Potential of Microalgae Extracts for Food and Feed Supplementation—A Promising Source of Antioxidant and Anti-Inflammatory Compounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Microalgae Biomass Source
2.2. Extraction Procedure
2.3. Biochemical Characterization
2.4. Antioxidant Capacity Assessment
2.5. Anti-Inflammatory Capacity Assessment
2.5.1. Human Red Blood Cell (HRBC) Membrane Stabilization by Heat-Induction
2.5.2. Cyclooxygenase (COX-2) Enzymatic Activity
2.6. Bioactive Compounds
2.7. Statistical Analysis
3. Results
3.1. Extraction Yield
3.2. Biochemical Composition
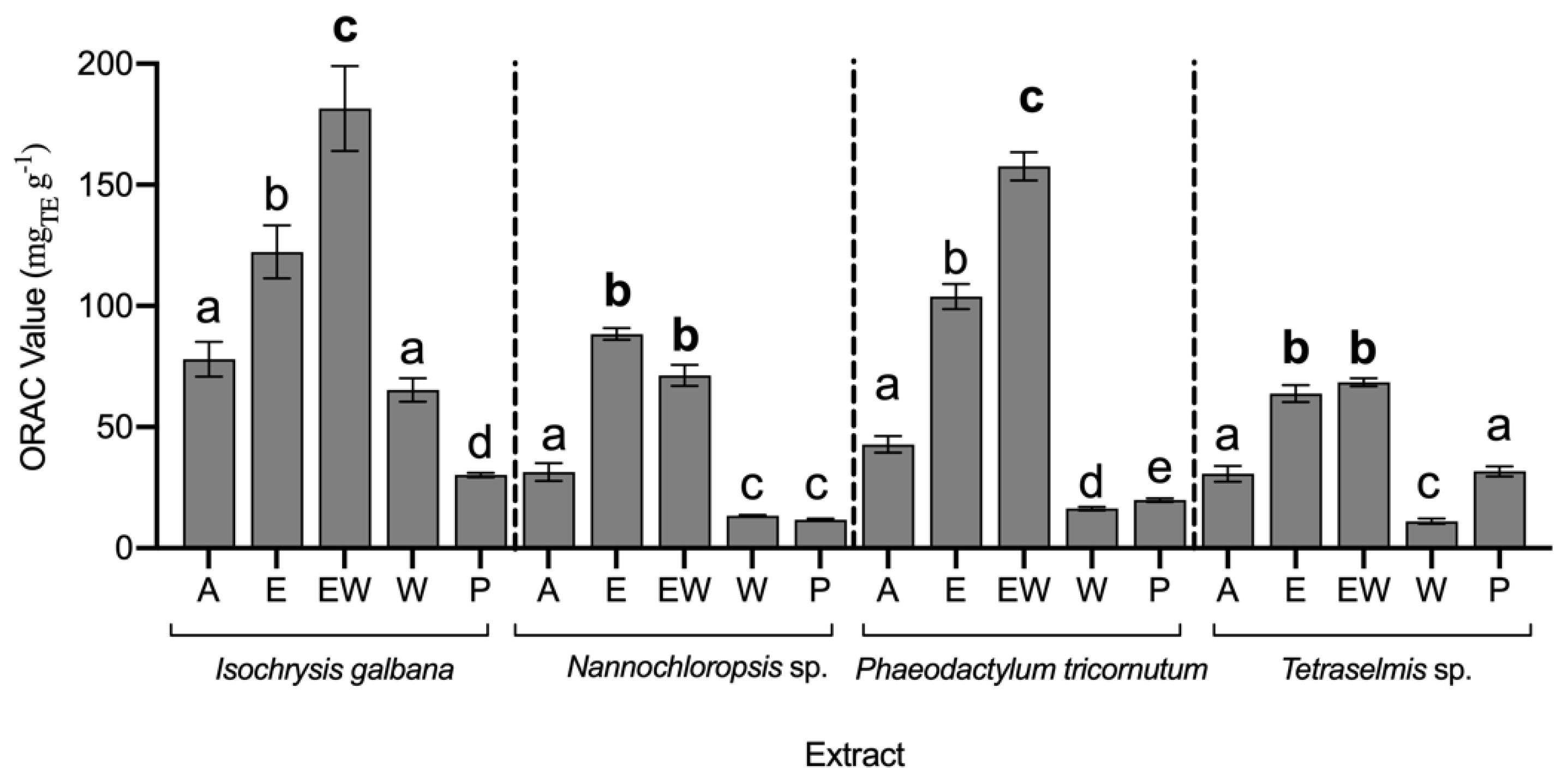
3.3. Antioxidant Capacity
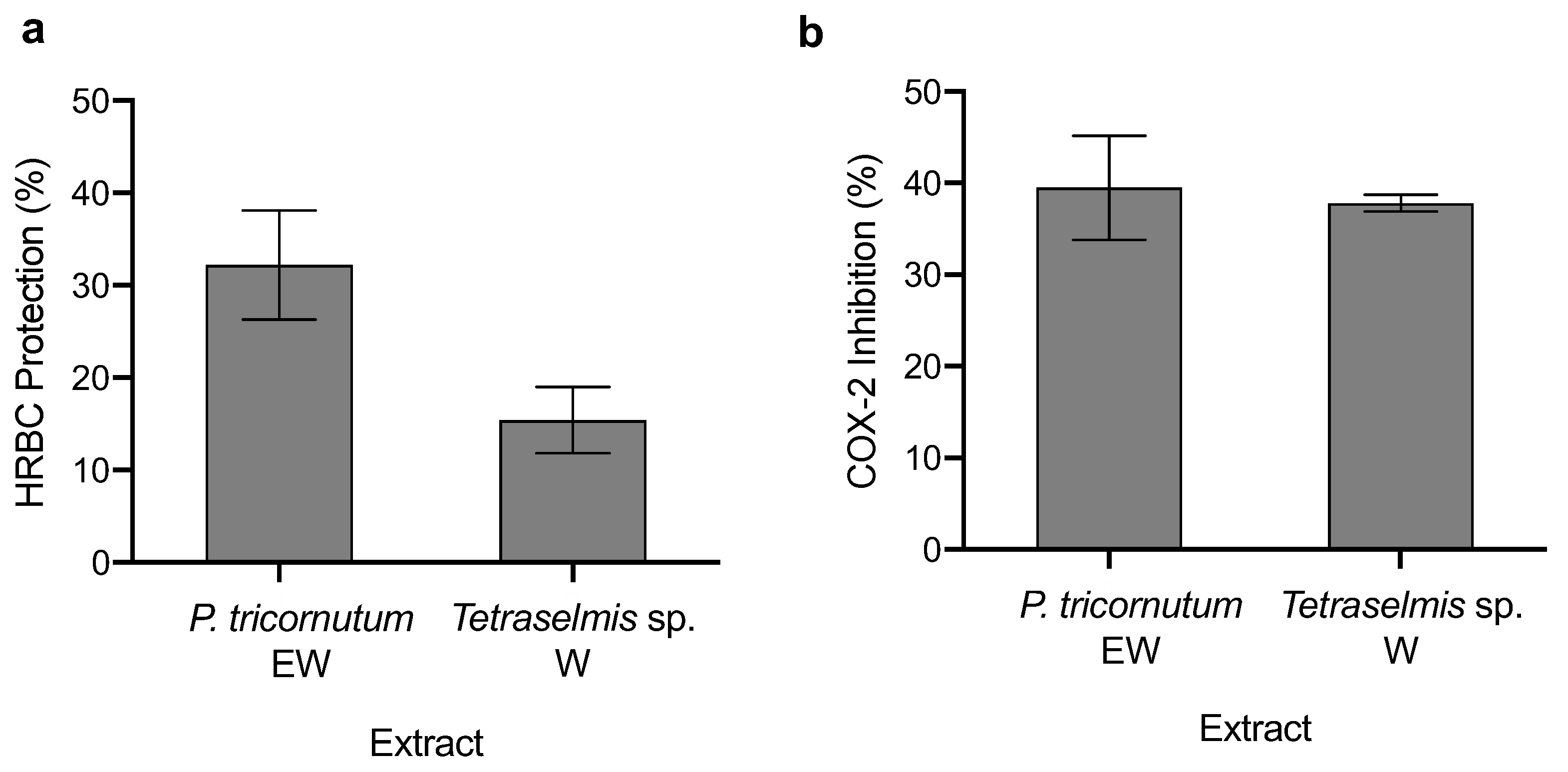
3.4. Anti-Inflammatory Capacity
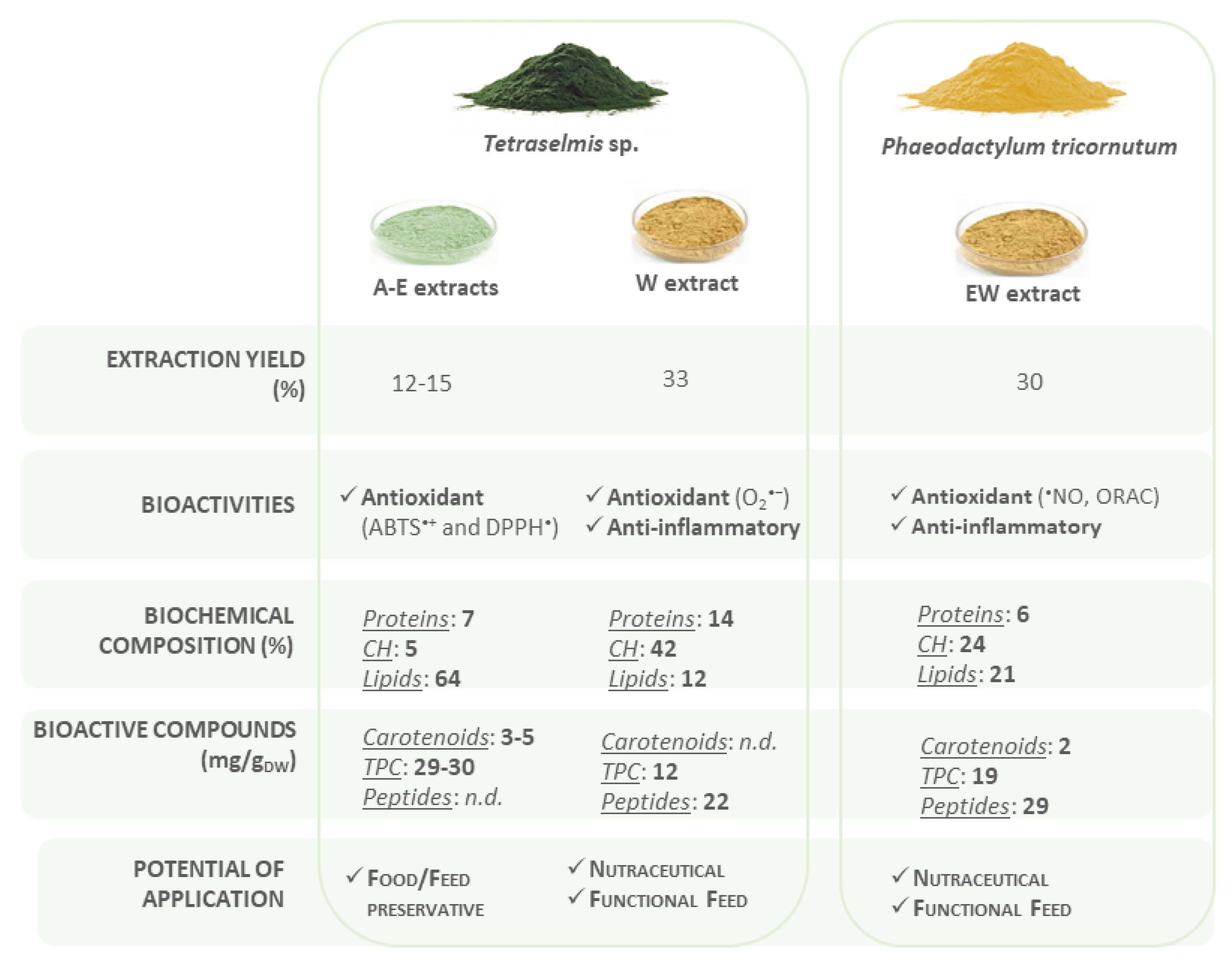
3.5. Bioactive Potential of Microalgae Extracts
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Borowitzka, M. Commercial-Scale Production of Microalgae for Bioproducts. In Blue Biotechnology; Wiley-VCH: Weinheim, Germany, 2018; Volume 1. [Google Scholar] [CrossRef]
- Muller-Feuga, A. Microalgae for Aquaculture: The Current Global Situation and Future Trends. In Handbook of Microalgal Culture: Applied Phycology and Biotechnology, 2nd ed.; Wiley: New York, NY, USA, 2013. [Google Scholar]
- Camacho, F.; Macedo, A.; Malcata, F. Potential Industrial Applications and Commercialization of Microalgae in the Functional Food and Feed Industries: A Short Review. Mar. Drugs 2019, 17, 312. [Google Scholar] [CrossRef] [PubMed]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–Nutritious, Sustainable Aqua- and Animal Feed Source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Ahmad, A.; Hassan, S.W.; Banat, F. An overview of microalgae biomass as a sustainable aquaculture feed ingredient: Food security and circular economy. Bioengineered 2022, 13, 9521–9547. [Google Scholar] [CrossRef]
- García, J.L.; de Vicente, M.; Galán, B. Microalgae, Old Sustainable Food and Fashion Nutraceuticals. Microb. Biotechnol. 2017, 10, 1017–1024. [Google Scholar] [CrossRef]
- Bishop, W.; Zubeck, H. Evaluation of Microalgae for Use as Nutraceuticals and Nutritional Supplements. J. Nutr. Food Sci. 2012, 2, 1–6. [Google Scholar] [CrossRef]
- Dellarosa, N.; Laghi, L.; Martinsdóttir, E.; Jónsdóttir, R.; Sveinsdóttir, K. Enrichment of Convenience Seafood with Omega-3 and Seaweed Extracts: Effect on Lipid Oxidation. LWT Food Sci. Technol. 2015, 62, 746–752. [Google Scholar] [CrossRef]
- Corsetto, P.A.; Montorfano, G.; Zava, S.; Colombo, I.; Ingadottir, B.; Jonsdottir, R.; Sveinsdottir, K.; Rizzo, A.M. Characterization of Antioxidant Potential of Seaweed Extracts for Enrichment of Convenience Food. Antioxidants 2020, 9, 249. [Google Scholar] [CrossRef]
- Deniz, I.; García-Vaquero, M.; Imamoglu, E. Trends in Red Biotechnology: Microalgae for Pharmaceutical Applications. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Elsevier: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Wu, J.; Gu, X.; Yang, D.; Xu, S.; Wang, S.; Chen, X.; Wang, Z. Bioactive Substances and Potentiality of Marine Microalgae. Food Sci. Nutr. 2021, 9, 5279–5292. [Google Scholar] [CrossRef]
- Araújo, R.; Vázquez Calderón, F.; Sánchez López, J.; Azevedo, I.C.; Bruhn, A.; Fluch, S.; Garcia Tasende, M.; Ghaderiardakani, F.; Ilmjärv, T.; Laurans, M.; et al. Current status of the algae production industry in Europe: An emerging sector of the blue bioeconomy. Front. Mar. Sci. 2021, 7, 626389. [Google Scholar] [CrossRef]
- Guedes, A.C.; Amaro, H.M.; Malcata, F.X. Microalgae as Sources of High Added-Value Compounds—A Brief Review of Recent Work. Biotechnol. Prog. 2011, 27, 597–613. [Google Scholar] [CrossRef]
- Pagels, F.; Pereira, R.N.; Vicente, A.A.; Guedes, A.C. Extraction of Pigments from Microalgae and Cyanobacteria—A Review on Current Methodologies. Appl. Sci. 2021, 11, 5187. [Google Scholar] [CrossRef]
- Hentati, F.; Delattre, C.; Ursu, A.V.; Desbrières, J.; Cerf, D.L.; Gardarin, C.; Abdelkafi, S.; Michaud, P.; Pierre, G. Structural Characterization and Antioxidant Activity of Water-Soluble Polysaccharides from the Tunisian Brown Seaweed Cystoseira Compressa. Carbohydr. Polym. 2018, 198, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Granados-Guzman, G.; Salazar-Aranda, R.; Garza-Tapia, M.; Castro-Rios, R.; Waksman de Torres, N. Optimization and Validation of Two High-Throughput Methods Indicating Antiradical Activity. Curr. Anal. Chem. 2017, 13, 499–507. [Google Scholar] [CrossRef]
- Bobo-García, G.; Davidov-Pardo, G.; Arroqui, C.; Vírseda, P.; Marín-Arroyo, M.R.; Navarro, M. Intra-Laboratory Validation of Microplate Methods for Total Phenolic Content and Antioxidant Activity on Polyphenolic Extracts, and Comparison with Conventional Spectrophotometric Methods. J. Sci. Food Agric. 2015, 95, 204–209. [Google Scholar] [CrossRef]
- Pinho, B.R.; Sousa, C.; Valentão, P.; Andrade, P.B. Is Nitric Oxide Decrease Observed with Naphthoquinones in LPS Stimulated RAW 264.7 Macrophages a Beneficial Property? PLoS ONE 2011, 6, e24098. [Google Scholar] [CrossRef]
- Dávalos, A.; Gómez-Cordovés, C.; Bartolomé, B. Extending Applicability of the Oxygen Radical Absorbance Capacity (ORAC-Fluorescein) Assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef]
- Moualek, I.; Iratni Aiche, G.; Mestar Guechaoui, N.; Lahcene, S.; Houali, K. Antioxidant and Anti-Inflammatory Activities of Arbutus Unedo Aqueous Extract. Asian Pac. J. Trop. Biomed. 2016, 6, 937–944. [Google Scholar] [CrossRef]
- Strickland, J.D.H.; Parsons, T.R. A Practical Handbook for Seawater Analysis, 2nd ed.; Fisheries Research Board: Ottawa, ON, Canada, 1972. [Google Scholar]
- Magalhães, L.M.; Santos, F.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Rapid Microplate High-Throughput Methodology for Assessment of Folin-Ciocalteu Reducing Capacity. Talanta 2010, 83, 441–447. [Google Scholar] [CrossRef]
- Sekar, S.; Chandramohan, M. Phycobiliproteins as a Commodity: Trends in Applied Research, Patents and Commercialization. J. Appl. Phycol. 2008, 20, 113–136. [Google Scholar] [CrossRef]
- Coulombier, N.; Jauffrais, T.; Lebouvier, N. Antioxidant Compounds from Microalgae: A Review. Mar. Drugs 2021, 19, 549. [Google Scholar] [CrossRef]
- Bonfanti, C.; Cardoso, C.; Afonso, C.; Matos, J.; Garcia, T.; Tanni, S.; Bandarra, N.M. Potential of Microalga Isochrysis Galbana: Bioactivity and Bioaccessibility. Algal Res. 2018, 29, 242–248. [Google Scholar] [CrossRef]
- Hafsa, M.B.; Ismail, M.B.; Garrab, M.; Aly, R.; Gagnon, J.; Naghmouchi, K. Antimicrobial, Antioxidant, Cytotoxic and Anticholinesterase Activities of Water-Soluble Polysaccharides Extracted from Microalgae Isochrysis Galbana and Nannochloropsis Oculata. J. Serb. Chem. Soc. 2017, 82, 509–522. [Google Scholar]
- Gilbert-López, B.; Mendiola, J.A.; Fontecha, J.; van den Broek, L.A.M.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream Processing of Isochrysis Galbana: A Step towards Microalgal Biorefinery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef]
- Zanella, L.; Vianello, F. Microalgae of the Genus Nannochloropsis: Chemical Composition and Functional Implications for Human Nutrition. J. Funct. Foods 2020, 68, 103919. [Google Scholar] [CrossRef]
- Cui, Y.; Thomas-Hall, S.R.; Schenk, P.M. Phaeodactylum Tricornutum Microalgae as a Rich Source of Omega-3 Oil: Progress in Lipid Induction Techniques towards Industry Adoption. Food Chem. 2019, 297, 124937. [Google Scholar] [CrossRef]
- Neumann, U.; Derwenskus, F.; Flister, V.F.; Schmid-Staiger, U.; Hirth, T.; Bischoff, S.C. Fucoxanthin, a Carotenoid Derived from Phaeodactylum Tricornutum Exerts Antiproliferative and Antioxidant Activities in Vitro. Antioxidants 2019, 8, 183. [Google Scholar] [CrossRef]
- Rico, M.; López, A.; Santana-Casiano, J.M.; González, A.G.; González-Dávila, M. Variability of the Phenolic Profile in the Diatom Phaeodactylum Tricornutum Growing under Copper and Iron Stress. Limnol. Oceanogr. 2013, 58, 144–152. [Google Scholar] [CrossRef]
- Patras, D.; Moraru, C.V.; Socaciu, C. Bioactive Ingredients from Microalgae: Food and Feed Applications. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca. Food Sci. Technol. 2019, 76, 2334. [Google Scholar] [CrossRef]
- Kashif, S.A.; Hwang, Y.J.; Park, J.K. Potent Biomedical Applications of Isolated Polysaccharides from Marine Microalgae Tetraselmis Species. Bioprocess Biosyst. Eng. 2018, 41, 1611–1620. [Google Scholar] [CrossRef] [PubMed]
- Tabarzad, M.; Atabaki, V.; Hosseinabadi, T. Anti-Inflammatory Activity of Bioactive Compounds from Microalgae and Cyanobacteria by Focusing on the Mechanisms of Action. Mol. Biol. Rep. 2020, 47, 6193–6205. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.S.; Choi, Y.J.; Kim, H.J.; Nam, B.H.; Hong, S.H.; Lee, G.A.; Lee, S.W.; Seo, S.Y.; Jeong, M.H. Anti-Inflammatory Effect of Microalgal Extracts from Tetraselmis Suecica. Food Sci. Biotechnol. 2010, 19, 1519–1528. [Google Scholar] [CrossRef]
- Assunção, J.; Amaro, H.M.; Lopes, G.; Tavares, T.; Malcata, F.X.; Guedes, A.C. Synechocystis salina: Potential bioactivity and combined extraction of added-value metabolites. J. Appl. Phycol. 2021, 33, 3731–3746. [Google Scholar] [CrossRef]
- Nunes, M.C.; Fernandes, I.; Vasco, I.; Sousa, I.; Raymundo, A. Tetraselmis chuii as a Sustainable and Healthy Ingredient to Produce Gluten-Free Bread: Impact on Structure, Colour and Bioactivity. Foods 2020, 9, 579. [Google Scholar] [CrossRef]
- Savio, S.; Farrotti, S.; Paris, D.; Arnaìz, E.; Díaz, I.; Bolado, S.; Muñoz, R.; Rodolfo, C.; Congestri, R. Value-added co-products from biomass of the diatoms Staurosirella pinnata and Phaeodactylum tricornutum. Algal Res. 2020, 47, 101830. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]

| Microalgae | Extract | Yield (%DW) |
|---|---|---|
| Isochrysis galbana | A | 19.46 ± 0.62 a |
| E | 25.44 ± 0.76 b | |
| EW | 33.84 ± 0.88 c | |
| W | 67.27 ± 1.24 d | |
| P | 21.47 ± 0.85 e | |
| Nannochloropsis sp. | A | 9.56 ± 1.02 a |
| E | 10.40 ± 0.23 a | |
| EW | 18.57 ± 0.02 b | |
| W | 26.07 ± 1.36 c | |
| P | 6.48 ± 0.44 d | |
| Phaeodactylum tricornutum | A | 12.12 ± 0.67 a |
| E | 15.67 ± 0.97 b | |
| EW | 29.90 ± 1.36 c | |
| W | 32.09 ± 1.03 c | |
| P | 0.40 ± 0.01 d | |
| Tetraselmis sp. | A | 12.03 ± 2.80 a |
| E | 14.94 ± 0.94 a | |
| EW | 21.96 ± 2.07 b | |
| W | 33.21 ± 1.28 c | |
| P | 13.59 ± 0.61 a |
| Microalgae | Extract | Content (%Extract) | ||
|---|---|---|---|---|
| Soluble Proteins | Carbohydrates | Lipids | ||
| Isochrysis galbana | A | 4.47 ± 0.04 a | 0.80 ± 0.05 a | 51.36 ± 1.46 a |
| E | 9.90 ± 0.31 b | 2.39 ± 0.08 b | 63.11 ± 2.28 b | |
| EW | 6.79 ± 0.47 c | 20.97 ± 0.34 c | 25.67 ± 0.60 c | |
| W | 9.65 ± 1.18 b | 53.26 ± 7.78 d | 13.77 ± 0.28 d | |
| P | 9.65 ± 0.17 b | 79.40 ± 0.28 e | 2.65 ± 1.17 e | |
| Nannochloropsis sp. | A | 3.59 ± 0.17 a | 2.41 ± 0.07 a | 71.82 ± 1.36 a |
| E | 6.99 ± 0.27 b | 3.49 ± 0.08 b | 71.12 ± 3.12 a | |
| EW | 6.26 ± 0.80 b | 24.48 ± 0.54 c | 45.66 ± 5.35 b | |
| W | 8.80 ± 0.96 c | 50.80 ± 2.46 d | 36.29 ± 2.57 b | |
| P | 11.10 ± 0.32 d | 77.51 ± 3.12 e | 8.33 ± 3.61 c | |
| Phaeodactylum tricornutum | A | 2.94 ± 0.41 a | 1.75 ± 0.05 a | 66.74 ± 3.79 a |
| E | 5.01 ± 0.61 b | 3.79 ± 0.11 b | 68.11 ± 0.53 a | |
| EW | 6.47 ± 0.44 c | 23.45 ± 0.44 c | 20.61 ± 1.93 b | |
| W | 11.38 ± 1.39 d | 38.95 ± 4.26 d | 26.86 ± 0.55 c | |
| P | 13.60 ± 1.25 d | 73.19 ± 5.56 e | 0.00 ± 0.00 d | |
| Tetraselmis sp. | A | 3.99 ± 0.30 a | 3.16 ± 0.05 a | 69.24 ± 3.09 a |
| E | 6.90 ± 0.38 b | 5.05 ± 0.07 b | 64.11 ± 2.66 a | |
| EW | 6.28 ± 0.68 b | 21.49 ± 0.23 c | 44.65 ± 1.69 b | |
| W | 13.78 ± 0.66 c | 41.82 ± 4.31 d | 11.57 ± 2.27 c | |
| P | 12.00 ± 0.53 d | 74.04 ± 5.51 e | 6.78 ± 1.55 c | |
| Microalgae | Extract | Highlighted Potential (Assay) | Carotenoids | Phenolic Compounds | Peptides |
|---|---|---|---|---|---|
| Isochrysis galbana | EW | ORAC-FL | 2.6 ± 0.2 a | 35.5 ± 1.6 a | 50.4 ± 5.3 a |
| Nannochloropsis sp. | E | ABTS•+ | 14.8 ± 0.3 b | 16.0 ± 0.6 b | - |
| Phaeodactylum tricornutum | E | •NO | 7.9 ± 0.2 c | 22.8 ± 1.2 c | - |
| EW | •NO, ORAC-FL, HBRC, COX | 2.0 ± 0.1 d | 19.0 ± 0.7 d | 29.6 ± 3.2 b | |
| Tetraselmis sp. | A | ABTS•+, DPPH• | 5.4 ± 0.2 e | 28.5 ± 1.6 e | - |
| E | ABTS•+, DPPH• | 3.4 ± 0.2 f | 30.7 ± 1.1 e | - | |
| W | O2•−, HBRC, COX | - | 12.5 ± 0.1 f | 22.4 ± 0.5 c | |
| P | ABTS•+ | - | 8.8 ± 0.2 g | 13.4 ± 1.0 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pagels, F.; Amaro, H.M.; Tavares, T.G.; Amil, B.F.; Guedes, A.C. Potential of Microalgae Extracts for Food and Feed Supplementation—A Promising Source of Antioxidant and Anti-Inflammatory Compounds. Life 2022, 12, 1901. https://doi.org/10.3390/life12111901
Pagels F, Amaro HM, Tavares TG, Amil BF, Guedes AC. Potential of Microalgae Extracts for Food and Feed Supplementation—A Promising Source of Antioxidant and Anti-Inflammatory Compounds. Life. 2022; 12(11):1901. https://doi.org/10.3390/life12111901
Chicago/Turabian StylePagels, Fernando, Helena M. Amaro, Tânia G. Tavares, Berta F. Amil, and A. Catarina Guedes. 2022. "Potential of Microalgae Extracts for Food and Feed Supplementation—A Promising Source of Antioxidant and Anti-Inflammatory Compounds" Life 12, no. 11: 1901. https://doi.org/10.3390/life12111901
APA StylePagels, F., Amaro, H. M., Tavares, T. G., Amil, B. F., & Guedes, A. C. (2022). Potential of Microalgae Extracts for Food and Feed Supplementation—A Promising Source of Antioxidant and Anti-Inflammatory Compounds. Life, 12(11), 1901. https://doi.org/10.3390/life12111901









