Butyrate Supplementation Exacerbates Myocardial and Immune Cell Mitochondrial Dysfunction in a Rat Model of Faecal Peritonitis
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Methodologies
2.3. Ex Vivo Human Peripheral Blood Mononuclear Cell Isolation and Incubation
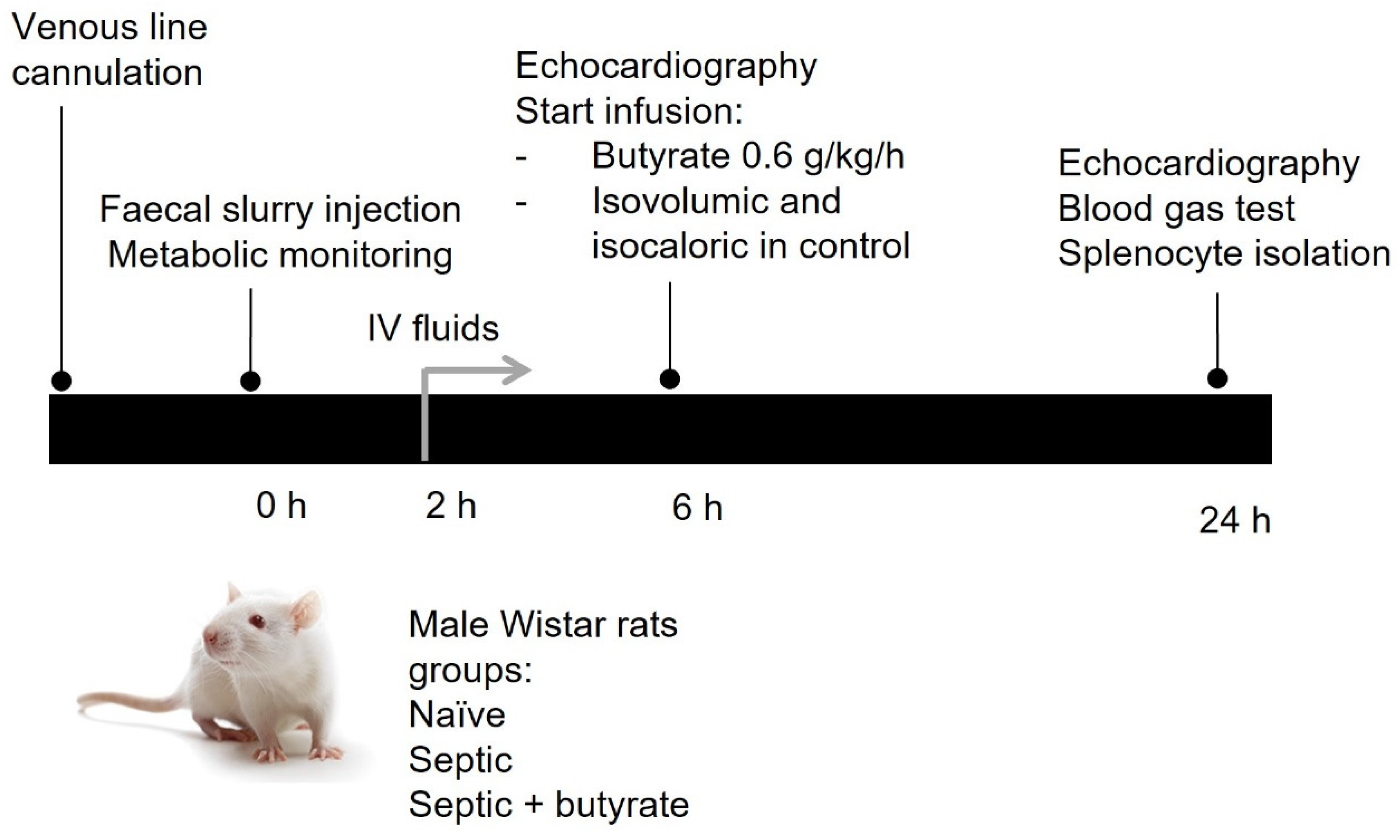
2.4. In Vivo Rat Model of Sepsis
2.5. Rat Splenocyte Isolation and Culture
2.6. High-Resolution Respirometry
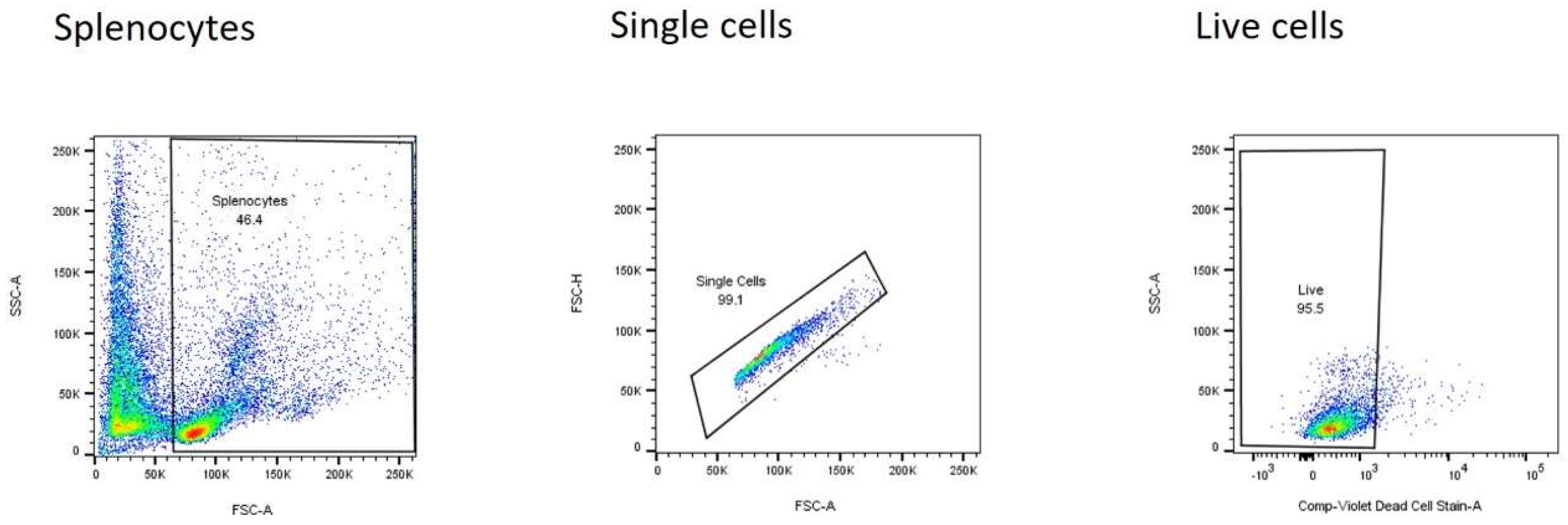
2.7. Flow Cytometry
2.8. Measurement of Released Cytokines
2.9. Statistical Analysis
3. Results
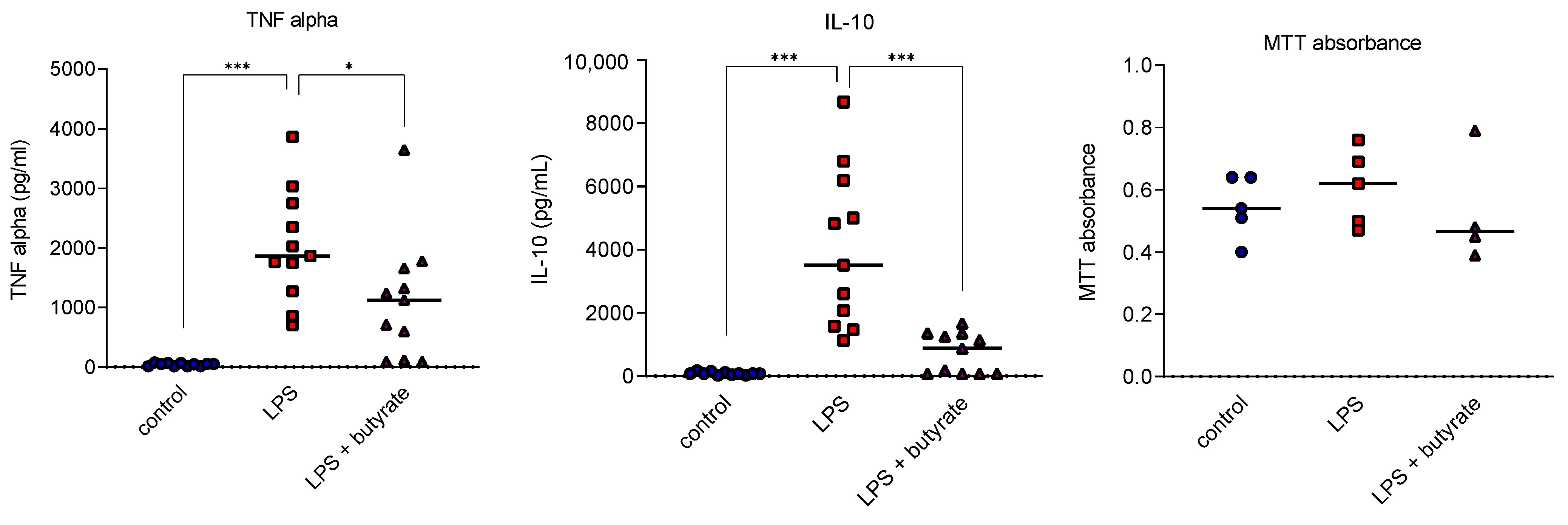
3.1. Butyrate Impaired Cytokine Release in Human Peripheral Blood Mononuclear Cell Studies
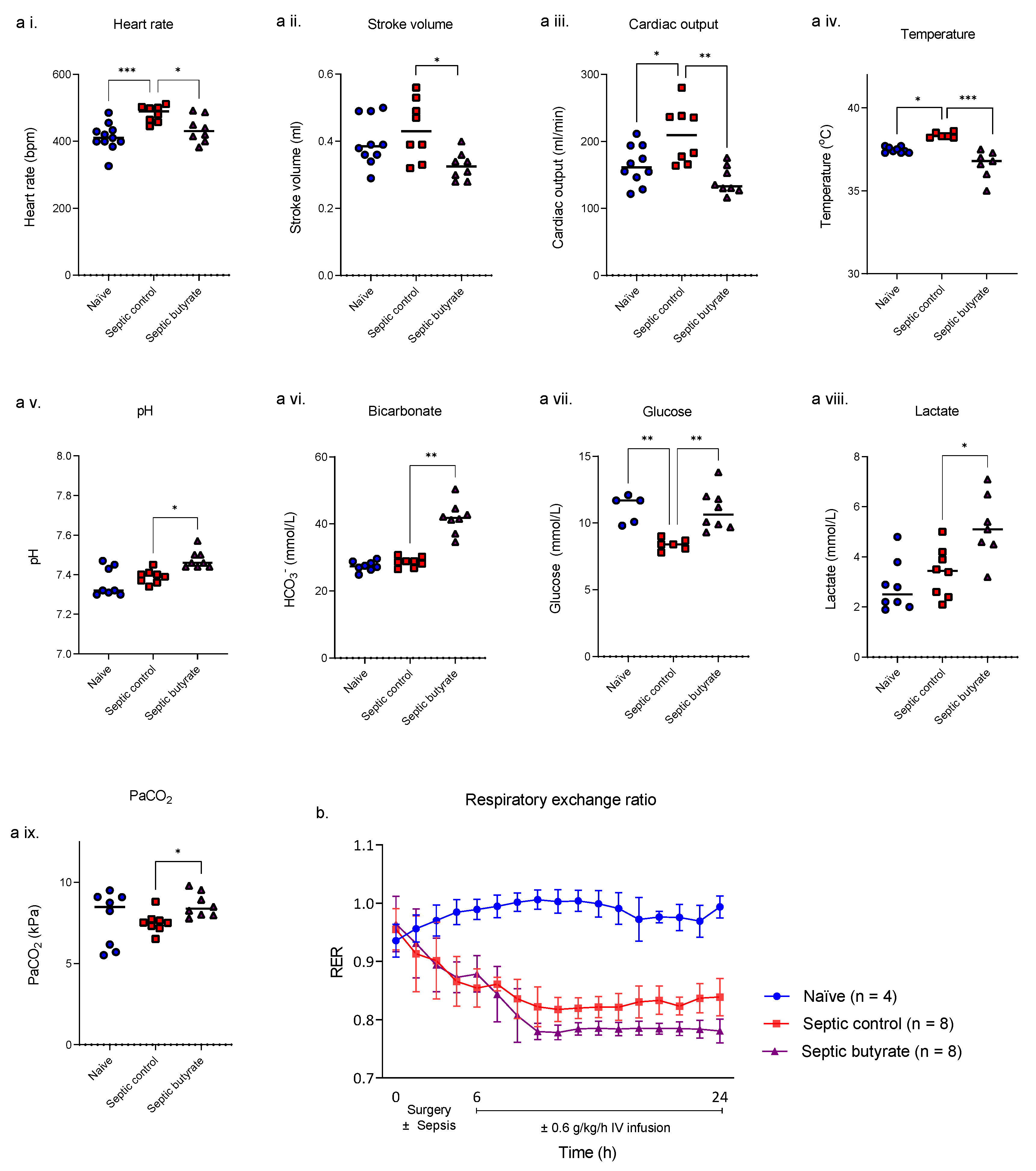
3.2. In Vivo Rat Model of Sepsis
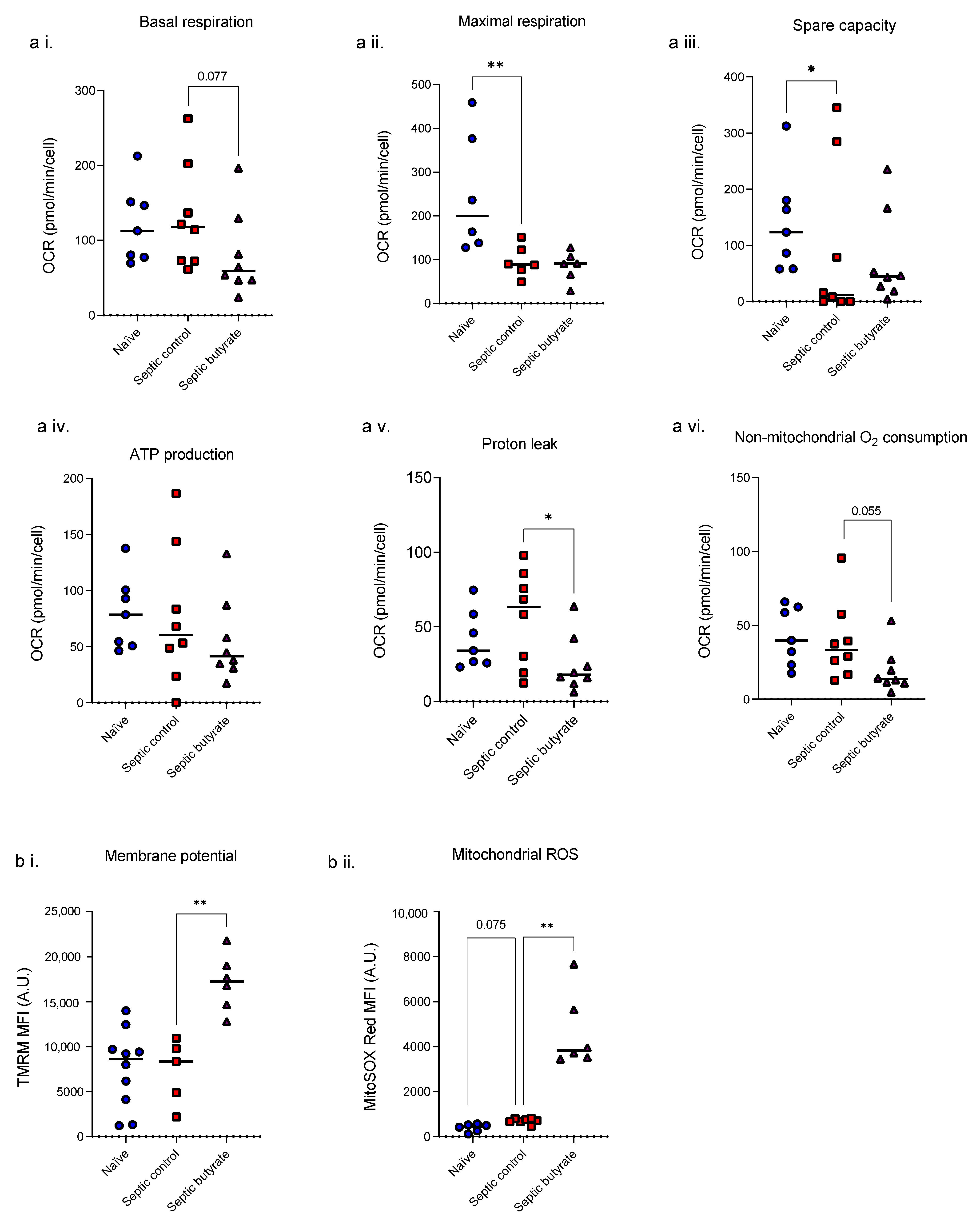
3.3. Butyrate Infusion Increased Mitochondrial Stress in Isolated Splenocytes
3.4. Butyrate Infusion Did Not Impact upon Sepsis-Induced Increased Intracellular Cytokine but Reduced LPS- Induced IL-10 Release
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A. Detailed Methods
A.1. Butyrate Stock Preparation
A.2. Human PBMC Isolation and Culture
A.3. MTT Assay
A.4. In Vivo Rat Model of Sepsis
A.5. Rat Splenocyte Isolation
A.6. Respirometry
A.7. Flow Cytometry
| Cell Marker | Cat. No | Clone | Laser | Filter | Fluorophore | Dilution |
|---|---|---|---|---|---|---|
| TNF-a | 11-7423-82 | TN3-19.12 | Blue (488 nm) | 530/30 | FITC | 1:50 |
| IL-10 | 555088 | A5-4 | Yellow (561 nm) | 585/15 | PE | 1:50 |
| Live dead | L34963 | Violet (405 nm) | 450/50 | violet | 1:1000 | |
| MitoSOX | Yellow (561 nm) | 585/15 | 2.5 µM | |||
| TMRM | T668 | Yellow (561 nm) | 585/15 | 25 nM |
References
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Johnson, S.C.; Agesa, K.M.; Shackelford, K.A.; Tsoi, D.; Kievlan, D.R.; Colombara, D.V.; Ikuta, K.S.; Kissoon, N.; Finfer, S.; et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: Analysis for the Global Burden of Disease Study. Lancet 2020, 395, 200–211. [Google Scholar] [CrossRef] [PubMed]
- Munford, R.S.; Pugin, J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am. J. Respir. Crit. Care Med. 2001, 163, 316–321. [Google Scholar] [CrossRef]
- van der Poll, T.; van de Veerdonk, F.L.; Scicluna, B.P.; Netea, M.G. The immunopathology of sepsis and potential therapeutic targets. Nat. Rev. Immunol. 2017, 17, 407–420. [Google Scholar] [CrossRef] [PubMed]
- Steinhorn, D.M.; Green, T.P. Severity of illness correlates with alterations in energy metabolism in the pediatric intensive care unit. Crit. Care Med. 1991, 19, 1503–1509. [Google Scholar] [CrossRef]
- Briassoulis, G.; Venkataraman, S.; Thompson, A.E. Energy expenditure in critically ill children. Crit. Care Med. 2000, 28, 1166–1172. [Google Scholar] [CrossRef]
- White, M.S.; Shepherd, R.W.; McEniery, J.A. Energy expenditure in 100 ventilated, critically ill children: Improving the accuracy of predictive equations. Crit. Care Med. 2000, 28, 2307–2312. [Google Scholar] [CrossRef]
- Gebara, B.M.; Gelmini, M.; Sarnaik, A. Oxygen consumption, energy expenditure, and substrate utilization after cardiac surgery in children. Crit. Care Med. 1992, 20, 1550–1554. [Google Scholar] [CrossRef]
- Singer, M. Mitochondrial function in sepsis: Acute phase versus multiple organ failure. Crit. Care Med. 2007, 35 (Suppl. S9), S441–S448. [Google Scholar] [CrossRef]
- Zolfaghari, P.S.; Pinto, B.B.; Dyson, A.; Singer, M. The metabolic phenotype of rodent sepsis: Cause for concern? Intensive Care Med. Exp. 2013, 1, 25. [Google Scholar] [CrossRef]
- Kreymann, G.; Grosser, S.; Buggisch, P.; Gottschall, C.; Matthaei, S.; Greten, H. Oxygen consumption and resting metabolic rate in sepsis, sepsis syndrome, and septic shock. Crit. Care Med. 1993, 21, 1012–1019. [Google Scholar] [CrossRef] [PubMed]
- Hart, D.W.; Chinkes, D.L.; Gore, D.C. Increased tissue oxygen extraction and acidosis with progressive severity of sepsis. J. Surg. Res. 2003, 112, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Adrie, C.; Bachelet, M.; Vayssier-Taussat, M.; Russo-Marie, F.; Bouchaert, I.; Adib-Conquy, M.; Cavaillon, J.-M.; Pinsky, M.R.; Dhainaut, J.-F.; Polla, B.S. Mitochondrial membrane potential and apoptosis peripheral blood monocytes in severe human sepsis. Am. J. Respir. Crit. Care Med. 2001, 164, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Japiassu, A.M.; Santiago, A.P.S.A.; D’Avila, J.d.C.P.; Garcia-Souza, L.F.; Galina, A.; Castro Faria-Neto, H.C.; Bozza, F.A.; Oliveira, M.F. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5′-triphosphate synthase activity. Crit. Care Med. 2011, 39, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Garrabou, G.; Morén, C.; López, S.; Tobías, E.; Cardellach, F.; Miró, Ò.; Casademont, J. The effects of sepsis on mitochondria. J. Infect. Dis. 2012, 205, 392–400. [Google Scholar] [CrossRef]
- D’Argenio, V.; Salvatore, F. The role of the gut microbiome in the healthy adult status. Clin. Chim. Acta 2015, 451 Pt A, 97–102. [Google Scholar] [CrossRef]
- Heintz-Buschart, A.; Wilmes, P. Human Gut Microbiome: Function Matters. Trends Microbiol. 2018, 26, 563–574. [Google Scholar] [CrossRef]
- Rios-Covian, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Peters, V.B.M.; Van De Steeg, E.; van Bilsen, J.; Meijerink, M. Mechanisms and immunomodulatory properties of pre- and probiotics. Benef. Microbes 2019, 10, 225–236. [Google Scholar] [CrossRef]
- Correa-Oliveira, R.; Fachi, J.L.; Vieira, A.; Sato, F.T.; Vinolo, M.A.R. Regulation of immune cell function by short-chain fatty acids. Clin. Transl. Immunol. 2016, 5, e73. [Google Scholar] [CrossRef]
- Donohoe, D.R.; Garge, N.; Zhang, X.; Sun, W.; O’Connell, T.M.; Bunger, M.K.; Bultman, S.J. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell Metab. 2011, 13, 517–526. [Google Scholar] [CrossRef]
- Gao, Z.; Yin, J.; Zhang, J.; Ward, R.E.; Martin, R.J.; Lefevre, M.; Cefalu, W.T.; Ye, J. Butyrate improves insulin sensitivity and increases energy expenditure in mice. Diabetes 2009, 58, 1509–1517. [Google Scholar] [CrossRef]
- den Besten, G.; Bleeker, A.; Gerding, A.; van Eunen, K.; Havinga, R.; van Dijk, T.H.; Oosterveer, M.H.; Jonker, J.W.; Groen, A.K.; Reijngoud, D.-J.; et al. Short-Chain Fatty Acids Protect Against High-Fat Diet-Induced Obesity via a PPARgamma-Dependent Switch From Lipogenesis to Fat Oxidation. Diabetes 2015, 64, 2398–2408. [Google Scholar] [CrossRef]
- Gallis, J.L.; Tissier, P.; Gin, H.; Beauvieux, M.-C. Decrease in oxidative phosphorylation yield in presence of butyrate in perfused liver isolated from fed rats. BMC Physiol. 2007, 7, 8. [Google Scholar] [CrossRef]
- Cummings, J.H.; Pomare, E.W.; Branch, W.J.; Naylor, C.P.; Macfarlane, G.T. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut 1987, 28, 1221–1227. [Google Scholar] [CrossRef]
- Dyson, A.; Rudiger, A.; Singer, M. Temporal changes in tissue cardiorespiratory function during faecal peritonitis. Intensive Care Med. 2011, 37, 1192–1200. [Google Scholar] [CrossRef]
- Brealey, D.; Karyampudi, S.; Jacques, T.S.; Novelli, M.; Stidwill, R.; Taylor, V.; Smolenski, R.; Singer, M. Mitochondrial dysfunction in a long-term rodent model of sepsis and organ failure. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R491–R497. [Google Scholar] [CrossRef]
- Egorin, M.J.; Yuan, Z.-M.; Sentz, D.L.; Plaisance, K.; Eiseman, J.L. Plasma pharmacokinetics of butyrate after intravenous administration of sodium butyrate or oral administration of tributyrin or sodium butyrate to mice and rats. Cancer Chemother. Pharmacol. 1999, 43, 445–453. [Google Scholar] [CrossRef]
- Divakaruni, A.S.; Paradyse, A.; Ferrick, D.A.; Murphy, A.N.; Jastroch, M. Analysis and Interpretation of Microplate-Based Oxygen Consumption and pH Data. Methods Enzymol. 2014, 547, 309–354. [Google Scholar]
- Zhang, L.T.; Yao, Y.-M.; Lu, J.-Q.; Yan, X.-J.; Yu, Y.; Sheng, Z.-Y. Sodium butyrate prevents lethality of severe sepsis in rats. Shock 2007, 27, 672–677. [Google Scholar] [CrossRef]
- McAndrew, H.F.; Lloyd, D.; Rintala, R.; van Saene, H. Intravenous glutamine or short-chain fatty acids reduce central venous catheter infection in a model of total parenteral nutrition. J. Pediatr. Surg. 1999, 34, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Ciarlo, E.; Heinonen, T.; Herderschee, J.; Fenwick, C.; Mombelli, M.; LE Roy, D.; Roger, T. Impact of the microbial derived short chain fatty acid propionate on host susceptibility to bacterial and fungal infections in vivo. Sci. Rep. 2016, 6, 37944. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, J.; Weng, T.; Shen, K.; Chen, Z.; Yu, Y.; Huang, Q.; Wang, G.; Liu, Z.; Jin, S.; et al. The Inflammation Induced by Lipopolysaccharide can be Mitigated by Short-chain Fatty Acid, Butyrate, through Upregulation of IL-10 in Septic Shock. Scand. J. Immunol. 2017, 85, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Askanazi, J.; Carpentier, Y.A.; Elwyn, D.H.; Nördenström, J.; Jeevanandam, M.; Rosenbaum, S.H.; Gump, F.E.; Kinney, J.M. Influence of Total Parenteral Nutrition on Fuel Utilization in Injury and Sepsis. Ann. Surg. 1980, 191, 40–46. [Google Scholar] [CrossRef] [PubMed]
- Shaw, J.H.; Wolfe, R.R. Influence of beta-hydroxybutyrate infusion on glucose and free fatty acid metabolism in dogs. Am. J. Physiol. 1984, 247, E756–E764. [Google Scholar] [CrossRef]
- Takasu, O.; Gaut, J.P.; Watanabe, E.; To, K.; Fagley, R.E.; Sato, B.; Jarman, S.; Efimov, I.R.; Janks, D.L.; Srivastava, A.; et al. Mechanisms of Cardiac and Renal Dysfunction in Patients Dying of Sepsis. Am. J. Respir. Crit. Care Med. 2013, 187, 509–517. [Google Scholar] [CrossRef]
- Wang, F.; Jin, Z.; Shen, K.; Weng, T.; Chen, Z.; Feng, J.; Zhang, Z.; Liu, J.; Zhang, X.; Chu, M. Butyrate pretreatment attenuates heart depression in a mice model of endotoxin-induced sepsis via anti-inflammation and anti-oxidation. Am. J. Emerg. Med. 2017, 35, 402–409. [Google Scholar] [CrossRef]
- MacLeod, J.J.R.; Hoover, D.H. Lactic acid production in the blood following the injection of alkaline solutions and dextrose or of alkaline solutions alone. Am. J. Physiol. 1917, 42, 460–465. [Google Scholar] [CrossRef]
- Relman, A.S. Metabolic consequences of acid-base disorders. Kidney Int. 1972, 1, 347–359. [Google Scholar] [CrossRef]
- Lowenstein, J.M.; Chance, B. The effect of hydrogen ions on the control of mitochondrial respiration. J. Biol. Chem. 1968, 243, 3940–3946. [Google Scholar] [CrossRef]
- Schonfeld, P.; Wojtczak, L. Short- and medium-chain fatty acids in energy metabolism: The cellular perspective. J. Lipid. Res. 2016, 57, 943–954. [Google Scholar] [CrossRef]
- Schonfeld, P.; Wojtczak, L. Fatty acids as modulators of the cellular production of reactive oxygen species. Free Radic. Biol. Med. 2008, 45, 231–241. [Google Scholar] [CrossRef]
- Rose, S.; Bennuri, S.C.; Davis, J.E.; Wynne, R.; Slattery, J.C.; Tippett, M.; Delhey, L.; Melnyk, S.; Kahler, S.G.; Macfabe, D.F.; et al. Butyrate enhances mitochondrial function during oxidative stress in cell lines from boys with autism. Transl. Psychiatry 2018, 8, 42. [Google Scholar] [CrossRef]
- Genders, A.J.; Martin, S.D.; McGee, S.L.; Bishop, D.J. A physiological drop in pH decreases mitochondrial respiration, and HDAC and Akt signaling, in L6 myocytes. Am. J. Physiol. Cell Physiol. 2019, 316, C404–C414. [Google Scholar] [CrossRef]
- Lachmandas, E.; Heuvel, C.N.A.M.V.D.; Damen, M.S.M.A.; Cleophas, M.C.P.; Netea, M.G.; van Crevel, R. Diabetes Mellitus and Increased Tuberculosis Susceptibility: The Role of Short-Chain Fatty Acids. J. Diabetes Res. 2015, 2016, 6014631. [Google Scholar] [CrossRef]
- Liu, T.F.; Li, J.; Liu, Y.; Xiao, N.; Suo, H.; Xie, K.; Yang, C.; Wu, C. Short-Chain Fatty Acids Suppress Lipopolysaccharide-Induced Production of Nitric Oxide and Proinflammatory Cytokines Through Inhibition of NF-kappa B Pathway in RAW264.7. Cells Inflamm. 2012, 35, 1676–1684. [Google Scholar] [CrossRef]
- Park, J.-S.; Lee, E.-J.; Lee, J.-C.; Kim, W.-K.; Kim, H.-S. Anti-inflammatory effects of short chain fatty acids in IFN-gamma-stimulated RAW 264.7 murine macrophage cells: Involvement of NF-kappa B and ERK signaling pathways. Int. Immunopharmacol. 2007, 7, 70–77. [Google Scholar] [CrossRef]
- Saemann, M.D.; Böhmig, G.A.; Osterreicher, C.H.; Burtscher, H.; Parolini, O.; Diakos, C.; Stöckl, J.; Hörl, W.H.; Zlabinger, G.J. Anti-inflammatory effects of sodium butyrate on human monocytes: Potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000, 14, 2380–2382. [Google Scholar] [CrossRef]
- Segain, J.P.; de la Blétière, D.R.; Bourreille, A.; Leray, V.; Gervois, N.; Rosales, C.; Ferrier, L.; Bonnet, C.; Blottière, H.M.; Galmiche, J.P. Butyrate inhibits inflammatory responses through NF kappa B inhibition: Implications for Crohn’s disease. Gut 2000, 47, 397–403. [Google Scholar] [CrossRef]
- Cleophas, M.C.; Crişan, T.O.; Lemmers, H.; Toenhake-Dijkstra, H.; Fossati, G.; Jansen, T.L.; Dinarello, C.A.; Netea, M.G.; Joosten, L.A.B. Suppression of monosodium urate crystal-induced cytokine production by butyrate is mediated by the inhibition of class I histone deacetylases. Ann. Rheum. Dis. 2016, 75, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Vinolo, M.A.; Rodrigues, H.G.; Hatanaka, E.; Sato, F.T.; Sampaio, S.C.; Curi, R. Suppressive effect of short-chain fatty acids on production of proinflammatory mediators by neutrophils. J. Nutr. Biochem. 2011, 22, 849–855. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; O’Garra, A. The regulation of IL-10 production by immune cells. Nat. Rev. Immunol. 2010, 10, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.J.; Goldsworthy, S.M.; Barnes, A.A.; Eilert, M.M.; Tcheang, L.; Daniels, D.; Muir, A.I.; Wigglesworth, M.J.; Kinghorn, I.; Fraser, N.J.; et al. The Orphan G Protein-coupled Receptors GPR41 and GPR43 Are Activated by Propionate and Other Short Chain Carboxylic Acids. J. Biol. Chem. 2003, 278, 11312–11319. [Google Scholar] [CrossRef] [PubMed]
- Le Poul, E.; Loison, C.; Struyf, S.; Springael, J.-Y.; Lannoy, V.; Decobecq, M.-E.; Brezillon, S.; Dupriez, V.; Vassart, G.; Van Damme, J.; et al. Functional Characterization of Human Receptors for Short Chain Fatty Acids and Their Role in Polymorphonuclear Cell Activation. J. Biol. Chem. 2003, 278, 25481–25489. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Gurav, A.; Sivaprakasam, S.; Brady, E.; Padia, R.; Shi, H.; Thangaraju, M.; Prasad, P.D.; Manicassamy, S.; Munn, D.H.; et al. Activation of Gpr109a, Receptor for Niacin and the Commensal Metabolite Butyrate, Suppresses Colonic Inflammation and Carcinogenesis. Immunity 2014, 40, 128–139. [Google Scholar] [CrossRef]
- Unsinger, J.; Kazama, H.; McDonough, J.S.; Griffith, T.S.; Hotchkiss, R.S.; Ferguson, T.A. Sepsis-Induced Apoptosis Leads to Active Suppression of Delayed-Type Hypersensitivity by CD8+ Regulatory T Cells through a TRAIL-Dependent Mechanism. J. Immunol. 2010, 184, 6766–6772. [Google Scholar] [CrossRef]
- Unsinger, J.; McDonough, J.S.; Shultz, L.D.; Ferguson, T.A.; Hotchkiss, R.S. Sepsis-induced human lymphocyte apoptosis and cytokine production in “humanized” mice. J. Leukoc. Biol. 2009, 86, 219–227. [Google Scholar] [CrossRef]
- Boomer, J.S.; Shuherk-Shaffer, J.; Hotchkiss, R.S.; Green, J.M. A prospective analysis of lymphocyte phenotype and function over the course of acute sepsis. Crit. Care 2012, 16, R112. [Google Scholar] [CrossRef]
- Boomer, J.S.; To, K.; Chang, K.C.; Takasu, O.; Osborne, D.F.; Walton, A.H.; Bricker, T.L.; Jarman, S.D.; Kreisel, D., 2nd; Krupnick, A.S.; et al. Immunosuppression in Patients Who Die of Sepsis and Multiple Organ Failure. JAMA 2011, 306, 2594–2605. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Swanson, P.E.; Freeman, B.D.; Tinsley, K.W.; Cobb, J.P.; Matuschak, G.M.; Buchman, T.; Karl, I.E. Apoptotic cell death in patients with sepsis, shock, and multiple organ dysfunction. Crit. Care Med. 1999, 27, 1230–1251. [Google Scholar] [CrossRef]
- Hotchkiss, R.S.; Tinsley, K.W.; Swanson, P.E.; Schmieg, R.E.; Hui, J.J.; Chang, K.C.; Osborne, D.F.; Freeman, B.D.; Cobb, J.P.; Buchman, T.G.; et al. Sepsis-Induced Apoptosis Causes Progressive Profound Depletion of B and CD4+T Lymphocytes in Humans. J. Immunol. 2001, 166, 6952–6963. [Google Scholar] [CrossRef]
- Sharpe, A.H.; Freeman, G.J. The B7-CD28 superfamily. Nat. Rev. Immunol. 2002, 2, 116–126. [Google Scholar] [CrossRef]
- Boomer, J.S.; Green, J.M.; Hotchkiss, R.S. The changing immune system in sepsis Is individualized immuno-modulatory therapy the answer? Virulence 2014, 5, 45–56. [Google Scholar] [CrossRef]
- Emre, Y.; Nubel, T. Uncoupling protein UCP2: When mitochondrial activity meets immunity. FEBS Lett. 2010, 584, 1437–1442. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peters, V.B.M.; Arulkumaran, N.; Melis, M.J.; Gaupp, C.; Roger, T.; Shankar-Hari, M.; Singer, M. Butyrate Supplementation Exacerbates Myocardial and Immune Cell Mitochondrial Dysfunction in a Rat Model of Faecal Peritonitis. Life 2022, 12, 2034. https://doi.org/10.3390/life12122034
Peters VBM, Arulkumaran N, Melis MJ, Gaupp C, Roger T, Shankar-Hari M, Singer M. Butyrate Supplementation Exacerbates Myocardial and Immune Cell Mitochondrial Dysfunction in a Rat Model of Faecal Peritonitis. Life. 2022; 12(12):2034. https://doi.org/10.3390/life12122034
Chicago/Turabian StylePeters, Vera B. M., Nishkantha Arulkumaran, Miranda J. Melis, Charlotte Gaupp, Thierry Roger, Manu Shankar-Hari, and Mervyn Singer. 2022. "Butyrate Supplementation Exacerbates Myocardial and Immune Cell Mitochondrial Dysfunction in a Rat Model of Faecal Peritonitis" Life 12, no. 12: 2034. https://doi.org/10.3390/life12122034
APA StylePeters, V. B. M., Arulkumaran, N., Melis, M. J., Gaupp, C., Roger, T., Shankar-Hari, M., & Singer, M. (2022). Butyrate Supplementation Exacerbates Myocardial and Immune Cell Mitochondrial Dysfunction in a Rat Model of Faecal Peritonitis. Life, 12(12), 2034. https://doi.org/10.3390/life12122034






