repanovici, A.; Cocuz, M.E. Analysis of Research Directions on the Rehabilitation of Patients with Stroke and Diabetes Using Scientometric Methods. Healthcare 2022, 10, 773. [Google Scholar] [CrossRef]
- VOSviewer—Visualizing Scientific Landscapes. Available online: https://www.vosviewer.com/ (accessed on 11 September 2022).
- Bucataru, C.; Savescu, D.; Repanovici, A.; Blaga, L.; Coman, E.; Cocuz, M.-E. The Implications and Effects of Medical Waste on Development of Sustainable Society—A Brief Review of the Literature. Sustainability 2021, 13, 3300. [Google Scholar] [CrossRef]
- VOSviewer Online. Available online: https://app.vosviewer.com/ (accessed on 30 October 2022).
- van Eck, N.J.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, S.; Brien, J.A.; Greenfield, J.; Aslani, P. A systematic review of interventions addressing adherence to anti-diabetic medications in patients with type 2 diabetes—Impact on adherence. PLoS ONE 2015, 10, e0118296. [Google Scholar] [CrossRef] [PubMed]
- Thieu, V.T.; Robinson, S.; Kennedy-Martin, T.; Boye, K.S.; Garcia-Perez, L.E. Patient preferences for glucagon-like peptide 1 receptor–agonist treatment attributes. Patient Prefer. Adherence 2019, 13, 561–576. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.H.; Chowdhury, T.A.; Hussain, S.; Syed, A.; Karamat, A.; Helmy, A.; Waqar, S.; Ali, S.; Dabhad, A.; Seal, S.T.; et al. Ramadan and Diabetes: A Narrative Review and Practice Update. Diabetes Ther. 2020, 11, 2477–2520. [Google Scholar] [CrossRef]
- Mühlbacher, A.C.; Juhnke, C.; Sadler, A. Pdb124—Personalized Diabetes Management: What Do Patients with Diabetes Mellitus Prefer? Value Health 2018, 21, S139. [Google Scholar] [CrossRef]
- Lambrinou, E.; Kyriakou, M.; Lakatamitou, I.; Angus, N.; Khatib, R.; Vellone, E.; Barrowcliff, A.; Hansen, T.B.; Lee, G.A. An integrative review on facilitators and barriers in delivering and managing injectable therapies in chronic conditions: A part of the ACNAP project ‘injectable medicines among patients with cardiovascular conditions’. Eur. J. Cardiovasc. Nurs. 2020, 19, 663–680. [Google Scholar] [CrossRef]
- Mühlbacher, A.C.; Sadler, A.; Juhnke, C. Personalized diabetes management: What do patients with diabetes mellitus prefer? A discrete choice experiment. Eur. J. Health Econ. 2021, 22, 425–443. [Google Scholar] [CrossRef]
- Stuckey, H.L.; Desai, U.; King, S.B.; Popadic, L.; Levinson, W.; Kirson, N.Y.; Hankosky, E.R.; Mitchell, B. The experience of a severe hypoglycaemic event from the perspective of people with diabetes and their caregivers: “What am I going to do?”. Diabet. Med. 2022, 39, 1–12. [Google Scholar] [CrossRef]
- Santos, T.; Lovell, J.; Shiell, K.; Johnson, M.; Ibrahim, J.E. The impact of cognitive impairment in dementia on self-care domains in diabetes: A systematic search and narrative review. Diabetes Metab. Res. Rev. 2018, 34, e3013. [Google Scholar] [CrossRef]
- Soto, C.; Strain, W.D. Tackling clinical inertia: Use of coproduction to improve patient engagement. J. Diabetes 2018, 10, 942–947. [Google Scholar] [CrossRef]
- Lauffenburger, J.C.; Ghazinouri, R.; Jan, S.; Makanji, S.; Ferro, C.A.; Lewey, J.; Wittbrodt, E.; Lee, J.; Haff, N.; Fontanet, C.P.; et al. Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: The ENhancing outcomes through Goal Assessment and Generating Engagement in Diabetes Mellitus (ENGAGE-DM) pragmatic randomized trial. PLoS ONE 2019, 14, e0214754. [Google Scholar] [CrossRef] [PubMed]
- Robert, A.; Al Dawish, M.; Braham, R.; Musallam, M.; Al Hayek, A.; Al Kahtany, N. Type 2 Diabetes Mellitus in Saudi Arabia: Major Challenges and Possible Solutions. Curr. Diabetes Rev. 2016, 13, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Guimaraes, C.; Marra, C.; Gill, S.; Simpson, S.; Meneilly, G.S.; Queiroz, R.H.; Lynd, L.D. A discrete choice experiment evaluation of patients’ preferences for different risk, benefit, and delivery attributes of insulin therapy for diabetes management. Patient Prefer. Adherence 2010, 4, 433–440. [Google Scholar] [CrossRef] [PubMed][Green Version]
- He, X.; Chen, L.; Wang, K.; Wu, H.; Wu, J. Insulin adherence and persistence among chinese patients with type 2 diabetes: A retrospective database analysis. Patient Prefer. Adherence 2017, 11, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Lally, J.; O’ Loughlin, A.; Stubbs, B.; Guerandel, A.; O’Shea, D.; Gaughran, F. Pharmacological management of diabetes in severe mental illness: A comprehensive clinical review of efficacy, safety and tolerability. Expert Rev. Clin. Pharmacol. 2018, 11, 411–424. [Google Scholar] [CrossRef]
- Giorgino, F.; Penfornis, A.; Pechtner, V.; Gentilella, R.; Corcos, A. Adherence to antihyperglycemic medications and glucagon-like peptide 1-receptor agonists in type 2 diabetes: Clinical consequences and strategies for improvement. Patient Prefer. Adherence 2018, 12, 707–719. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, D.G.; Ozcan, S.; Deyneli, O. Adherence to insulin treatment in insulin-naïve type 2 diabetic patients initiated on different insulin regimens. Patient Prefer. Adherence 2007, 9, 1225–1231. [Google Scholar] [CrossRef]
- Zozaya, N.; Capel, M.; Simón, S.; Soto-González, A. A systematic review of economic evaluations in non-insulin antidiabetic treatments for patients with type 2 diabetes mellitus. Glob. Reg. Health Technol. Assess. 2019, 2019, 228424031987657. [Google Scholar] [CrossRef]
- Tejedor, M.; Woldaregay, A.Z.; Godtliebsen, F. Reinforcement learning application in diabetes blood glucose control: A systematic review. Artif. Intell. Med. 2020, 104, 1–37. [Google Scholar] [CrossRef]
- García-Lorenzo, B.; Rivero-Santana, A.; Vallejo-Torres, L.; Castilla-Rodríguez, I.; García-Pérez, S.; García-Pérez, L.; Perestelo-Pérez, L. Cost-effectiveness analysis of real-time continuous monitoring glucose compared to self-monitoring of blood glucose for diabetes mellitus in Spain. J. Eval. Clin. Pract. 2018, 24, 772–781. [Google Scholar] [CrossRef]
- Litwak, L.E.; Querzoli, I.; Musso, C.; Dain, A.; Houssay, S.; Proietti, A.; Gil, J.E.C. Monitoreo Continuo De Glucosa. Utilidad E Indicaciones. Medicina 2019, 79, 44–52. [Google Scholar] [PubMed]
- Bellei, E.A.; Biduski, D.; Lisboa, H.R.K.; De Marchi, A.C.B. Development and Assessment of a Mobile Health Application for Monitoring the Linkage Among Treatment Factors of Type 1 Diabetes Mellitus. Telemed. J. e-Health 2020, 26, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Alenazi, H.A.; Jamal, A.; Batais, M.A. Identification of type 2 diabetes management mobile app features and engagement strategies: Modified delphi approach. JMIR mHealth uHealth 2020, 8, 1–16. [Google Scholar] [CrossRef]
- Grunberger, G.; Sherr, J.; Allende, M.; Blevins, T.; Bode, B.; Handelsman, Y.; Hellman, R.; Lajara, R.; Roberts, V.L.; Rodbard, D.; et al. American Association of Clinical Endocrinology Clinical Practice Guideline: The Use of Advanced Technology in the Management of Persons with Diabetes Mellitus. Endocr. Pract. 2021, 27, 505–537. [Google Scholar] [CrossRef] [PubMed]
- Campbell, M.D.; Babic, D.; Bolcina, U.; Smirčić-Duvnjak, L.; Tankova, T.; Mitrakou, A.; Kempler, P.; Janez, A. High level of clinical inertia in insulin initiation in type 2 diabetes across Central and South-Eastern Europe: Insights from SITIP study. Acta Diabetol. 2019, 56, 1045–1049. [Google Scholar] [CrossRef]
- Bailey, R.A.; Shillington, A.C.; Harshaw, Q.; Funnell, M.M.; VanWingen, J.; Col, N. Changing Patients’ Treatment Preferences and Values with a Decision Aid for Type 2 Diabetes Mellitus: Results from the Treatment Arm of a Randomized Controlled Trial. Diabetes Ther. 2018, 9, 803–814. [Google Scholar] [CrossRef]
- Ireland, S.; Endacott, R.; Cameron, P.; Fitzgerald, M.; Paul, E. The incidence and significance of accidental hypothermia in major trauma—A prospective observational study. Resuscitation 2011, 82, P300–P306. [Google Scholar] [CrossRef]
- Dankoly, U.S.; Vissers, D.; El Farkouch, Z.; Kolasa, E.; Ziyyat, A.; Van Rompaey, B.; Maamri, A. Perceived Barriers, Benefits, Facilitators, and Attitudes of Health Professionals Towards Multidisciplinary Team Care in Type 2 Diabetes Management: A Systematic Review. Curr. Diabetes Rev. 2021, 17, 1–27. [Google Scholar] [CrossRef]
- Parekh, W.; Streeton, S.E.; Baker-Knight, J.; Montagnoli, R.; Nicoziani, P.; Marchesini, G. The Economic Burden of Insulin-Related Hypoglycemia in Adults with Diabetes: An Analysis from the Perspective of the Italian Healthcare System. Diabetes Ther. 2018, 9, 1037–1047. [Google Scholar] [CrossRef]
- Lambert-Obry, V.; Lafrance, J.P.; Savoie, M.; Lachaine, J. The Impact of Hypoglycemia on Productivity Loss and Utility in Patients with Type 2 Diabetes Treated with Insulin in Real-world Canadian Practice: Protocol for a Prospective Study. JMIR Res. Protoc. 2022, 11, 1–11. [Google Scholar] [CrossRef]
- Hermanns, N.; Mahr, M.; Kulzer, B.; Skovlund, S.E.; Haak, T. Barriers towards insulin therapy in type 2 diabetic patients: Results of an observational longitudinal study. Health Qual. Life Outcomes 2010, 8, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Biderman, A.; Noff, E.; Harris, S.B.; Friedman, N.; Levy, A. Treatment satisfaction of diabetic patients: What are the contributing factors? Fam. Pract. 2009, 26, 102–108. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, S.; Makuch, S.; Dróżdż, M.; Dudzik, T.; Domański, I.; Poręba, R.; Mazur, G. The Impact of Hypoglycemia on Patients with Diabetes Mellitus: A Cross-Sectional Analysis. J. Clin. Med. 2022, 11, 626. [Google Scholar] [CrossRef]
- Sims, T.J.; Boye, K.S.; Robinson, S.; Kennedy-Martin, T. Treatment-Related Attributes of Diabetes Therapies and How People with Type 2 Diabetes Report Their Impact on Indicators of Medication-Taking Behaviors. Patient Prefer. Adherence 2022, 16, 1919–1939. [Google Scholar] [CrossRef]
- Shiju, R.; Akhil, A.; Thankachan, S.; Tuomilehto, J.; Al Arouj, M.; Bennakhi, A. Safety Assessment of Glucose-Lowering Drugs and Importance of Structured Education during Ramadan: A Systematic Review and Meta-Analysis. J. Diabetes Res. 2022, 2022, 3846253. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.A.; Levy, C.J. Emerging technologies for the management of type 2 diabetes mellitus. J. Diabetes 2021, 13, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Byrne, J.; Willis, A.; Dunkley, A.; Fitzpatrick, C.; Campbell, S.; Sidhu, M.S.; Choudhary, P.; Davies, M.J.; Khunti, K. Individual, healthcare professional and system-level barriers and facilitators to initiation and adherence to injectable therapies for type 2 diabetes: A systematic review and meta-ethnography. Diabet. Med. 2022, 39, e14678. [Google Scholar] [CrossRef]
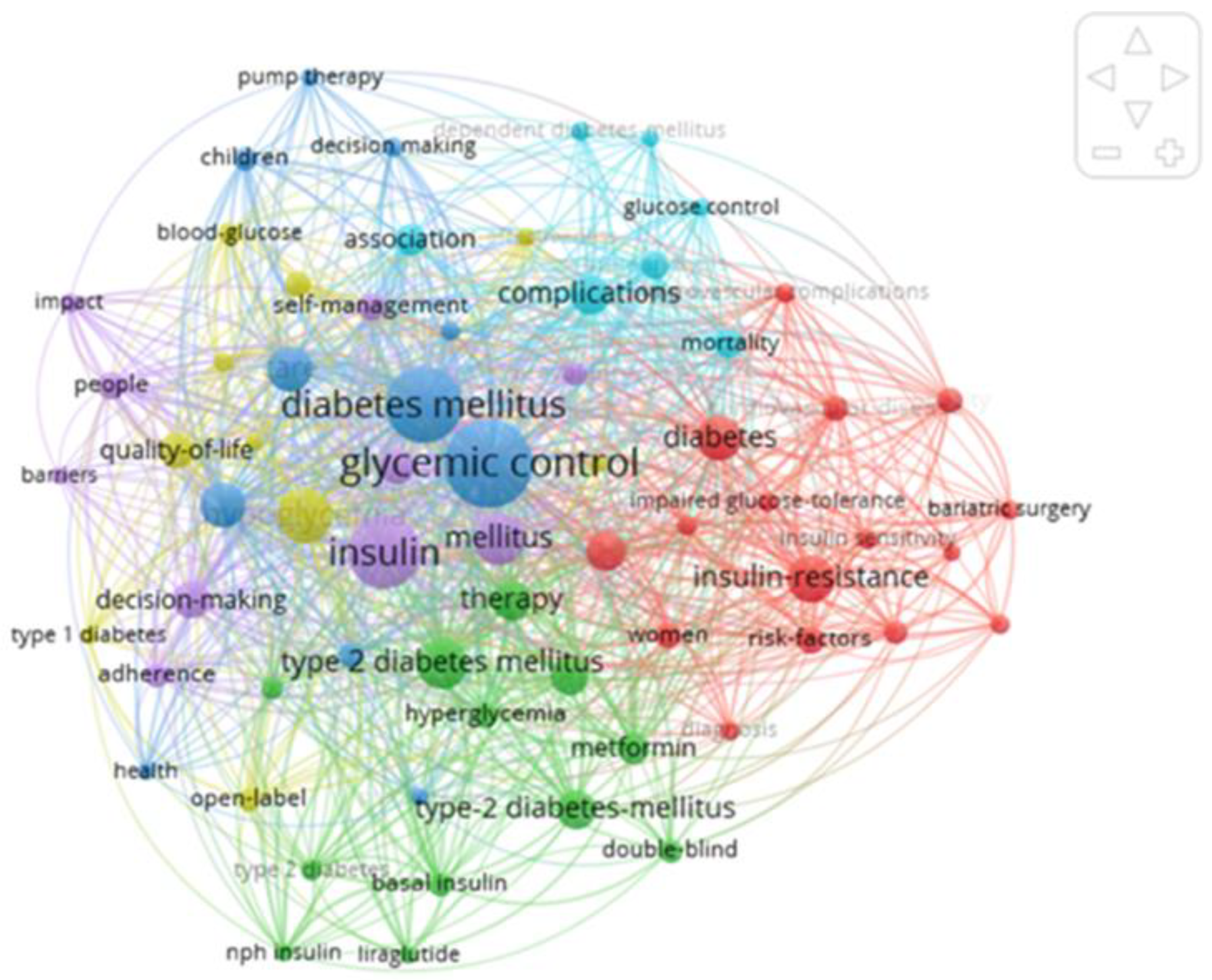
| No. | Steps | Description |
|---|---|---|
| 1 | Formulation of the problem | Mapping, bibliometric analysis of publications using descriptors, and research direction identification. |
| 2 | Research criteria | Subject: “((ALL = (decision making)) AND ALL = (diabetes Mellitus)) AND ALL = (insulin)” |
| 3 | Database used for research | Claryvate analytics, WEB OF SCIENCE—WOS Accessed on |
| 4 | Eligibility criteria | Filter 1: years of publication (2017–2022) |
| Result: 237 documents. | ||
| Filter 2: articles | ||
| Filter 3: English | ||
| Filter 4: open access | ||
| Result: 46 documents. | ||
| 5 | Data extraction | Bilingual format |
| 6 | Analysis and synthesis of results | Qualitative (descriptive) and quantitative (bibliometric) using VOS Viewer |
| 7 | Discussions | Analysis of the data gained |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pantea, I.; Roman, N.; Repanovici, A.; Drugus, D. Diabetes Patients’ Acceptance of Injectable Treatment, a Scientometric Analysis. Life 2022, 12, 2055. https://doi.org/10.3390/life12122055
Pantea I, Roman N, Repanovici A, Drugus D. Diabetes Patients’ Acceptance of Injectable Treatment, a Scientometric Analysis. Life. 2022; 12(12):2055. https://doi.org/10.3390/life12122055
Chicago/Turabian StylePantea, Ileana, Nadinne Roman, Angela Repanovici, and Daniela Drugus. 2022. "Diabetes Patients’ Acceptance of Injectable Treatment, a Scientometric Analysis" Life 12, no. 12: 2055. https://doi.org/10.3390/life12122055
APA StylePantea, I., Roman, N., Repanovici, A., & Drugus, D. (2022). Diabetes Patients’ Acceptance of Injectable Treatment, a Scientometric Analysis. Life, 12(12), 2055. https://doi.org/10.3390/life12122055








