Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Placental Biopsy
2.3. Genomic DNA Extraction and Methylation Analysis
2.4. Total RNA Extraction and Gene Expression Analysis
2.5. Statistical Analysis
3. Results
3.1. Study Participants
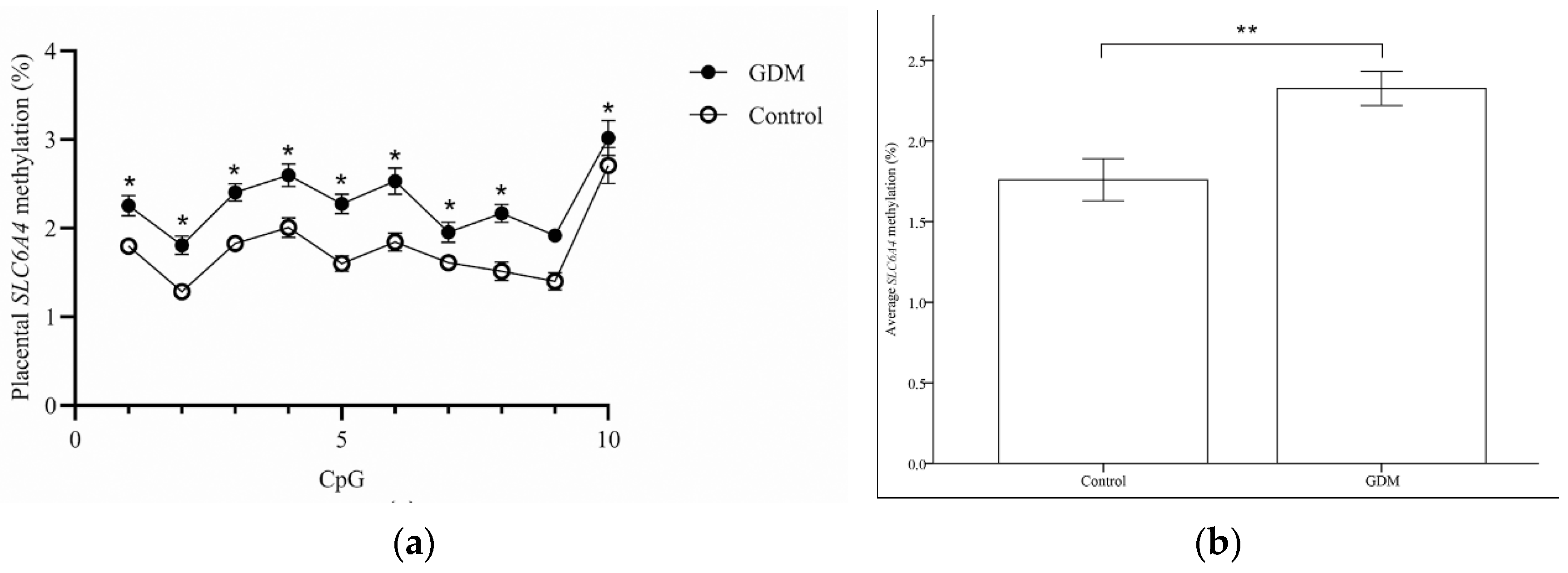
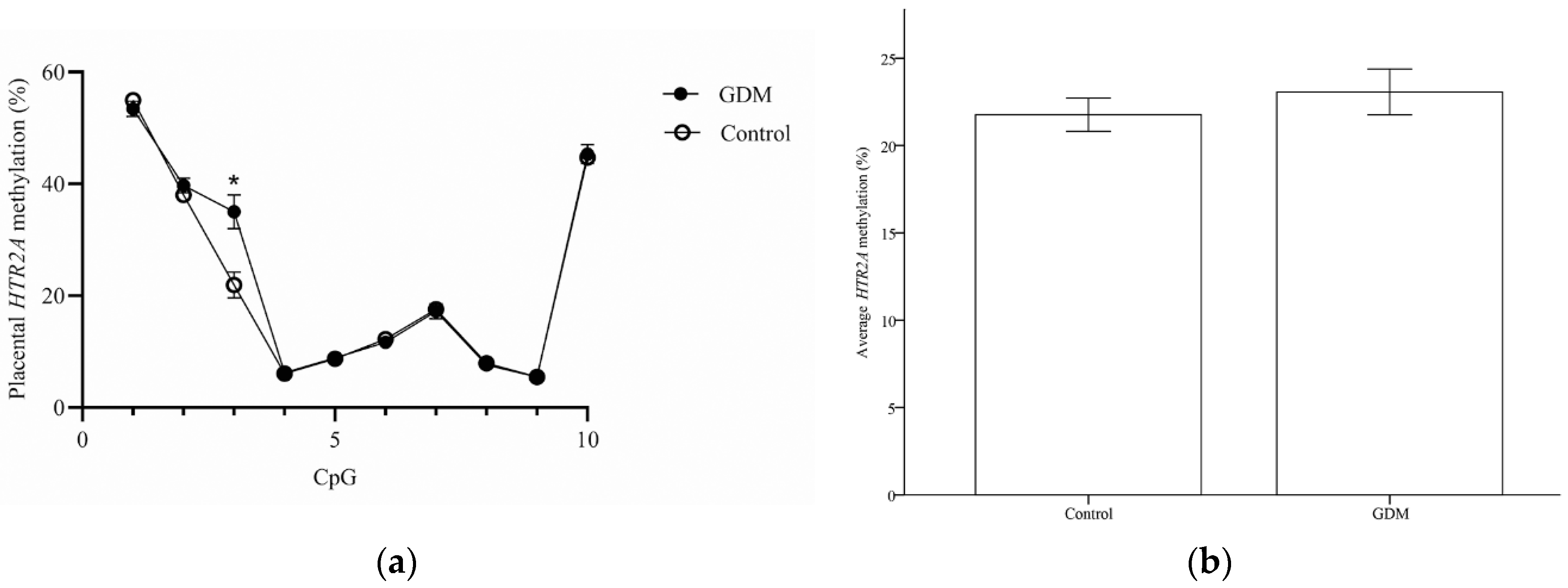
3.2. Methylation Levels of SLC6A4 and HTR2A in Placental Tissues
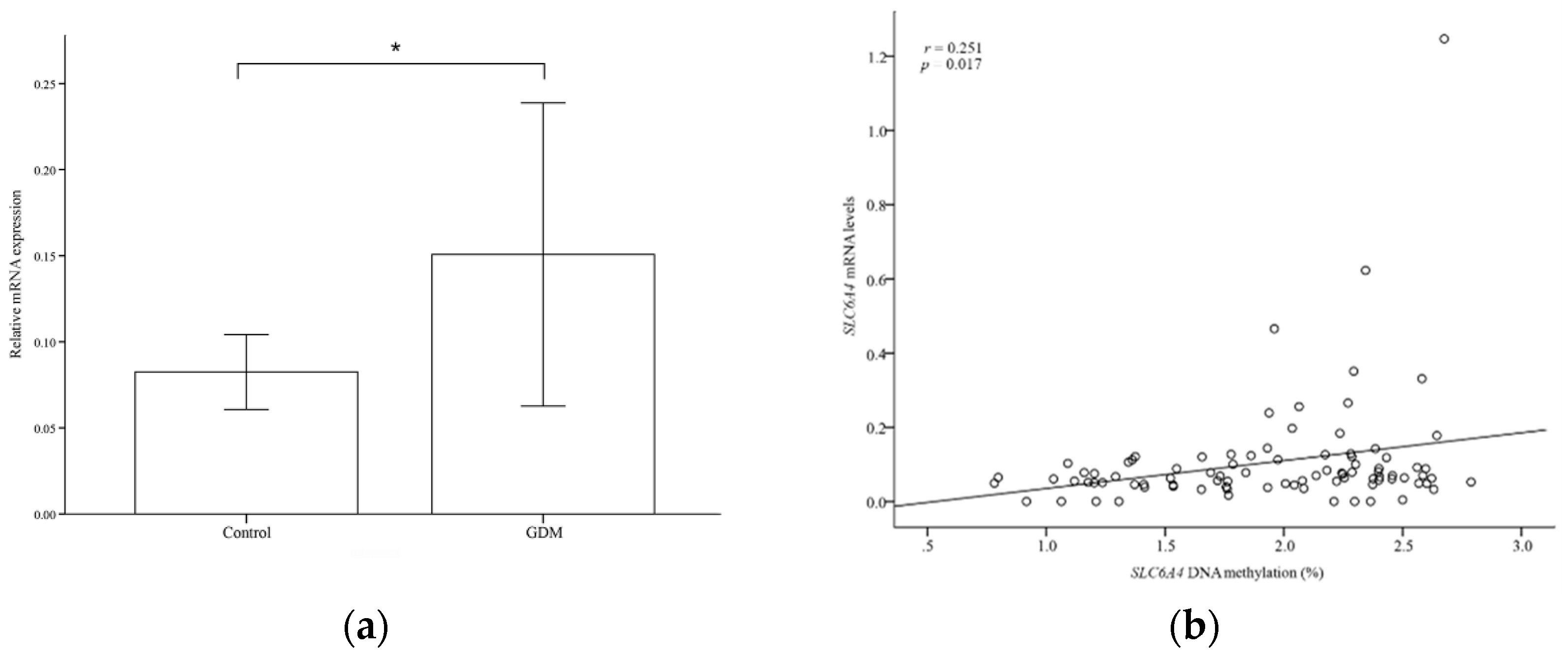
3.3. mRNA Expression of the Serotonin System
3.4. Correlations between Placental HTR2A and SLC6A4 Methylation Levels and Clinical Factors
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.S.; Hong, S.; Han, K.; Park, C.Y. The Clinical characteristics of Gestational Diabetes Mellitus in Korea: A National Health Information Database Study. Endocrinol. Metab. 2021, 36, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during pregnancy: A maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. A clinical review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef] [PubMed]
- Dalrymple, K.V.; El-Heis, S.; Godfrey, K.M. Maternal weight and gestational diabetes impacts on child health. Curr. Opin. Clin. Nutr. Metab. Care 2022, 25, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Banik, A.; Kandilya, D.; Ramya, S.; Stünkel, W.; Chong, Y.S.; Dheen, S.T. Maternal factors that induce epigenetic changes contribute to neurological disorders in offspring. Genes 2017, 8, 150. [Google Scholar] [CrossRef]
- Xiang, A.H.; Wang, X.; Martinez, M.P.; Walthall, J.C.; Curry, E.S.; Page, K.; Buchanan, T.A.; Coleman, K.J.; Getahun, D. Association of maternal diabetes with autism in offspring. JAMA 2015, 313, 1425–1434. [Google Scholar] [CrossRef] [PubMed]
- Miguel, P.M.; Pereira, L.O.; Silveira, P.P.; Meaney, M.J. Early environmental influences on the development of children’s brain structure and function. Dev. Med. Child Neurol. 2019, 61, 1127–1133. [Google Scholar] [CrossRef]
- Franzago, M.; Fraticelli, F.; Marchioni, M.; Di Nicola, M.; Di Sebastiano, F.; Liberati, M.; Stuppia, L.; Vitacolonna, E. Fat mass and obesity-associated (FTO) gene epigenetic modifications in gestational diabetes: New insights and possible pathophysiological connections. Acta Diabetol. 2021, 58, 997–1007. [Google Scholar] [CrossRef]
- Franzago, M.; Fraticelli, F.; Stuppia, L.; Vitacolonna, E. Nutrigenetics, epigenetics and gestational diabetes: Consequences in mother and child. Epigenetics 2019, 14, 215–235. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. The placenta-brain-axis. J. Neurosci. Res. 2021, 99, 271–283. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging roles for serotonin in regulating metabolism: New implications for an ancient molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef]
- Muller, C.L.; Anacker, A.M.J.; Veenstra-VanderWeele, J. The serotonin system in autism spectrum disorder: From biomarker to animal models. Neuroscience 2016, 321, 24–41. [Google Scholar] [CrossRef] [PubMed]
- Hanswijk, S.I.; Spoelder, M.; Shan, L.; Verheij, M.M.M.; Muilwijk, O.G.; Li, W.; Liu, C.; Kolk, S.M.; Homberg, J.R. Gestational factors throughout fetal neurodevelopment: The serotonin link. Int. J. Mol. Sci. 2020, 21, 5850. [Google Scholar] [CrossRef] [PubMed]
- Muller, C.L.; Anacker, A.M.; Rogers, T.D.; Goeden, N.; Keller, E.H.; Forsberg, C.G.; Kerr, T.M.; Wender, C.; Anderson, G.M.; Stanwood, G.D.; et al. Impact of maternal serotonin transporter genotype on placental serotonin, fetal forebrain serotonin, and neurodevelopment. Neuropsychopharmacology 2017, 42, 427–436. [Google Scholar] [CrossRef]
- Crane, J.D.; Palanivel, R.; Mottillo, E.P.; Bujak, A.L.; Wang, H.; Ford, R.J.; Collins, A.; Blümer, R.M.; Fullerton, M.D.; Yabut, J.M.; et al. Inhibiting peripheral serotonin synthesis reduces obesity and metabolic dysfunction by promoting brown adipose tissue thermogenesis. Nat. Med. 2015, 21, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Nakano, T.; Saito, R.; Akasaka, D.; Saito, K.; Ogasawara, H.; Minashima, T.; Miyazawa, K.; Kanaya, T.; Takakura, I.; et al. Serotonin improves high fat diet induced obesity in mice. PLoS ONE 2016, 11, e0147143. [Google Scholar] [CrossRef]
- Binetti, J.; Bertran, L.; Riesco, D.; Aguilar, C.; Martínez, S.; Sabench, F.; Porras, J.A.; Camaron, J.; Castillo, D.D.; Richart, C.; et al. Deregulated serotonin pathway in women with morbid obesity and NAFLD. Life 2020, 10, 245. [Google Scholar] [CrossRef]
- Horvatiček, M.; Perić, M.; Bečeheli, I.; Klasić, M.; Žutić, M.; Kesić, M.; Desoye, G.; Nakić Radoš, S.; Ivanišević, M.; Hranilovic, D.; et al. Maternal metabolic state and fetal sex and genotype modulate methylation of the serotonin receptor type 2A gene (HTR2A) in the human placenta. Biomedicines 2022, 10, 467. [Google Scholar] [CrossRef]
- Viau, M.; Lafond, J.; Vaillancourt, C. Expression of placental serotonin transporter and 5-HT 2A receptor in normal and gestational diabetes mellitus pregnancies. Reprod. Biomed. Online 2009, 19, 207–215. [Google Scholar] [CrossRef]
- Gynecologists, A.C.O.O. Practice Bulletin No. 180: Gestational Diabetes Mellitus. Obstet. Gynecol. 2017, 130, e17–e37. [Google Scholar]
- Institute of Medicine (US) and National Research Council (US) Committee to Reexamine IOM Pregnancy Weight Guidelines. The National Academies Collection: Reports funded by National Institutes of Health. In Weight Gain During Pregnancy: Reexamining the Guidelines; Rasmussen, K.M., Yaktine, A.L., Eds.; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Fenton, T.R.; Kim, J.H. A systematic review and meta-analysis to revise the Fenton growth chart for preterm infants. BMC Pediatr. 2013, 13, 59. [Google Scholar] [CrossRef]
- Blazevic, S.; Horvaticek, M.; Kesic, M.; Zill, P.; Hranilovic, D.; Ivanisevic, M.; Desoye, G.; Stefulj, J. Epigenetic adaptation of the placental serotonin transporter gene (SLC6A4) to gestational diabetes mellitus. PLoS ONE 2017, 12, e0179934. [Google Scholar] [CrossRef]
- Paquette, A.G.; Lesseur, C.; Armstrong, D.A.; Koestler, D.C.; Appleton, A.A.; Lester, B.M.; Marsit, C.J. Placental HTR2A methylation is associated with infant neurobehavioral outcomes. Epigenetics 2013, 8, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Traube, F.R.; Carell, T. The chemistries and consequences of DNA and RNA methylation and demethylation. RNA Biol. 2017, 14, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.H.; Oh, C.M.; Kim, H. Serotonin in the regulation of systemic energy metabolism. J. Diabetes Investig. 2022, 13, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Fumagalli, M.; Provenzi, L.; De Carli, P.; Dessimone, F.; Sirgiovanni, I.; Giorda, R.; Cinnante, C.; Squarcina, L.; Pozzoli, U.; Triulzi, F.; et al. From early stress to 12-month development in very preterm infants: Preliminary findings on epigenetic mechanisms and brain growth. PLoS ONE 2018, 13, e0190602. [Google Scholar] [CrossRef]
- Rosenfeld, C.S. Placental serotonin signaling, pregnancy outcomes, and regulation of fetal brain development†. Biol. Reprod. 2020, 102, 532–538. [Google Scholar] [CrossRef]
- Sato, K. Placenta-derived hypo-serotonin situations in the developing forebrain cause autism. Med. Hypotheses 2013, 80, 368–372. [Google Scholar] [CrossRef]
- Yang, C.J.; Tan, H.P.; Du, Y.J. The developmental disruptions of serotonin signaling may involved in autism during early brain development. Neuroscience 2014, 267, 1–10. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, J.H.; Noh, S.; Hur, H.J.; Sung, M.J.; Hwang, J.T.; Park, J.H.; Yang, H.J.; Kim, M.S.; Kwon, D.Y.; et al. Metabolomic analysis of livers and serum from high-fat diet induced obese mice. J. Proteome Res. 2011, 10, 722–731. [Google Scholar] [CrossRef]
- Lillycrop, K.A.; Garratt, E.S.; Titcombe, P.; Melton, P.E.; Murray, R.J.S.; Barton, S.J.; Clarke-Harris, R.; Costello, P.M.; Holbrook, J.D.; Hopkins, J.C.; et al. Differential SLC6A4 methylation: A predictive epigenetic marker of adiposity from birth to adulthood. Int. J. Obes. 2019, 43, 974–988. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Goldberg, J.; Vaccarino, V. Promoter methylation of serotonin transporter gene is associated with obesity measures: A monozygotic twin study. Int. J. Obes. 2013, 37, 140–145. [Google Scholar] [CrossRef] [PubMed]
| GDM (N = 30) | Control (N = 60) | p-Value | |
|---|---|---|---|
| Maternal characteristics | |||
| Maternal age, years | 35.77 ± 2.89 | 33.68 ± 4.06 | 0.014 * |
| Nulliparous, % | 15 (50) | 40 (66) | 0.262 |
| Prepregnancy BMI, kg/m2 | 26.84 ± 4.52 | 22.07 ± 4.04 | <0.001 * |
| Gestation at birth, weeks | 36.91 ± 2.46 | 37.13 ± 2.31 | 0.683 |
| Delivery < 37 weeks, % | 10 (33.3) | 18 (30) | 0.747 |
| Gestational weight gain | 10.97 ± 7.58 | 13.75 ± 5.65 | 0.054 |
| % low category | 13 (43.3) | 19 (31.7) | 0.548 |
| % normal category | 9 (30.0) | 21 (35.0) | |
| % high category | 8 (28.6) | 20 (33.3) | |
| Fasting glucose, mg/dL | 96.47 ± 18.59 | 86.62 ± 15.62 | 0.01 * |
| 50 g OGTT | 186.06 ± 30.03 | 122.70 ± 21.61 | < 0.001 * |
| Total cholesterol | 256.07 ± 41.85 | 267.26 ± 50.75 | 0.431 |
| Neonate and placenta characteristics | |||
| Birth weight, kg | 3.03 ± 0.76 | 2.86 ± 0.72 | 0.3 |
| Birthweight percentile | 54.63 ± 28.53 | 40.18 ± 26.28 | 0.019 * |
| SGA, % | 1 (3.3) | 6 (10.0) | 0.205 |
| LGA, % | 5 (16.7) | 4 (6.7) | |
| Apgar < 7 at 5 min, % | 1 (3.3) | 2 (3.3) | 1 |
| Infant gender (% male) | 13 (43.3) | 33 (55.0) | 0.297 |
| Fasting glucose | 79.14 ± 55.11 | 64.25 ± 28.51 | 0.21 |
| TSH | 3.63 ± 2.17 | 3.77 ± 2.14 | 0.769 |
| 17alpha-OHP | 1.83 ± 1.15 | 2.04 ± 1.20 | 0.433 |
| Placenta weight, kg | 0.70 ± 0.21 | 0.64 ± 0.20 | 0.192 |
| Placental weight < 10th percentile | 2 (6.7) | 9 (15.0) | 0.255 |
| β | p | R2 | |
|---|---|---|---|
| Mean methylation levels of SLC6A4 (%) | |||
| Fasting plasma glucose | 0.007 | 0.036 * | 0.196 |
| Birthweight percentile | 0.008 | 0.002 * | 0.197 |
| HC percentile | −0.006 | 0.017 * | 0.226 |
| Mean methylation levels of HTR2A (%) | |||
| Fasting plasma glucose | 0.037 | 0.132 | 0.061 |
| Birthweight percentile | 0.015 | 0.441 | 0.006 |
| HC percentile | −0.018 | 0.350 | 0.072 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, J.Y.; Lee, K.E.; Byeon, E.J.; Choi, J.; Kim, S.J.; Shin, J.E. Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta. Life 2022, 12, 1869. https://doi.org/10.3390/life12111869
Song JY, Lee KE, Byeon EJ, Choi J, Kim SJ, Shin JE. Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta. Life. 2022; 12(11):1869. https://doi.org/10.3390/life12111869
Chicago/Turabian StyleSong, Jae Yen, Kyung Eun Lee, Eun Jeong Byeon, Jieun Choi, Sa Jin Kim, and Jae Eun Shin. 2022. "Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta" Life 12, no. 11: 1869. https://doi.org/10.3390/life12111869
APA StyleSong, J. Y., Lee, K. E., Byeon, E. J., Choi, J., Kim, S. J., & Shin, J. E. (2022). Maternal Gestational Diabetes Influences DNA Methylation in the Serotonin System in the Human Placenta. Life, 12(11), 1869. https://doi.org/10.3390/life12111869






