The Life Cycle of the Xylophagous Beetle, Steraspis speciosa (Coleoptera, Buprestidae), Feeding on Acacia Trees in Saudi Arabia
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Areas and Insect Infection
2.2. Effect of Habitat on Infection
2.3. Parameters Measured
2.4. Statics Analysis
3. Results
3.1. Acacia Populations in the Ha’il Area
3.2. Effects of Tree Ages and Habitats on the Infection by Steraspis speciosa
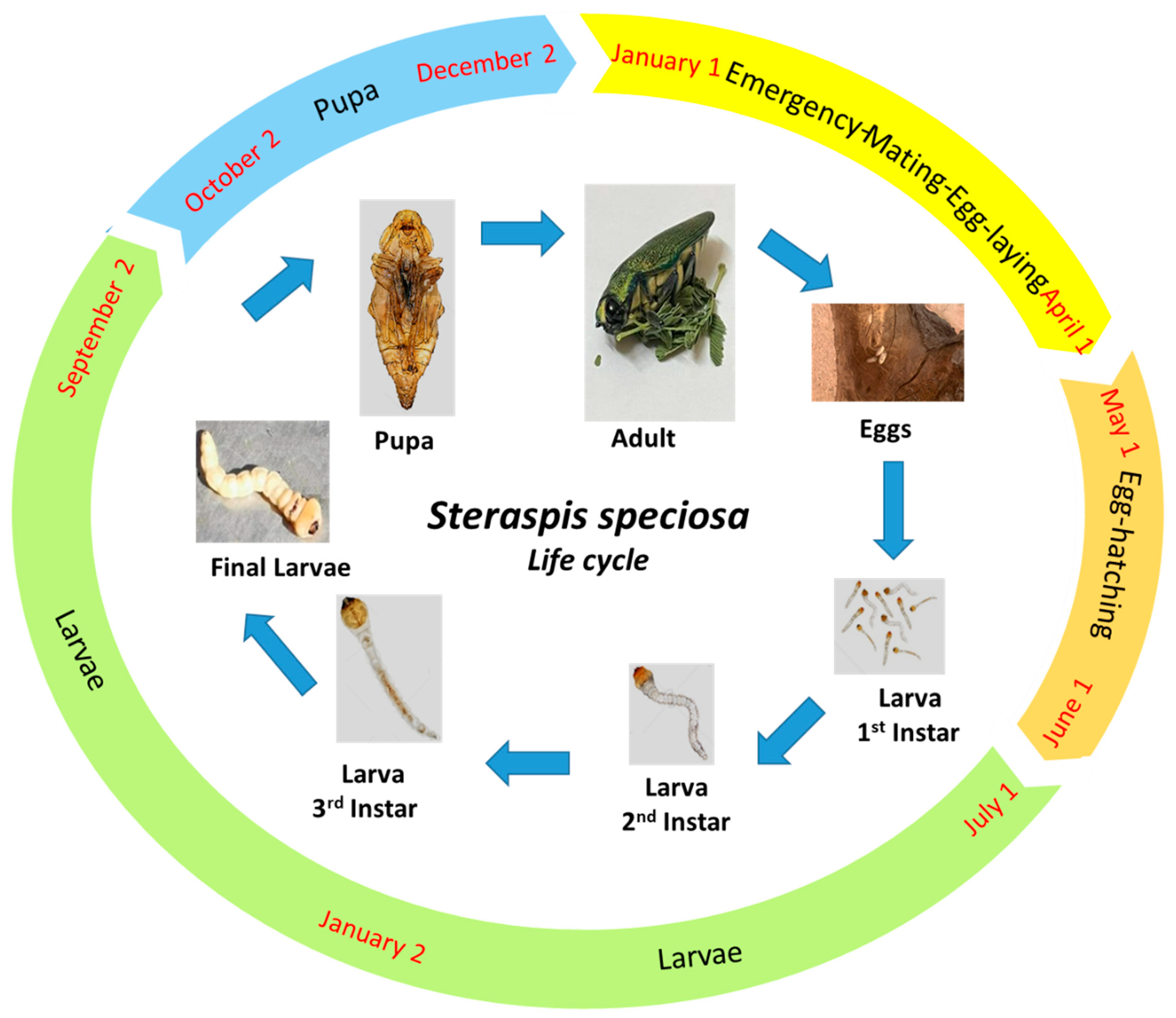
3.3. The Life Cycle of Steraspis speciosa
3.3.1. Adult Mating
3.3.2. Oviposition and Egg Hatching
3.3.3. Larval Stage
3.3.4. Pupation Stage
3.3.5. Emergence and Imago
4. Discussions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bellamy, C.L.; Westcott, R.L.; Verity, D.S. New synonymy, distributional and adult and larval host records for some southern African Buprestidae (Coleoptera). Coleopt. Bull. 1988, 42, 73–83. [Google Scholar]
- Westcott, R.L. A world catalogue and bibliography of the jewel beetles (Coleoptera: Buprestoidea), Volume 1–5. Coleopt. Bull. 2009, 63, 117–118. [Google Scholar] [CrossRef]
- Dejean, P. Catalogue des coléopteres de la collection de M. Le Comte Dejean, Vol. 2; Méquignon-Marvis Père Fils: Paris, France, 1833; pp. 97–176. [Google Scholar]
- Curletti, G. Sul Genere Steraspis Dejean, 1833 (Coleoptera, Buprestidae). Bol. Mus. Reg. Sci. Nat. Torino 2009, 26, 83–153. [Google Scholar]
- Klug, F.; Hemprich, F.W.; Ehrenberg, C.G. Symbolae Physicae seu Icones et Descriptiones Insectorum Quae Ex Itinere per Africam Borealem et Asiam Occidentalem Friderici Guilelmi Hemprich et Christiani Godofredi Ehrenberg. Studio Novae Aut Illustratae Redierunt; Officina Academica: São Paulo, Brazil, 1829. [Google Scholar]
- Mateu, J. Les Insectes Xylophages des Acacias dans les Régions Sahariennes; Instituto de Zoología “Dr. Augusto Nobre”: Porto, Portugal, 1972. [Google Scholar]
- Gardner, J.C.M. An Annotated List of East African Forest Insects; EAAFRO For Tech Note 7, Gov’t Printer; EAAFRO: Nairobi, Kenya, 1957; p. 34. [Google Scholar]
- De Meyer, M. Forest entomology in East Africa—Forest insects of Tanzania. J. East Afr. Nat. Hist. 2006, 95, 241–242. [Google Scholar] [CrossRef]
- Yasin, E.; Has, E.; Mulyana, B. Spatial Distribution of tree species composition and carbon stock in Tozi tropical dry forest, Sinnar State, Sudan. Biodiversitas J. Biol. Divers. 2022, 23, 2359–2368. [Google Scholar] [CrossRef]
- Hosny, A.M.; Shawky, R.A.; Hashim, A.A. Size structure and floristic diversity of Acacia trees population in Taif area, Saudi Arabia. J. Biodivers Endanger Species 2018, 6, 2. [Google Scholar] [CrossRef]
- Shaltout, K.H.; Mady, M.A. Analysis of Raudhas vegetation in Central Saudi Arabia. J. Arid Environ. 1996, 34, 441–454. [Google Scholar] [CrossRef]
- Migahid, A.M. Flora of Saudi Arabia; University Libraries, K.S.U.: Helsinki, Finland, 1988. [Google Scholar]
- El-Sheikh, M.A.; Thomas, J.; Alatar, A.A.; Hegazy, A.K.; Abbady, G.A.; Alfarhan, A.H.; Okla, M.I. Vegetation of Thumamah Nature Park: A managed arid land site in Saudi Arabia. Rend. Lincei 2013, 24, 349–367. [Google Scholar] [CrossRef]
- Thomas, J.; Sivadasan, M.; Al-Ansari, A.M.; Alfarhan, A.; El-Sheikh, M.; Basahi, M.; Alatar, A.A. New generic and species records for the flora of Saudi Arabia. Saudi J. Biol. Sci. 2014, 21, 457–464. [Google Scholar] [CrossRef]
- Alatar, A.A.; El-Sheikh, M.A.R.; Thomas, J.; Hegazy, A.K.; El Adawy, H.A. Vegetation, floristic diversity, and size-classes of Acacia gerrardii in an arid wadi ecosystem. Arid L. Res. Manag. 2015, 29, 335–359. [Google Scholar] [CrossRef]
- Wickens, G.E.; El Din, A.G.S.; Nahal, I.; Sita, G. Role of Acacia Species in the Rural Economy of Dry Africa and the Near East; Food & Agriculture Organization: Nishi Ward, Yokohama, 1995; ISBN 9251036519. [Google Scholar]
- Aref, I.M.; El-Juhany, L.I.; Hegazy, S.S. Comparison of the growth and biomass production of six Acacia species in Riyadh, Saudi Arabia after 4 years of irrigated cultivation. J. Arid Environ. 2003, 54, 783–792. [Google Scholar] [CrossRef]
- Haggag, S.M.; Girgis, G.N.; Batt, A.M.; Okil, A.M. Biological studies on the tamarisk Jewel borer, Steraspis squamosa, Klug (Cleoptera: Buprestidae) in Egypt. Egypt. J. Agric. Res. 1996, 74, 1013–1020. [Google Scholar]
- Ahmed, E.M. Infestation of the longhorned beetles species (Cerambycidae) on Acacia mellifera (Vahl) Benth in the gum arabic belt of Sudan. Asian J. Res. Biosci. 2022, 62–67. [Google Scholar]
- Vayssières, J.-F.; Bellamy, C.L. About the biology of Steraspis infuscata Théry and data on additional species of Steraspis collected in Benin (Coleoptera, Buprestidae). Bull. Société Entomol. Fr. 2012, 117, 179–185. [Google Scholar] [CrossRef]
- Khider, M.M.; Al Abjar, Z.A.; Eltigani, A. Studies on ecology and biology of some insect pests of the mesquite Trees, Prosopis juliflora in Sudan. Egypt. J. Biol. Pest Control 2011, 21, 353–359. [Google Scholar]
- Mohammed Ali, R. Infestation of Mesquite Trees Prosobis Chilenesis by Sudanese Native Beetle Steraspis Speciosa; University of Khartoum: Khartoum, Sudan, 2012. [Google Scholar]
- Klug, J.C.F. Symbolae Physicae seu Icones et Descriptions Insectorum Quae Exitinere Africam Borealem et Asiam Occidentalem FG Hemprich et CH Ehrenberg Studio Novae aut Illustratae Rediereunt; Mittler: Berlin, Germany, 1832; p. 185. [Google Scholar]
- Bousquet, Y.; Bouchard, P. The genera in the third catalogue (1836–1837) of Dejean’s coleoptera collection. Zookeys 2013, 282, 221–239. [Google Scholar]
- Ghahari, H.; Volkovitsh, M.G.; Bellamy, C.L. An annotated catalogue of the Buprestidae of Iran (Coleoptera: Buprestoidea). Zootaxa 2015, 3984, 1–141. [Google Scholar] [CrossRef]
- Noumi, Z. Acacia Tortilis (Forssk.) Hayne Subsp. Raddiana (Savi) Brenan en Tunisie Pré-Saharienne: Structure du Peuplement, Réponses et Effets Biologiques et Environnementaux; University of Sfax: Sfax, Tunisia, 2010. [Google Scholar]
- Migahid, A.M. Flora of Saudi Arabia: Cryptogams and Dicotyledons, Equisetaceae to Neuradaceae; University Libraries, King Saud University: Riyadh, Saudi Arabia, 1988; Volume 1. [Google Scholar]
- Blair, K.G. A collection of Coleoptera from the Rub’al Khali, Southern Arabia, with descriptions of some new species and varieties. Entomol. Mon. Mag. 1931, 67, 269–271. [Google Scholar]
- Bily, S. Insects of Saudi Arabia: Coleoptera: Fam. Buprestidae. Fauna Saudi Arab. 1979, 215–222. [Google Scholar]
- Evans, H.F.; Moraal, L.G.; Pajares, J.A. Biology, ecology and economic importance of Buprestidae and Cerambycidae. In Bark and Wood Boring Insects in Living Trees in EUROPE, a Synthesis; Springer: Berlin/Heidelberg, Germany, 2007; pp. 447–474. [Google Scholar]
- Schabel, H.G. Shoot, bark and wood borers. In Forest Entomology in East Africa; Springer: Dordrecht, The Netherlands, 2006; pp. 119–178. [Google Scholar]
- Reed, K.; Denman, S.; Leather, S.R.; Forster, J.; Inward, D.J.G. The lifecycle of Agrilus biguttatus: The role of temperature in its development and distribution, and implications for acute oak decline. Agric. For. Entomol. 2018, 20, 334–346. [Google Scholar] [CrossRef]
- Klausnitzer, B. (Ed.) Familie Elateridae. In Die Larven der Käfer Mitteleuropas; Goecke & Evers: Baden-Wuerttemberg, Germany, 1994; Volume 2, pp. 118–189. [Google Scholar]
- Moraal, L.G.; Hilszczanski, J. The oak Buprestid beetle, Agrilus biguttatus (F.)(Col., Buprestidae), a recent factor in oak decline in Europe. Anz. Für Schädlingskunde = J. Pest Sci. 2000, 73, 134–138. [Google Scholar] [CrossRef]
- Habermann, M.; Preller, J. Studies on the biology and control of two-spotted lichen Buprestid (Agrilus biguttatus Fabr.). Forst Holz 2003, 58, 215–220. [Google Scholar]
- Rosenberger, D.W.; Venette, R.C.; Aukema, B.H. Susceptibility of Eurasian scots pine, Pinus sylvestris L., to the aggressive North American mountain pine beetle, Dendroctonus ponderosae Hopkins. For. Ecol. Manag. 2019, 445, 20–25. [Google Scholar] [CrossRef]
- Kolb, T.; Keefover-Ring, K.; Burr, S.J.; Hofstetter, R.; Gaylord, M.; Raffa, K.F. Drought-mediated changes in tree physiological processes weaken tree defenses to bark beetle attack. J. Chem. Ecol. 2019, 45, 888–900. [Google Scholar] [CrossRef]
- Vayssières, J.-F.; Bellamy, C.L.; Goergen, G.; Adandonon, A.; Sinzogan, A.; Curletti, G. About the biology of Steraspis fastuosa Gerstaecker in Atacora region, northern Benin (Coleoptera, Buprestidae). Bull. Société Entomol. Fr. 2013, 118, 427–432. [Google Scholar] [CrossRef]
- Hanks, L.M. Influence of the larval host plant on reproductive strategies of Cerambycid beetles. Annu. Rev. Entomol. 1999, 44, 483–505. [Google Scholar] [CrossRef]
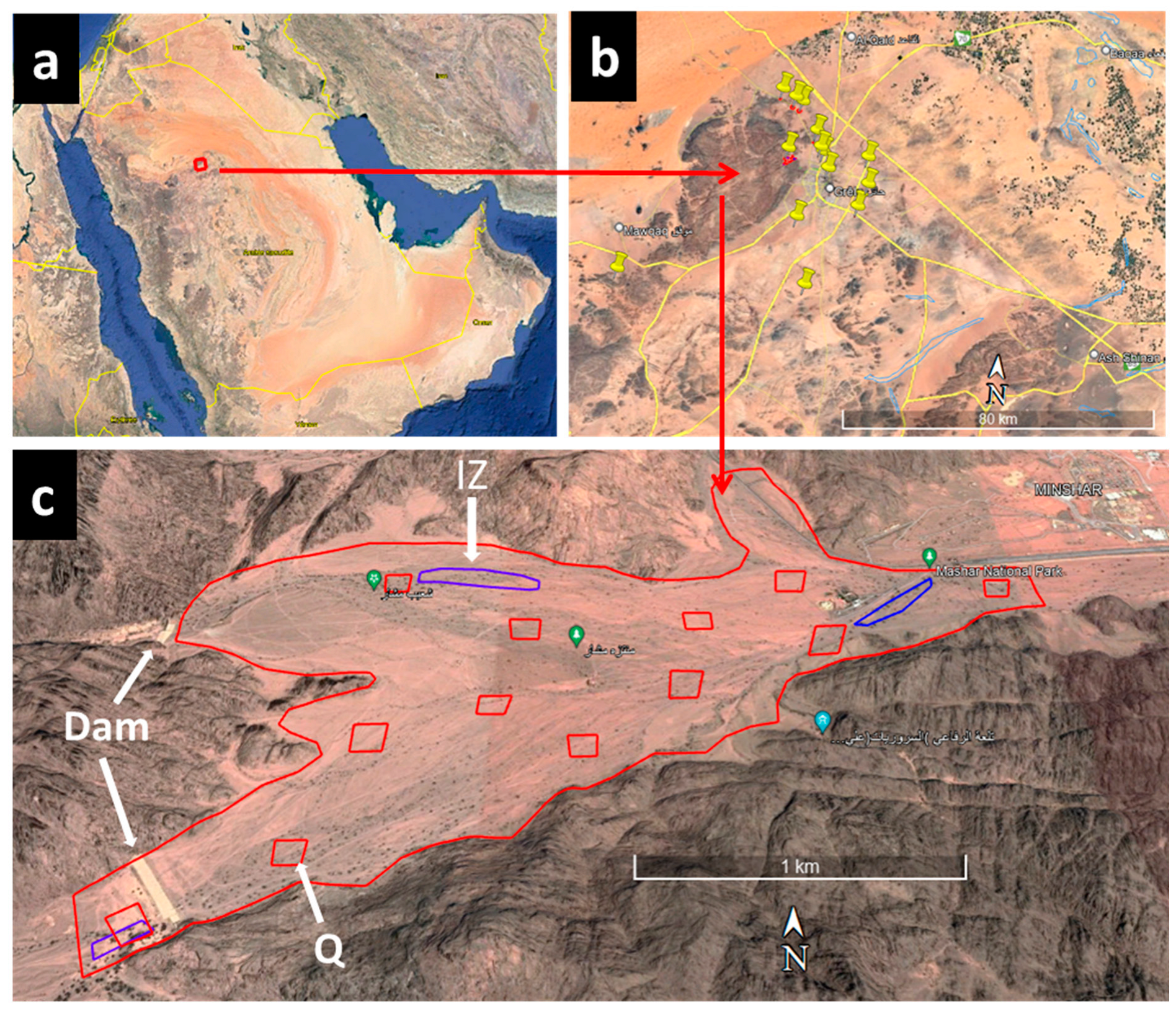


| Zone | Geographic Coordinates | Rate of Infection by S. speciosa | Non Infected(N) Infected (I) | Trunk Perimeter at 1 m Length (cm) | Number of Branches | Status of Tree | Richness (%) Rate of Acacia spp. | ||
|---|---|---|---|---|---|---|---|---|---|
| A. ger | A. ehr | A. rad | |||||||
| 1 | 27 34 418 N | 23% | N (77) | 55 ± 6 | 6 ± 1 | Flowering | 95 | 5 | 0 |
| 41 39 681 E | I (23) | 75 ± 3 | 7 ± 1 | Flowering Insect holes | |||||
| 2 | 27 34 808 N | 16% | N (84) | 42 ± 3 | 5 ± 1 | Flowering | 97 | 3 | 0 |
| 41 39 146 E | I (16) | 54 ± 7 | 7 ± 3 | Flowering Insect holes Reviviscence | |||||
| 3 | 27 27 315 N | 22% | N (78) | 60 ± 5 | 6 ± 2 | Flowering | 75 | 21 | 4 |
| 41 48 983 E | I (22) | 66 ± 4 | 7 ± 3 | Flowering Insect holes Insect collect | |||||
| 4 | 27 35 305 N | 18% | N (82) | 59 ± 5 | 5 ± 3 | Flowering | 88 | 9 | 3 |
| 41 43 039 E | I (18) | 72 ± 3 | 7 ± 1 | Flowering Insect holes Insect collect | |||||
| 5 | 27 32 479 N | 21% | N (79 | 67 ± 6 | 5 ± 1 | Flowering | 92 | 8 | 0 |
| 41 46 565 E | I (21) | 69 ± 5 | 7 ± 1 | Flowering Insect holes Reviviscence | |||||
| 6 | 27 35 825 N | 17% | N (83) | 58 ± 2 | 3 ± 2 | Flowering | 84 | 10 | 6 |
| 41 41 855 E | I (17) | 80 ± 1 | 8 ± 1 | Flowering Insect holes Insect collect | |||||
| 7 | 27 36 585 N | 12% | N (88) | 54 ± 3 | 4 ± 2 | Flowering | 87 | 8 | 5 |
| 41 41 755 E | I (12) | 61 ± 3 | 5 ± 1 | Flowering Insect holes Insect collect | |||||
| 8 | 27 37 043 N | 25% | N (75) | 56 ± 1 | 6 ± 2 | Flowering | 99 | 1 | 0 |
| 41 42 239 E | I (25) | 75 ± 2 | 9 ± 1 | Flowering Insect holes Reviviscence | |||||
| 9 | 27 37 043 N | 23% | N (77) | 58 ± 1 | 6 ± 2 | Flowering | 95 | 3 | 2 |
| 41 42 239 E | I (23) | 74 ± 4 | 8 ± 1 | Flowering Insect holes | |||||
| 10 | 27 18 680 N | 10% | N (90) | 56 ± 5 | 6 ± 1 | Flowering | 94 | 6 | 0 |
| 41 40 767 E | I (10) | 90 ± 4 | 8 ± 2 | Flowering Insect holes Insect collect | |||||
| 11 | 27 33 657 N | 16% | N (84) | 71 ± 4 | 10 ± 1 | Flowering | 84 | 12 | 4 |
| 41 42 862 E | I (16) | 56 ± 3 | 6 ± 2 | Flowering Insect holes Insect collect Tree death | |||||
| 12 | 27 26 828 N | 33% | N (67) | 54 ± 2 | 6 ± 1 | Flowering | 97 | 3 | 0 |
| 41 49 742 E | I (33) | 78 ± 4 | 10 ± 2 | Flowering Insect holes Insect collect | |||||
| 13 | 27 24 596 N | 37% | N (63) | 36 ± 7 | 6 ± 1 | Flowering | 88 | 9 | 3 |
| 41 49 8252 E | I (37) | 82 ± 9 | 10 ± 2 | Flowering Insect holes Insect collect Reviviscence | |||||
| Average | N (79 ± 5.0) | 55.8 ± 9.0 | 5.7 ± 1.6 | ||||||
| I (21 ± 7.6) | 71.7 ± 10.3 | 7.6 ± 1.4 | |||||||
| Average (df = 23) | ** 21 ± 7.6% | 63.4 ± 9.7 | 6.6 ± 1.5 | Average (df = 12) | 90.3 ± 6.8 | 7.5 ± 5.2 | 2.2 ± 2.1 | ||
| ** | ** | NS | NS | NS | |||||
| Number of Trees (Trees/ha) According to Age in Three Habitats (Dam, Plateau, Wadi) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young | Adult | Old | Total | |||||||||
| Dam | 8.8 | ± | 5.7 | 16.0 | ± | 4,1 | 19.3 | ± | 1.7 | 44.0 | ± | 5.4 |
| Plateau | 1.0 | ± | 1.2 | 5.5 | ± | 1.3 | 4.0 | ± | 2.9 | 10.5 | ± | 2.3 |
| Wadi | 15.8 | ± | 1.0 | 7.3 | ± | 2.2 | 9.0 | ± | 5.5 | 32.0 | ± | 4.5 |
| Average (Trees/ha) | 8.5 | ± | 2.6 | 9.6 | ± | 2.5 | 10.8 | ± | 3.4 | 28.8 | ± | 1.1 |
| Population size | 2949.5 | ± | 412.2 | 3331.2 | ± | 576.7 | 3747.6 | ± | 456.9 | 10,028.3 | ± | 513.4 |
| Percentage (%) | 29.4 | ± | 4.0 | 33.2 | ± | 6.5 | 37.3 | ± | 5.1 | 100% | ||
| df | 11 | 11 | 11 | |||||||||
| F. value | 18.935 | 16.333 | 17.441 | |||||||||
| Sig. | 0.001 | 0.001 | 0.001 | |||||||||
| Percentage of Infection (%) by Steraspis speciosa | Dimensions of Infected Trees | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young | Adult | Old | Total | TL (m) | Tr Pe (cm) | Br Pe (cm) | |||||||||||||||
| Dam | 0.2 | ± | 0.1 | 0.6 | ± | 0.0 | 8.6 | ± | 0.1 | 9.4 | ± | 0.2 | 4.7 | ± | 0.4 | 110.3 | ± | 1.9 | - | ||
| Wadi | 4.8 | ± | 0.3 | 5.6 | ± | 0.6 | 17.6 | ± | 0.3 | 28.0 | ± | 4.8 | 4.3 | ± | 0.6 | 91.5 | ± | 35.6 | - | ||
| Plateau | 1.9 | ± | 0.4 | 2.7 | ± | 0.4 | 34.1 | ± | 0.8 | 38.8 | ± | 1.9 | 3.9 | ± | 0.3 | 66.7 | ± | 19.7 | 36.2 | ± | 13.8 |
| Average | 2.3 | ± | 0.2 | 3.0 | ± | 0.3 | 20.1 | ± | 0.4 | 25.4 | ± | 2.3 | 4.2 | ± | 0.5 | 91.5 | ± | 27.1 | |||
| df | 11 | 11 | 11 | 11 | 11 | ||||||||||||||||
| F. value | 216.0 | 100.4 | 386.5 | 2.4 | 2.3 | ||||||||||||||||
| Sig. | 0.000 | 0.000 | 0.000 | 0.145 (NS) | 0.147 (NS) | ||||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alanazi, N.A.; Ghorbel, M.; Brini, F.; Mseddi, K. The Life Cycle of the Xylophagous Beetle, Steraspis speciosa (Coleoptera, Buprestidae), Feeding on Acacia Trees in Saudi Arabia. Life 2022, 12, 2015. https://doi.org/10.3390/life12122015
Alanazi NA, Ghorbel M, Brini F, Mseddi K. The Life Cycle of the Xylophagous Beetle, Steraspis speciosa (Coleoptera, Buprestidae), Feeding on Acacia Trees in Saudi Arabia. Life. 2022; 12(12):2015. https://doi.org/10.3390/life12122015
Chicago/Turabian StyleAlanazi, Naimah Asid, Mouna Ghorbel, Faiçal Brini, and Khalil Mseddi. 2022. "The Life Cycle of the Xylophagous Beetle, Steraspis speciosa (Coleoptera, Buprestidae), Feeding on Acacia Trees in Saudi Arabia" Life 12, no. 12: 2015. https://doi.org/10.3390/life12122015
APA StyleAlanazi, N. A., Ghorbel, M., Brini, F., & Mseddi, K. (2022). The Life Cycle of the Xylophagous Beetle, Steraspis speciosa (Coleoptera, Buprestidae), Feeding on Acacia Trees in Saudi Arabia. Life, 12(12), 2015. https://doi.org/10.3390/life12122015






