Content of Cadmium and Nickel in Soils and Assimilatory Organs of Park Woody Species Exposed to Polluted Air
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil Analyses
2.3. Analyses of Assimilatory Organs of Woody Plants
2.4. Data Analyses
3. Results
3.1. Soil Properties
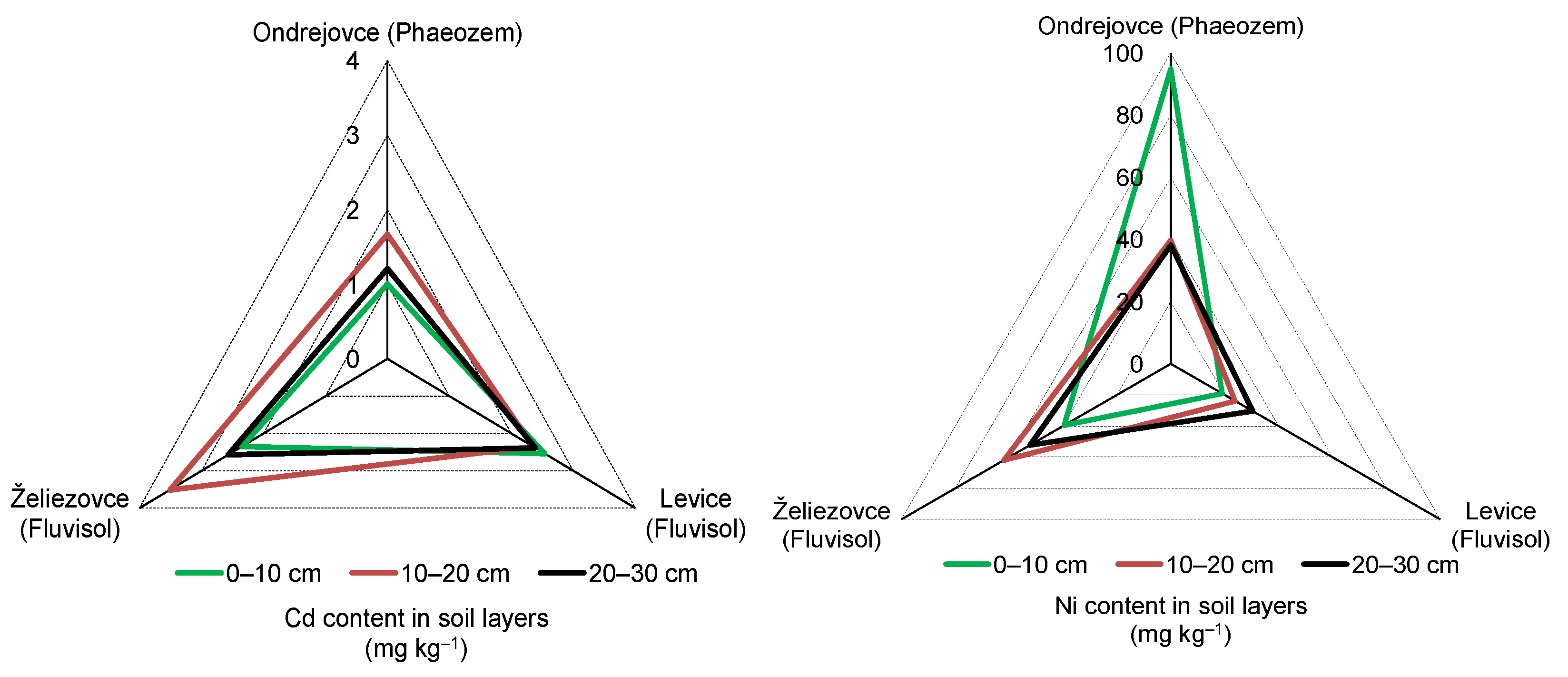
3.2. Cadmium Content in Soil Samples
3.3. Nickel Content in Soil Samples
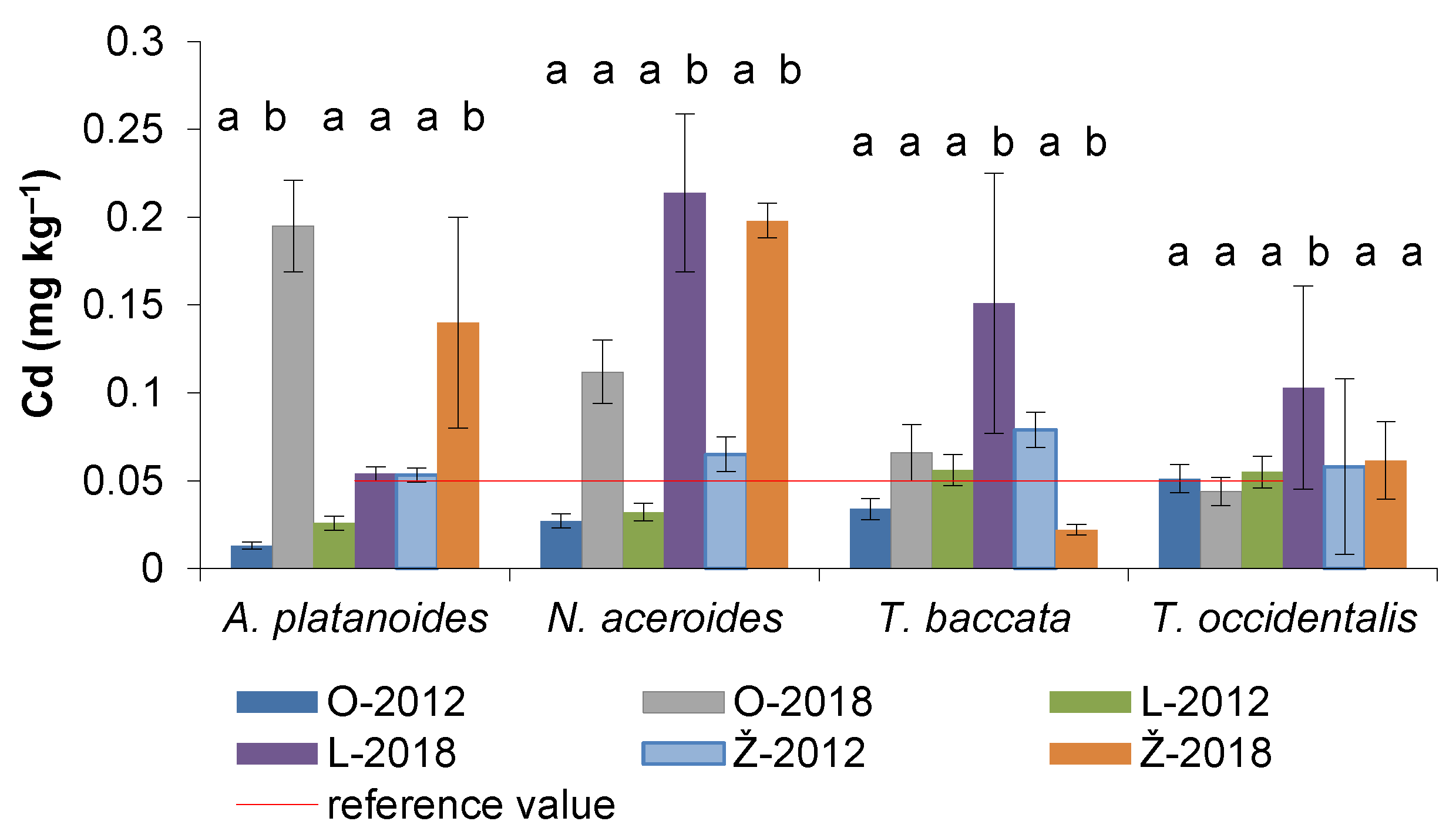
3.4. Cadmium Content in the Assimilatory Organs of Woody Plants
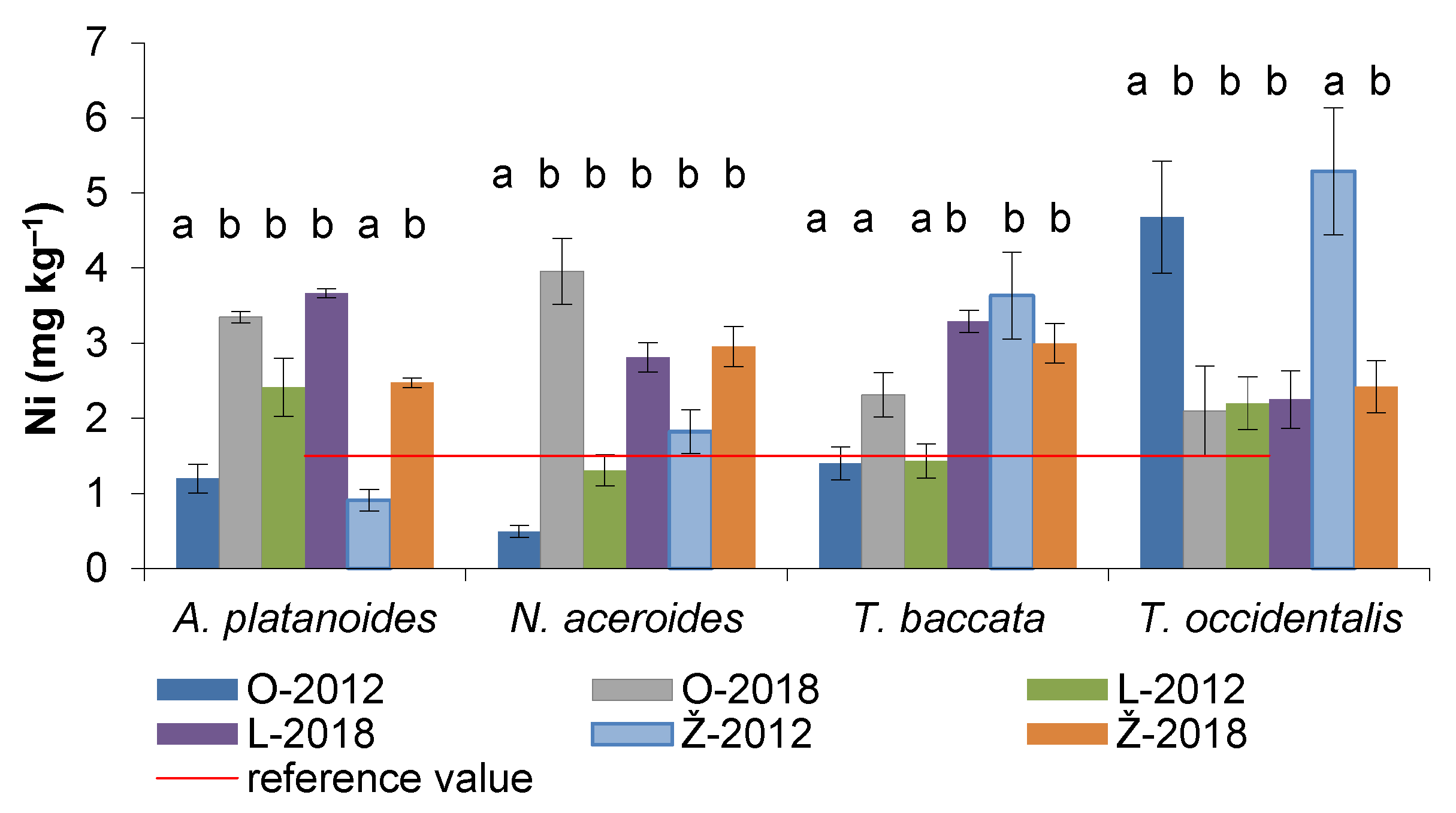
3.5. Nickel Content in the Assimilatory Organs of Woody Plants
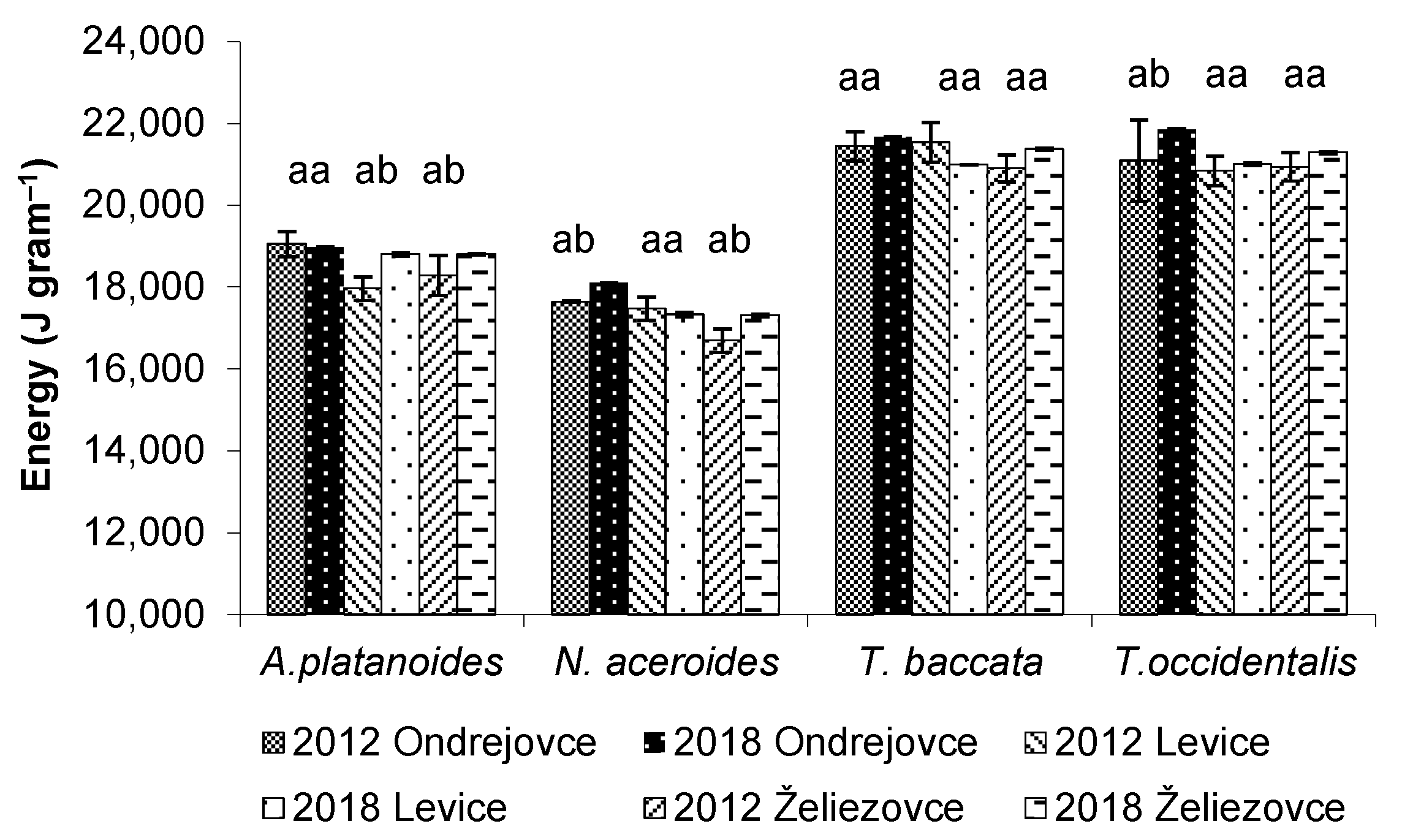
3.6. Energy Content in the Assimilatory Organs of Woody Plants
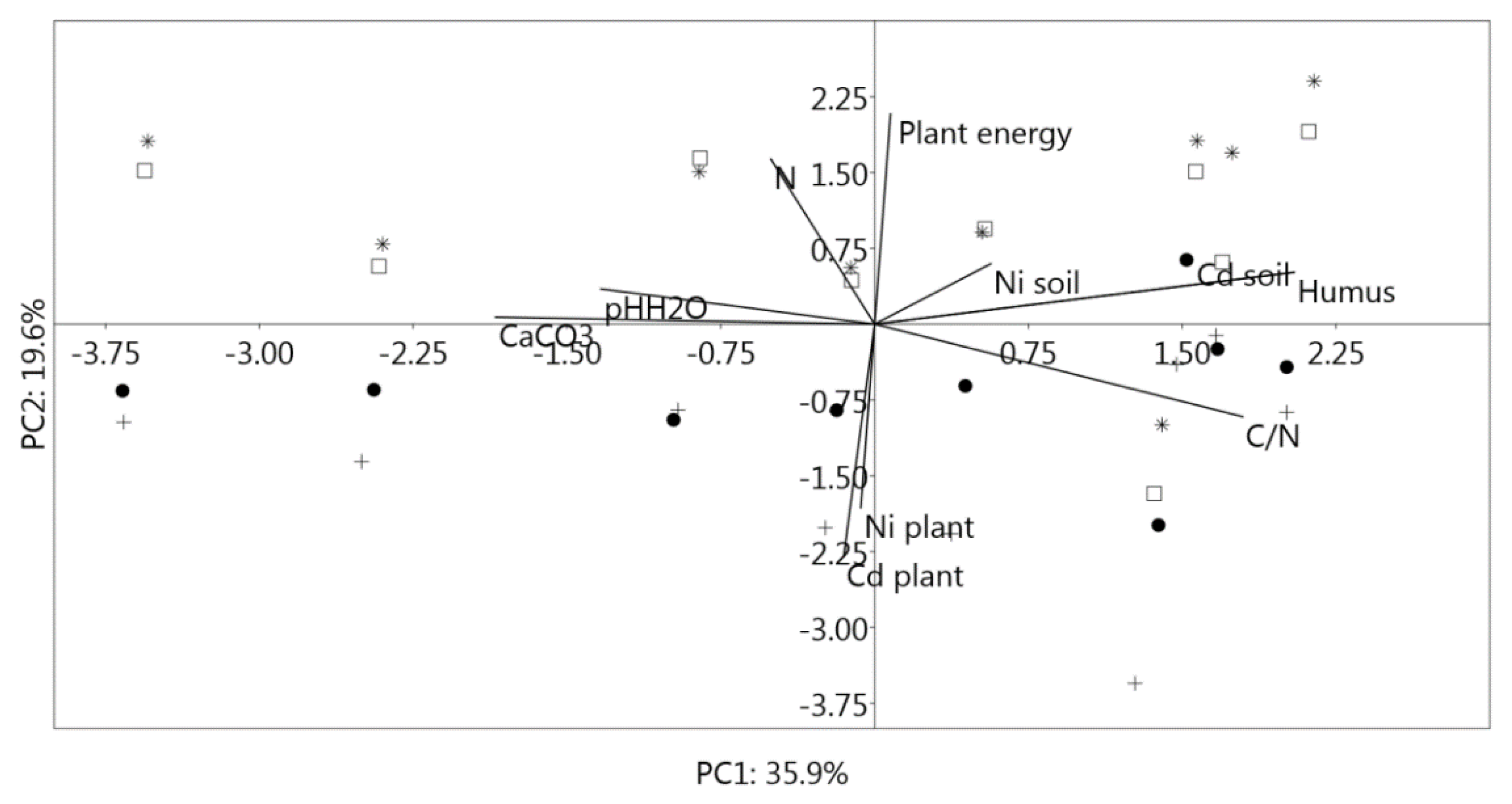
3.7. Principal Component Analysis (PCA)
3.8. Relationships between Plant and Soil Characteristics
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acosta, J.A.; Gabarrón, M.; Faz, A.; Martínez-Martínez, S.; Zornoza, R.; Arocena, J.M. Influence of population density on the concentration and speciation of metals in the soil and street dust from urban areas. Chemosphere 2015, 134, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Addo, M.A.; Darko, E.O.; Gordon, C.; Nyarko, J.B.; Gbadago, K. Heavy metal concentrations in road deposited dust at Ketu-South District, Ghana. Int. J. Sci. Technol. 2012, 2, 28–29. [Google Scholar]
- Wei, B.G.; Yang, L.S. A review of heavy metal contaminations in urban soils, urban road dusts and agricultural soils from China. Microchem. J. 2010, 94, 99–107. [Google Scholar] [CrossRef]
- Facchinelli, A.; Sacchi, E.; Mallen, L. Multivariate statistical and GIS-based approach to identify heavy metal sources in soils. Environ. Pollut. 2001, 114, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Simon, E.; Braun, M.; Vidic, A.; Bogyó, D.; Fábián, I.; Tóthmérész, B. Air pollution assessment based on elemental concentration of leaves tissue and foliage dust along an urbanization gradient in Vienna. Environ. Pollut. 2011, 159, 1229–1233. [Google Scholar] [CrossRef] [PubMed]
- Igewgbe, A.O.; Agukwe, C.H.; Negbenebo, C.A. A survey of Heavy metal (Lead, Cadmium and Copper) Contents of Selected Fruits and Vegetable crops from Borno State of Nigeria. Int. J. Eng. Sci. 2013, 2, 1–9. [Google Scholar]
- Maňkovská, B.; Godzik, B.; Badea, O.; Shparyk, Y.; Moravčík, P. Chemical and morphological characteristics of key tree species of the Carpathian Mountains. Environ. Pollut. 2004, 130, 41–54. [Google Scholar] [CrossRef]
- Benavides, M.P.; Gallego, S.M.; Tomaro, M.L. Cadmium toxicity in plants. Braz. J. Plant Physiol. 2005, 17, 21–34. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Hou, H.; Shangguan, Y.; Li, F. Source identification and health risk assessment of metals in urban soils around the Tanggu chemical industrial district, Tianjin, China. Sci. Total Environ. 2014, 468–469, 654–662. [Google Scholar] [CrossRef]
- Flexas, J.; Medrano, H. Drought-inhibition of photosynthesis in C3 plants: Stomatal and non-stomatal limitations revisited. Ann. Bot. 2002, 89, 183–189. [Google Scholar] [CrossRef]
- Seregin, I.V.; Ivanov, V.B. Physiological aspects of cadmium and lead toxic effects on higher plants. Russ. J. Plant Physiol. 2001, 48, 523–544. [Google Scholar] [CrossRef]
- Zhang, G.P.; Fukami, M.; Sekimoto, H. Influence of cadmium on mineral concentrations and yield components in wheat genotypes differing in Cd tolerance at seedling stage. Field Crop. Res. 2002, 77, 93–98. [Google Scholar] [CrossRef]
- Perronnet, K.; Schwartz, C.; Morel, J.L. Distribution of cadmium and zinc in the hyperaccumulator Thlaspi caerulescens grown on multicontaminated soil. Plant and Soil 2003, 249, 19–25. [Google Scholar] [CrossRef]
- Xu, Z.; Peng, J.; Zhu, Z.; Yu, P.; Wang, M.; Huang, Z.; Huang, Y.; Li, Z. Screening of Leafy Vegetable Varieties with Low Lead and Cadmium Accumulation Based on Foliar Uptake. Life 2022, 12, 339. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.U.; Chattha, M.U.; Khan, I.; Chattha, M.B.; Aamer, M.; Nawaz, M.; Ali, A.; Khan, M.A.U.; Khan, T.A. Nickel toxicity in plants: Reasons, toxic effects, tolerance mechanisms, and remediation possibilities—A review. Environ. Sci. Pollut. Res. 2019, 26, 12673–12688. [Google Scholar] [CrossRef] [PubMed]
- Pacyna, J.M.; Pacyna, E.G. An assessment of global and regional emissions of trace metals to the atmosphere from anthropogenic sources worldwide. Environ. Rev. 2001, 9, 269–298. [Google Scholar] [CrossRef]
- Chojnacka, K.; Chojnacki, A.; Górecka, H.; Górecki, H. Bioavailability of heavy metals from polluted soils to plants. Sci. Total Environ. 2005, 337, 175–182. [Google Scholar] [CrossRef]
- Gawel, J.E.; Trick, C.G.; Morel, F.M.M. Phytochelatins are bioindicators of atmospheric metal exposure via direct foliar uptake in trees near Sudbury, Ontario, Canada. Environ. Sci. Technol. 2001, 35, 2108–2113. [Google Scholar] [CrossRef]
- Wood, B.W. Nickel deficiency symptoms are influenced by foliar Zn:Ni or Cu:Ni concentration ratio. Acta Hort. 2010, 868, 163–170. [Google Scholar] [CrossRef]
- Solgi, E.; Keramaty, M.; Solgi, M. Biomonitoring of airborne Cu, Pb, and Zn in an urban area employing a broad leaved and a conifer tree species. J. Geochem. Explor. 2020, 208, 106400. [Google Scholar] [CrossRef]
- Liang, J.; Fang, H.L.; Zhang, T.L.; Wang, X.X.; Liu, Y.D. Heavy metal in leaves of twelve plant species from seven different areas in Shanghai, China. Urban For. Urban Green. 2017, 27, 390–398. [Google Scholar] [CrossRef]
- Madejón, P.; Marañón, T.; Murillo, J.M.; Robinson, B. White poplar (Populus alba) as a biomonitor of trace elements in contaminated riparian forests. Environ. Pollut. 2004, 132, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Dmuchowski, W.; Bytnerowicz, A. Monitoring environmental pollution in Poland by chemical analysis of Scots Pine (Pinus sylvestris L.) needles. Environ. Pollut. 1995, 87, 84–104. [Google Scholar] [CrossRef]
- Djingova, R.; Ivanova, J.; Wagner, G.; Korhammer, S.; Markert, B. Distribution of lanthanoids, Be, Bi, Ga, Te, Tl, Th and U on the territory of Bulgaria using Populus nigra “Italica” as an indicator. Sci. Total Environ. 2001, 280, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Sawidis, T.; Chettri, M.K.; Papaioannou, A.; Zachariadis, G.; Stratis, J. A study of metal distribution from lignite fuels using trees as biological monitors. Ecotoxicol. Environ. Saf. 2001, 48, 27–35. [Google Scholar] [CrossRef] [PubMed]
- André, O.; Vollenweider, P.; Günthardt-Goerg, M.S. Foliage response to heavy metal contamination in Sycamore Maple (Acer pseudoplatanus L.). For. Snow Landsc. Res. 2006, 80, 275–288. [Google Scholar]
- Ugolini, F.; Tognetti, R.; Raschi, A.; Bacci, L. Quercus ilex L. as bioaccumulator for heavy metals in urban areas: Effectiveness of leaf washing with distilled water and considerations on the trees distance from traffic. Urban For. Urban Green. 2013, 12, 576–584. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Korzeniowska, J.; Krąż, P.; Dorocki, S. Heavy Metal Content in the Plants (Pleurozium schreberi and Picea abies) of Environmentally Important Protected Areas of the Tatra National Park (the Central Western Carpathians, Poland). Minerals 2021, 11, 1231. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, J.; Xia, X.; Chu, J.; Wei, Y.; Shi, S.; Chang, E.; Yin, W.; Jiang, Z. The evaluation of heavy metal accumulation and application of a comprehensive bio-concentration index for woody species on contaminated sites in Hunan, China. Environ. Sci. Pollut. Res. 2014, 21, 5076–5085. [Google Scholar] [CrossRef]
- Futák, J. Phytogeographic division of Slovakia. In Flóra Slovenska IV/1; Bertová, L., Ed.; Veda: Bratislava, Slovakia, 1984; pp. 418–419. [Google Scholar]
- Miklós, L. Landscape atlas of the Slovak Republic, 1st ed.; Ministry of the Environment of the Slovak Republic: Bratislava, Slovakia; Slovak Environmental Agency: Bratislava, Slovakia, 2002; p. 342. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports no. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed.; CRC Press: Boca Raton, FL, USA, 2011. [Google Scholar]
- Hakanson, L. An ecological risk index for aquatic pollution control. A Sedimentological approach. Water Res. 1980, 14, 975–1001. [Google Scholar] [CrossRef]
- Markert, B. Instrumental Multielement Analysis in Plant Materials—A Modern Method in Environmental Chemistry and Tropical Systems Research; (Série 8); CETEM/CNPq: Rio de Janeiro, Brazil, 1995. [Google Scholar]
- Jung, M.C. Heavy metal concentration in soils and factors affecting metal uptake by plants in the vicinity of a Korean Cu–W mine. Sensors 2008, 8, 2413–2423. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Akther, S.M.; Hossain, M.F.; Parveen, Z. Spatial distribution and ecological risk assessment of potentially toxic metals in the Sundarbans mangrove soils of Bangladesh. Sci. Rep. 2022, 21, 10422. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.W.; Mclaren, R.G.; Roberts, A.H.C.; Condron, L.M. Effect of soil pH on cadmium phytoavailability in some New Zealand soils. N. Z. J. Crop Hortic. Sci. 1999, 27, 169–179. [Google Scholar] [CrossRef]
- Wolińska, A.; Banach, A.; Szafranek-Nakonieczna, A.; Stępniewska, Z.; Błaszczyk, M. Easily degradable carbon—An indicator of microbial hotspots and soil degradation. Int. Agrophys. 2018, 32, 123–131. [Google Scholar] [CrossRef]
- Al-Taani, A.A.; Nazzal, Y.; Howari, F.M.; Iqbal, J.; Bou Orm, N.; Xavier, C.M.; Bărbulescu, A.; Sharma, M.; Dumitriu, C.S. Contamination Assessment of Heavy Metals in Agricultural Soil, in the Liwa Area (UAE). Toxics 2021, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Uminska, R. Cadmium contents of cultivated soil exposed to contamination in Poland. Environ. Geochem. Health 1993, 15, 15–19. [Google Scholar] [CrossRef]
- Čurlík, J.; Šefčík, P. Geochemical Atlas of the Slovak Republic, Part V: Soils; State Geological Institute of Dionýz Štúr: Bratislava, Slovakia, 2012; Available online: http://apl.geology.sk/atlaspody (accessed on 10 May 2021).
- Fazekašová, D.; Petrovič, F.; Fazekaš, J.; Štofejová, L.; Baláž, I.; Tulis, F.; Tóth, T. Soil Contamination in the Problem Areas of Agrarian Slovakia. Land 2021, 10, 1248. [Google Scholar] [CrossRef]
- Šefčík, P.; Pramuka, S.; Gluch, A. Assessment of soil contamination in Slovakia according index of geoaccumulation. Agriculture 2008, 54, 119–130. [Google Scholar]
- Chen, T.B.; Zheng, Y.M.; Lei, M.; Huang, Z.C.; Wu, H.T.; Chen, H.; Fan, K.K.; Yu, K.; Wu, X.; Tian, Q.Z. Assessment of heavy metal pollution in surface soils of urban parks in Beijing, China. Chemosphere 2005, 60, 542–551. [Google Scholar] [CrossRef]
- Kirkham, M.B. Cadmium in plants on polluted soils: Effects of soil factors, hyperaccumulation, and amendments. Geoderma 2006, 137, 19–32. [Google Scholar] [CrossRef]
- Ladipo, M.K.; Doherty, V.F. Heavy metal levels in vegetables from selected markets in Lagos, Nigeria. Afr. J. Food Sci. Technol. 2011, 2, 18–21. [Google Scholar]
- Liu, W.X.; Li, H.H.; Li, S.R.; Wang, Y.W. Heavy metal accumulation of edible vegetables cultivated in agricultural soil in the Suburb of Zhengzhou city, People’s Republic of China. Bull. Environ. Contam. Toxicol. 2006, 76, 163–170. [Google Scholar]
- Molnárová, M.; Ružičková, J.; Lehotská, B.; Takáčová, A.; Fargašová, A. Determining As, Cd, Cu, Pb, Sb, and Zn in Leaves of Trees Collected near Mining Locations of Malé Karpaty Mts. in the Slovak Republic. Pol. J. Environ. Stud. 2018, 27, 2179–2191. [Google Scholar] [CrossRef]
- Alfaro, M.R.; Ugarte, O.M.; Lima, L.H.V.; Silva, J.R.; da Silva, F.B.V.; da Silva Lins, S.A.; do Nascimento, C.W.A. Risk assessment of heavy metals in soils and edible parts of vegetables grown on sites contaminated by an abandoned steel plant in Havana. Environ. Geochem. Health 2022, 44, 43–56. [Google Scholar] [CrossRef] [PubMed]
- Ismael, M.A.; Elyamine, A.M.; Moussa, M.G.; Cai, M.; Zhao, X.; Hu, C. Cadmium in plants: Uptake, toxicity, and its interactions with selenium fertilizers. Metallomics 2019, 11, 255–277. [Google Scholar] [CrossRef]
- Fröhlichová, A.; Száková, J.; Najmanová, J.; Tlustoš, P. An assessment of the risk of element contamination of urban and industrial areas using Taraxacum sect. Ruderalia as a bioindicator. Environ. Monit. Assess. 2018, 190, 150. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Schat, H.; Aarts, M.G.M. Thlaspi caerulescens, an attractive model species to study heavy metal hyperaccumulation in plants. New Phytol. 2003, 159, 351–360. [Google Scholar] [CrossRef]
- Agyarko, K.; Darteh, E.; Berlinger, B. Metal levels in some refuse dump soils and plants in Ghana. Plant Soil Environ. 2010, 56, 244–251. [Google Scholar] [CrossRef]
- Adamo, P.; Iavazzo, P.; Albanese, S.; Agrelli, D.; De Vivo, B.; Lima, A. Bioavailability and soil-to-plant transfer factors as indicators of potentially toxic element contamination in agricultural soils. Sci. Total Environ. 2014, 500, 11–22. [Google Scholar] [CrossRef]
- Menon, M.; Hermle, S.; Abbaspour, K.C.; Oswald, S.E.; Schulin, R. Water regime of metal-contaminated soil under juvenile forest vegetation. Plant Soil 2005, 271, 227–241. [Google Scholar] [CrossRef][Green Version]
- Pňakovič, Ľ.; Dzurenda, L. Combustion characteristics of fallen fall leaves from ornamental trees in city and forest parks. BioRes 2015, 10, 5563–5572. [Google Scholar] [CrossRef]
- Nurmi, J. Heating values of the above ground biomass of small-sized trees. Acta For. Fenn. 1993, 236, 7682. [Google Scholar] [CrossRef][Green Version]
- Font, R.; Conesa, J.A.; Moltó, J.; Muňoz, M. Kinetics of pyrolysis and combustion of pine needles and cones. J. Anal. Appl. Pyrolysis 2009, 85, 276–286. [Google Scholar] [CrossRef]
- Kelsey, R.G.; Shafizadeh, F.; Lowery, D.P. Heat Content of Bark, Twigs, and Foliage of Nine Species of Western Conifers. Forestry, Paper 69, 1979. USDA Forest Service, Research Note INT-261. 1979. Available online: https://digitalcommons.usu.edu/govdocs_forest/69 (accessed on 20 August 2020).
- Golia, E.E.; Dimirkou, A.; Mitsios, I.K. Influence of Some Soil Parameters on Heavy Metals Accumulation by Vegetables Grown in Agricultural Soils of Different Soil Orders. Bull. Environ. Contam. Toxicol. 2008, 81, 80–84. [Google Scholar] [CrossRef]
- Hinesly, T.D.; Redborg, K.E.; Pietz, R.I.; Ziegler, E.L. Cadmium and zinc uptake by corn (Zea mays L.) with repeated applications of sewage sludge. J. Agric. Food Chem. 1984, 32, 155–163. [Google Scholar] [CrossRef]
- Chiy, P.C.; Phillips, C.J.C. The effects of mild water stress and soil type on the responses of grass and clover to sodium fertilizer. J. Sci. Food Agric. 1999, 79, 1399–1405. [Google Scholar] [CrossRef]
- Rooney, C.P.; Zhao, F.-J.; McGrath, S.P. Phytotoxicity of nickel in a range of European soils: Influence of soil properties, Ni solubility and speciation. Environ. Pollut. 2007, 145, 596–605. [Google Scholar] [CrossRef]
- Xie, M.; Li, H.; Zhu, Y.; Xue, J.; You, Q.; Jin, B.; Shi, Z. Predicting Bioaccumulation of Potentially Toxic Element in Soil–Rice Systems Using Multi-Source Data and Machine Learning Methods: A Case Study of an Industrial City in Southeast China. Land 2021, 10, 558. [Google Scholar] [CrossRef]
- Alegría, A.; Barberá, R.; Boluda, R.; Errecalde, F.; Farré, R.; Lagarda, M.J. Environmental cadmium, lead and nickel contamination: Possible relationship between soil and vegetable content. Fresenius J. Anal. Chem. 1991, 339, 654–657. [Google Scholar] [CrossRef]
- Lopushniak, V.; Polutrenko, M.; Hrytsuliak, H.; Plevinskis, P.; Tonkha, O.; Pikovska, O.; Bykina, N.; Karabach, K.; Voloshin, Y. Accumulation of Heavy Metals in Silphium Perfoliatum L for the Cultivation of Oil-Contaminated Soils. Ecol. Eng. Environ. Tech. 2022, 23, 30–39. [Google Scholar] [CrossRef]
- Zhao, K.L.; Zhang, W.W.; Zhou, L.; Liu, X.M.; Xu, J.M.; Huang, P.M. Modeling transfer of heavy metals in soil–rice system and their risk assessment in paddy fields. Environ. Earth Sci. 2009, 59, 519–527. [Google Scholar] [CrossRef]


| Park Object | Categorization | Altitude [m] | Geographical Coordinates |
|---|---|---|---|
| Levice city | historical, origin 1879 | 160 | 48°12′ N, 18°36′ E |
| Želiezovce city | historical, origin 1875 | 145 | 48°02′ N, 18°39′ E |
| Ondrejovce village | historical, origin 1900 | 163 | 48°08′ N, 18°31′ E |
| Park Object | Soil Unit | Soil Layer | Soil Reaction | CaCO3 Equiv. | Humus | Ct | Nt | C/N | |
|---|---|---|---|---|---|---|---|---|---|
| [cm] | [pHH2O] | [pHKCl] | [%] | ||||||
| Levice | Pantofluvic Endostagnic Katocalcaric Hypereutric Fluvisol | 0–10 | 6.75 | 7.20 | 1.17 ± 0.13 | 3.81 ± 0.11 | 2.21 ± 0.10 | 0.28 ± 0.3 | 7.9 ± 0.6 |
| 10–20 | 6.82 | 7.23 | 1.51 ± 0.17 | 2.93 ± 0.09 | 1.70 ± 0.10 | 0.13 ± 0.1 | 12.9 ± 1.0 | ||
| 20–30 | 7.09 | 7.34 | 2.40 ± 0.26 | 1.98 ± 0.06 | 1.15 ± 0.05 | 0.24 ± 0.2 | 4.7 ± 0.4 | ||
| Želiezovce | 0–10 | 7.10 | 7.15 | 1.50 ± 0.16 | 4.12 ± 0.12 | 2.39 ± 0.11 | 0.26 ± 0.3 | 9.3 ± 0.7 | |
| 10–20 | 7.22 | 7.26 | 2.10 ± 0.23 | 3.55 ± 0.11 | 2.06 ± 0.10 | 0.21 ± 0.2 | 10.0 ± 0.8 | ||
| 20–30 | 7.25 | 7.42 | 2.46 ± 0.27 | 3.21 ± 0.10 | 1.86 ± 0.09 | 0.17 ± 0.2 | 10.6 ± 0.9 | ||
| Ondrejovce | Pantohypocalcic Phaeozem (Pantosiltic) | 0–10 | 6.94 | 7.16 | 1.90 ± 0.21 | 4.21 ± 0.13 | 2.44 ± 0.12 | 0.24 ± 0.2 | 10.1 ± 0.8 |
| 10–20 | 7.10 | 7.33 | 1.98 ± 0.22 | 2.79 ± 0.08 | 1.62 ± 0.09 | 0.26 ± 0.1 | 6.2 ± 0.5 | ||
| 20–30 | 7.24 | 7.38 | 2.88 ± 0.32 | 1.72 ± 0.05 | 1.00 ± 0.04 | 0.26 ± 0.1 | 3.7 ± 0.3 | ||
| Park/Soil Unit | Cadmium | Nickel | ||||
|---|---|---|---|---|---|---|
| Average (0–30 cm) | CF ± SD | The Level of Contamination, | Average (0–30 cm) | CF ± SD | The Level of Contamination, | |
| [mg kg−1 ±SD] | Hakanson [35] | [mg kg−1 ±SD] | Hakanson [35] | |||
| Ondrejovce/ Phaeozem | 1.29 ± 0.34 a | 3.2 ± 0.8 | considerable | 57.63 ± 32.3 b | 1.9 ± 1.1 | moderate |
| Levice/ Fluvisol | 2.43 ± 0.10 b | 5.9 ± 0.3 | 24.47 ± 5.7 a | 0.8 ± 0.2 | low | |
| Želiezovce/ Fluvisol | 2.81 ± 0.62 b | 6.9 ± 1.5 | very high | 51.30 ± 11.2 b | 1.8 ± 0.4 | moderate |
| Characteristic | Ni Content in Plant | Cd Content in Plant | |||||
|---|---|---|---|---|---|---|---|
| F-Ratio | R-Squared (R2) | Correlation Coefficient (R) | F-ratio | R-Squared (R2) | Correlation Coefficient (R) | ||
| A. platanoides | |||||||
| Soil layer 0–30 cm | Ni/Cd | 0.877 | 0.111 | −0.334 | 0.686 | 0.005 | −0.076 |
| pHH2O | 5.269 | 0.429 | −0.655 * | 3.849 | 0.355 | 0.596 * | |
| CaCO3 equivalent | 0.143 | 0.020 | −0.142 | 2.679 | 0.277 | 0.526 * | |
| Humus | 1.248 | 0.151 | −0.389 | 0.041 | 0.006 | −0.077 | |
| N | 0.082 | 0.012 | 0.108 | 0.231 | 0.032 | 0.179 | |
| C/N | 0.577 | 0.076 | −0.276 | 0.368 | 0.049 | −0.223 | |
| Leaf energy | 0.002 | 0.000 | −0.001 | 9.775 | 0.583 | 0.763 ** | |
| N. aceroides | |||||||
| Soil layer 0–30 cm | Ni/Cd | 5.464 | 0.438 | 0.662 * | 4.902 | 0.031 | −0.178 |
| pHH2O | 0.279 | 0.038 | 0.196 | 0.005 | 0.0007 | 0.002 | |
| CaCO3 equivalent | 1.537 | 0.180 | 0.424 * | 0.005 | 0.001 | −0.009 | |
| Humus | 0.070 | 0.010 | −0.099 | 0.230 | 0.032 | −0.178 | |
| N | 0.358 | 0.049 | 0.221 | 17.59 | 0.715 | −0.846 ** | |
| C/N | 0.426 | 0.057 | −0.240 | 2.412 | 0.256 | 0.506 * | |
| Leaf energy | 2.392 | 0.255 | 0.505 * | 0.099 | 0.014 | −0.118 | |
| T. baccata | |||||||
| Soil layer 0–30 cm | Ni/Cd | 1.731 | 0.198 | −0.445 * | 0.073 | 0.120 | −0.347 |
| pHH2O | 0.735 | 0.095 | −0.308 | 4.989 | 0.416 | −0.645 * | |
| CaCO3 equivalent | 3.137 | 0.309 | −0.556 * | 0.399 | 0.054 | −0.232 | |
| Humus | 0.960 | 0.121 | 0.347 | 0.957 | 0.120 | −0.347 | |
| N | 1.231 | 0.149 | −0.387 | 0.942 | 0.119 | −0.344 | |
| C/N | 2.979 | 0.299 | 0.546 * | 0.050 | 0.007 | 0.084 | |
| Needle energy | 0.136 | 0.020 | 0.138 | 3.697 | 0.356 | 0.589 * | |
| T. occidentalis | |||||||
| Soil layer 0–30 cm | Ni/Cd | 0.866 | 0.110 | −0.331 | 1.635 | 0.084 | −0.289 |
| pHH2O | 1.079 | 0.134 | 0.365 | 0.962 | 0.121 | −0.348 | |
| CaCO3 equivalent | 0.111 | 0.016 | 0.125 | 0.237 | 0.033 | −0.181 | |
| Humus | 0.620 | 0.081 | −0.285 | 0.639 | 0.084 | −0.289 | |
| N | 2.777 | 0.284 | −0.533 * | 7.022 | 0.501 | −0.708 ** | |
| C/N | 0.279 | 0.038 | 0.196 | 1.073 | 0.133 | 0.364 | |
| Needle energy | 0.376 | 0.051 | 0.226 | 0.008 | 0.0001 | −0.011 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pivková, I.; Kukla, J.; Hniličková, H.; Hnilička, F.; Krupová, D.; Kuklová, M. Content of Cadmium and Nickel in Soils and Assimilatory Organs of Park Woody Species Exposed to Polluted Air. Life 2022, 12, 2033. https://doi.org/10.3390/life12122033
Pivková I, Kukla J, Hniličková H, Hnilička F, Krupová D, Kuklová M. Content of Cadmium and Nickel in Soils and Assimilatory Organs of Park Woody Species Exposed to Polluted Air. Life. 2022; 12(12):2033. https://doi.org/10.3390/life12122033
Chicago/Turabian StylePivková, Ivica, Ján Kukla, Helena Hniličková, František Hnilička, Danica Krupová, and Margita Kuklová. 2022. "Content of Cadmium and Nickel in Soils and Assimilatory Organs of Park Woody Species Exposed to Polluted Air" Life 12, no. 12: 2033. https://doi.org/10.3390/life12122033
APA StylePivková, I., Kukla, J., Hniličková, H., Hnilička, F., Krupová, D., & Kuklová, M. (2022). Content of Cadmium and Nickel in Soils and Assimilatory Organs of Park Woody Species Exposed to Polluted Air. Life, 12(12), 2033. https://doi.org/10.3390/life12122033







