Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Cell Culture
2.3. Cellular Viability Assessment
2.4. Cellular Morphology
2.5. Immunofluorescence
2.6. Chorioallantoic Membrane (CAM) Assay
2.7. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Assay
2.8. Combination Index Calculation
2.9. Statistical Analysis
3. Results
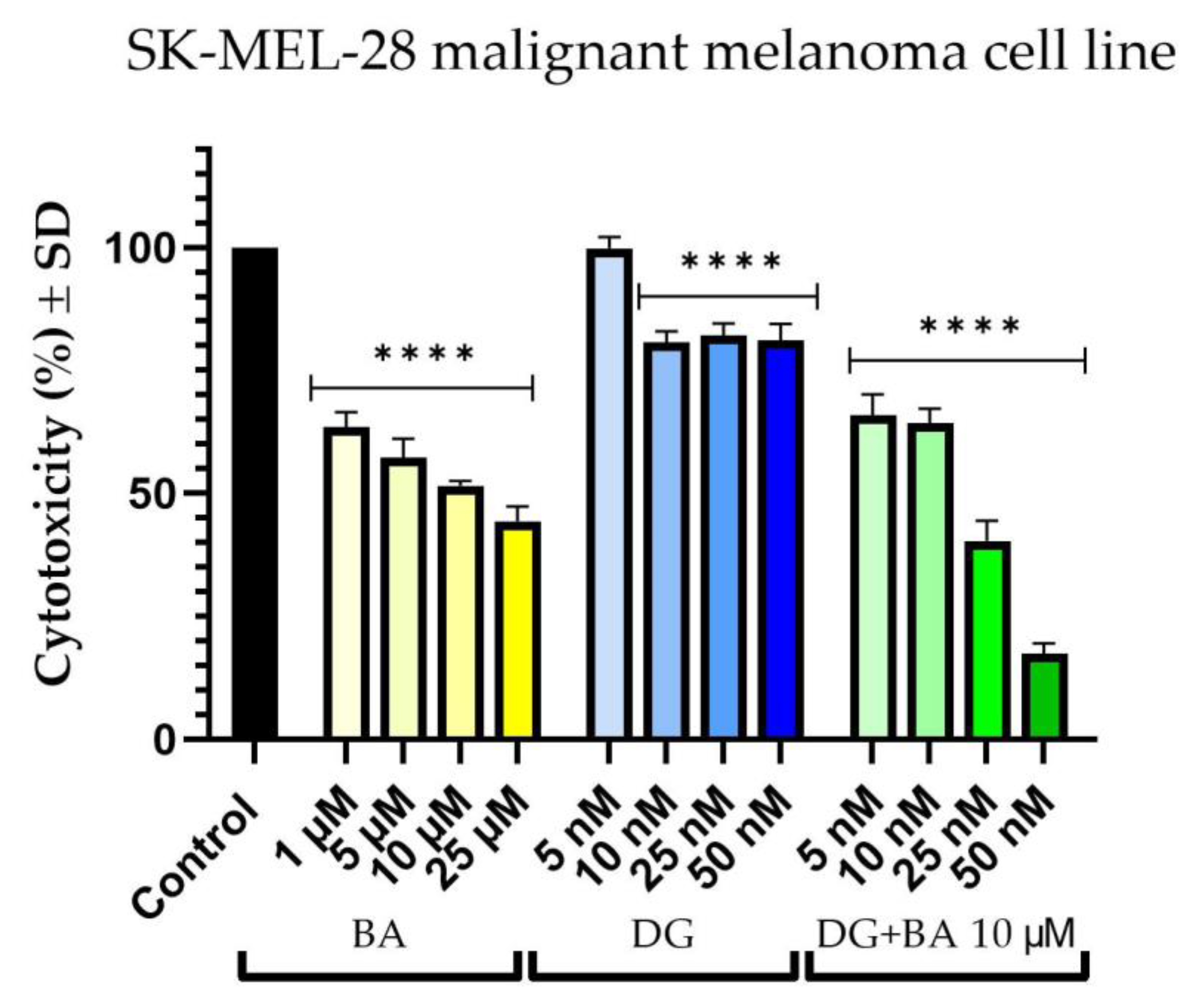
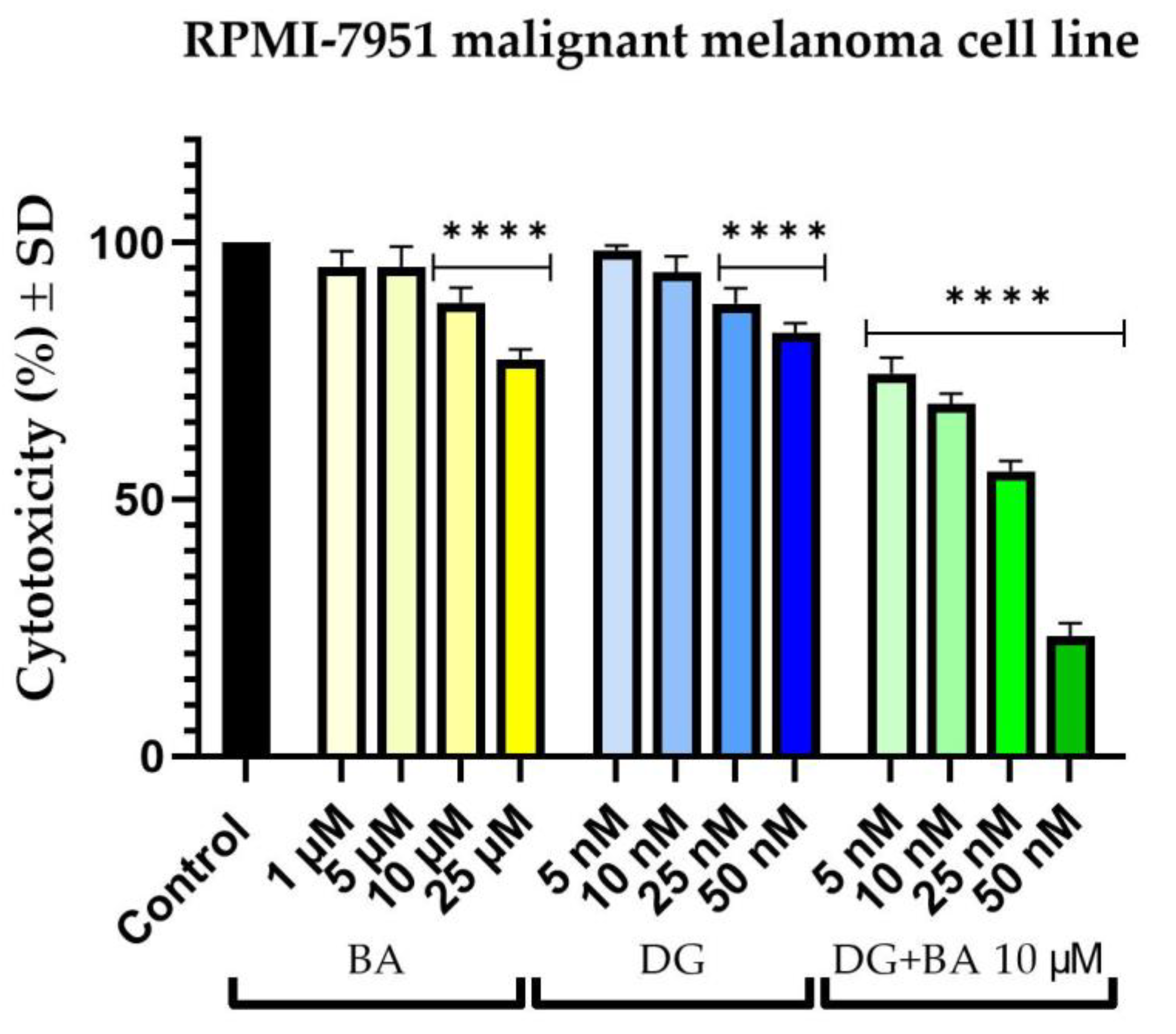
3.1. Cellular Viability Assessment
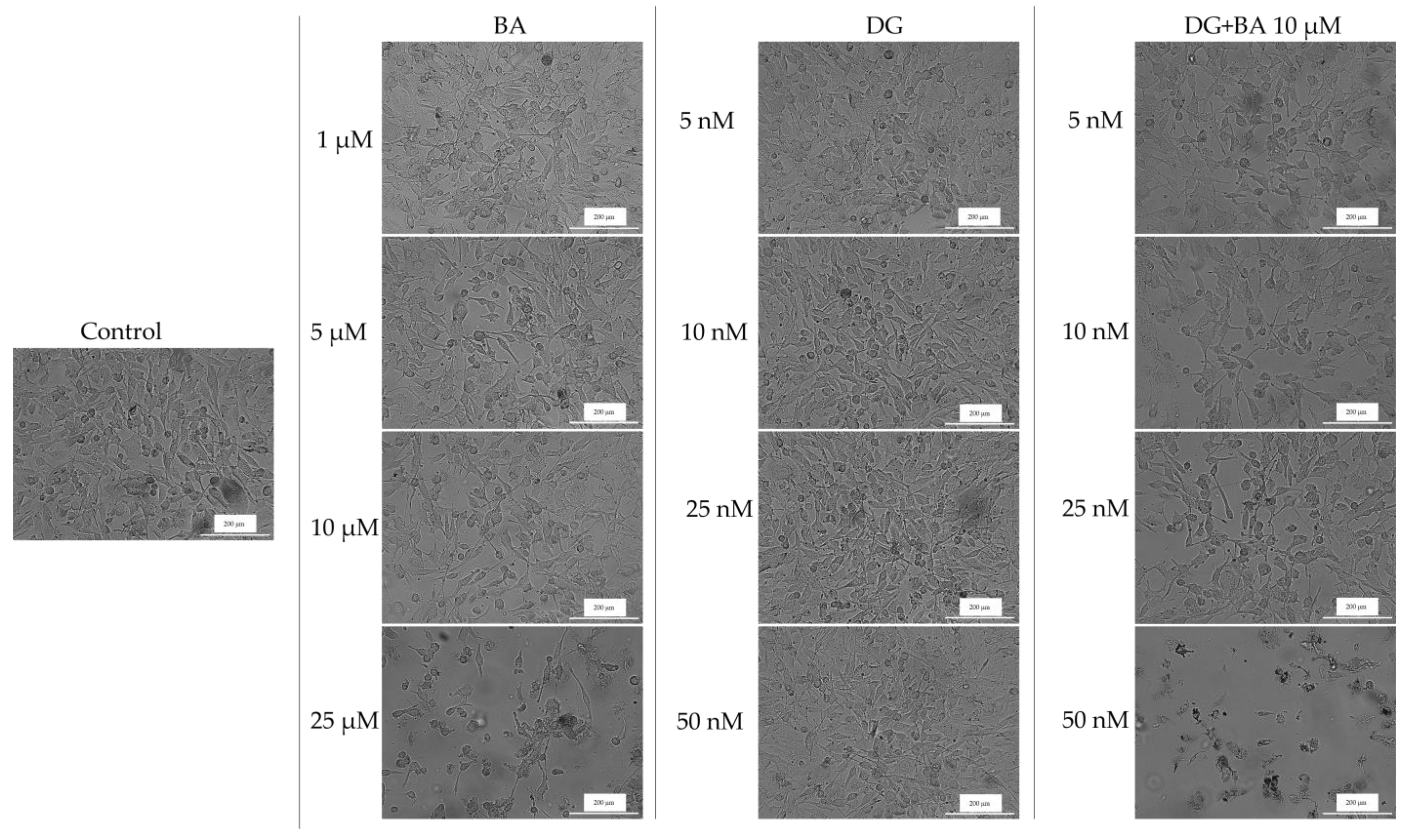
3.2. Cellular Morphology
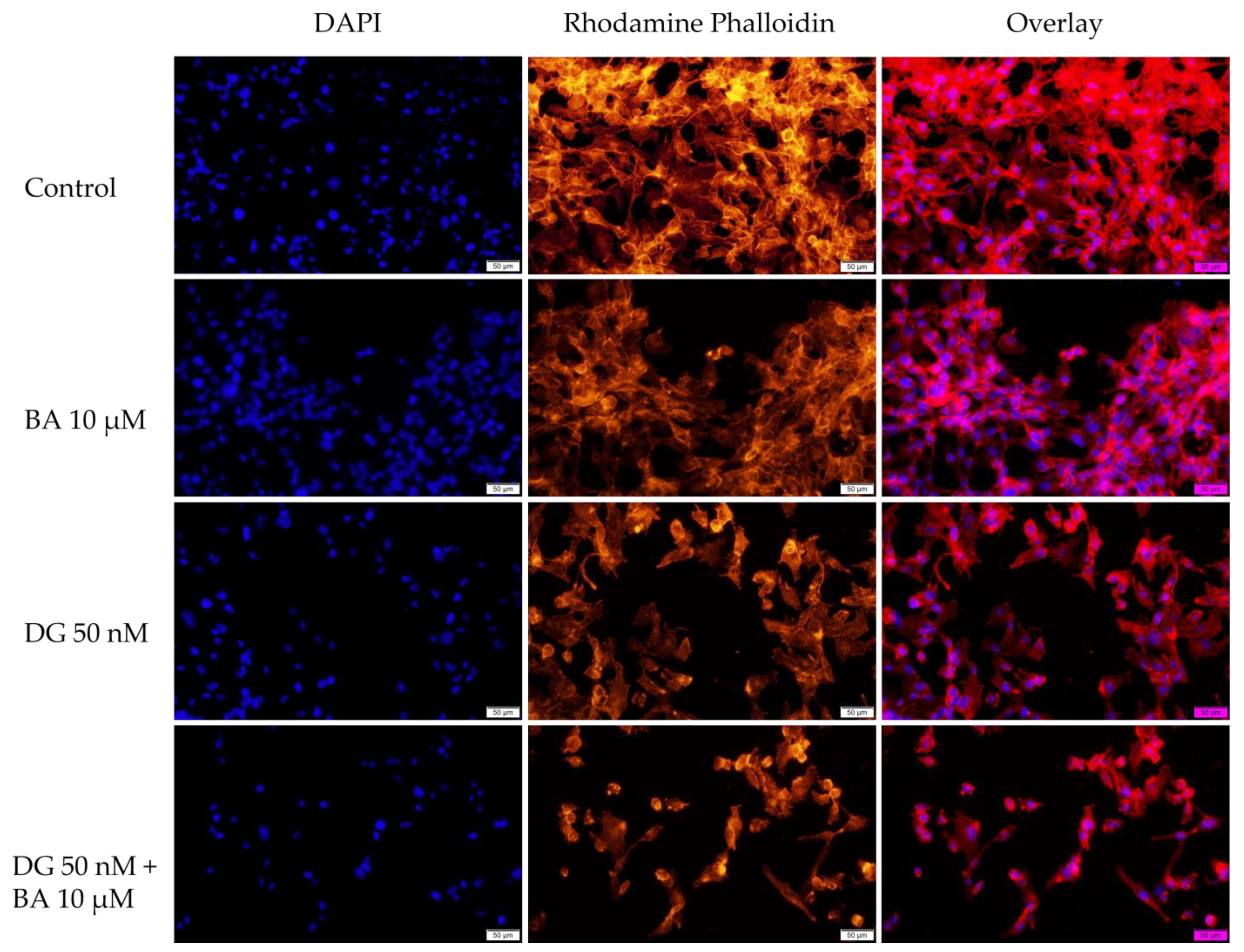
3.3. Immunofluorescence
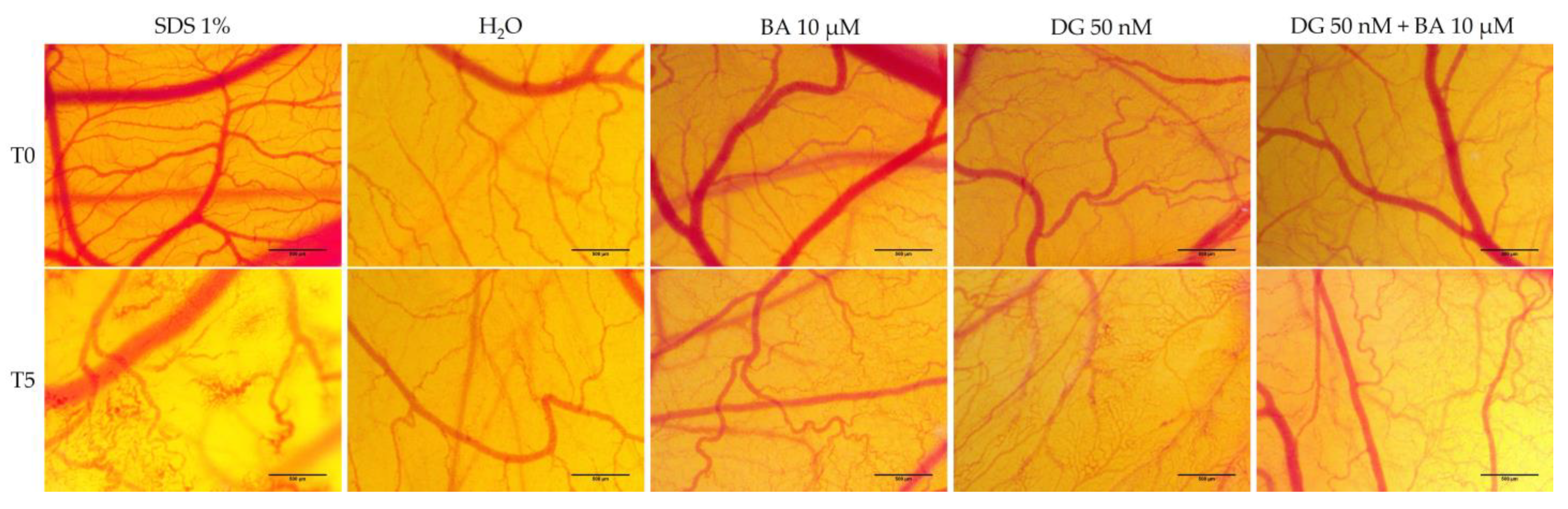
3.4. Hen’s Egg Test—Chorioallantoic Membrane (HET-CAM) Assay
3.5. Combination Index Calculation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deo, S.V.S.; Sharma, J.; Kumar, S. GLOBOCAN 2020 Report on Global Cancer Burden: Challenges and Opportunities for Surgical Oncologists. Ann. Surg. Oncol. 2022, 29, 6497–6500. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.H.; Mir, M.; Qian, L.; Baloch, M.; Ali Khan, M.F.; Rehman, A.; Ngowi, E.E.; Wu, D.-D.; Ji, X.-Y. Skin Cancer Biology and Barriers to Treatment: Recent Applications of Polymeric Micro/Nanostructures. J. Adv. Res. 2022, 36, 223–247. [Google Scholar] [CrossRef]
- Saginala, K.; Barsouk, A.; Aluru, J.S.; Rawla, P.; Barsouk, A. Epidemiology of Melanoma. Med. Sci. 2021, 9, 63. [Google Scholar] [CrossRef]
- Fijałkowska, M.; Koziej, M.; Antoszewski, B. Detailed Head Localization and Incidence of Skin Cancers. Sci. Rep. 2021, 11, 12391. [Google Scholar] [CrossRef] [PubMed]
- Domingues, B.; Lopes, J.M.; Soares, P.; Pópulo, H. Melanoma Treatment in Review. ImmunoTargets Ther. 2018, 7, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, M.; Heinrich, M.; Booker, A. Medicinal Plant Analysis: A Historical and Regional Discussion of Emergent Complex Techniques. Front. Pharmacol. 2020, 10, 1480. [Google Scholar] [CrossRef]
- Ullah, A.; Ullah, N.; Nawaz, T.; Aziz, T. Molecular Mechanisms of Sanguinarine in Cancer Prevention and Treatment. Anticancer. Agents Med. Chem. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Isaković-Vidović, S.; Dariš, B.; Knez, Ž.; Vidović, K.; Oprić, D.; Ferk, P. Antiproliferative Activity of Selected Triterpene Acids from Rosemary on Metastatic Melanoma Cell Line WM-266-4. Open Access Maced. J. Med. Sci. 2021, 9, 515–521. [Google Scholar] [CrossRef]
- Weber, L.A.; Meißner, J.; Delarocque, J.; Kalbitz, J.; Feige, K.; Kietzmann, M.; Michaelis, A.; Paschke, R.; Michael, J.; Pratscher, B.; et al. Betulinic Acid Shows Anticancer Activity against Equine Melanoma Cells and Permeates Isolated Equine Skin in Vitro. BMC Vet. Res. 2020, 16, 44. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.; Fong, H.H.; Kinghorn, A.D.; Brown, D.M. Discovery of Betulinic Acid as a Selective Inhibitor of Human Melanoma That Functions by Induction of Apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Lou, H.; Li, H.; Zhang, S.; Lu, H.; Chen, Q. A Review on Preparation of Betulinic Acid and Its Biological Activities. Molecules 2021, 26, 5583. [Google Scholar] [CrossRef] [PubMed]
- Patocka, J.; Nepovimova, E.; Wu, W.; Kuca, K. Digoxin: Pharmacology and Toxicology—A Review. Environ. Toxicol. Pharmacol. 2020, 79, 103400. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.-H.; Tsai, M.-F.; Su, K.-Y.; Wu, S.-G.; Huang, C.-P.; Yu, S.-L.; Yu, Y.-L.; Lan, C.-C.; Yang, C.-H.; Lin, S.-B.; et al. Slug Confers Resistance to the Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor. Am. J. Respir. Crit. Care Med. 2011, 183, 1071–1079. [Google Scholar] [CrossRef]
- Tahervand, A.; Parnian, B.; Habibi Roudkenar, M.; Mohammadi Roushandeh, A. Digoxin Effectively Inhibited Cell Growth and Induced Senescence in Cervical Cancer Cell Line. Gene Cell Tissue 2017, 4, e14216. [Google Scholar] [CrossRef]
- Eskiocak, U.; Ramesh, V.; Gill, J.G.; Zhao, Z.; Yuan, S.W.; Wang, M.; Vandergriff, T.; Shackleton, M.; Quintana, E.; Frankel, A.E.; et al. Erratum: Synergistic Effects of Ion Transporter and MAP Kinase Pathway Inhibitors in Melanoma. Nat. Commun. 2016, 7, 13080. [Google Scholar] [CrossRef]
- Frankel, A.E.; Eskiocak, U.; Gill, J.G.; Yuan, S.; Ramesh, V.; Froehlich, T.W.; Ahn, C.; Morrison, S.J. Digoxin Plus Trametinib Therapy Achieves Disease Control in BRAF Wild-Type Metastatic Melanoma Patients. Neoplasia 2017, 19, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Narayan, R.S.; Molenaar, P.; Teng, J.; Cornelissen, F.M.G.; Roelofs, I.; Menezes, R.; Dik, R.; Lagerweij, T.; Broersma, Y.; Petersen, N.; et al. A Cancer Drug Atlas Enables Synergistic Targeting of Independent Drug Vulnerabilities. Nat. Commun. 2020, 11, 2935. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.C.; Sorger, P.K. Combination Cancer Therapy Can Confer Benefit via Patient-to-Patient Variability without Drug Additivity or Synergy. Cell 2017, 171, 1678–1691.e13. [Google Scholar] [CrossRef]
- Liang, Z.; Xie, H.; Shen, W.; Shao, L.; Zeng, L.; Huang, X.; Zhu, Q.; Zhai, X.; Li, K.; Qiu, Z.; et al. The Synergism of Natural Compounds and Conventional Therapeutics against Colorectal Cancer Progression and Metastasis. FBL 2022, 27, 263. [Google Scholar] [CrossRef]
- Lin, S.-R.; Fu, Y.-S.; Tsai, M.-J.; Cheng, H.; Weng, C.-F. Natural Compounds from Herbs That Can Potentially Execute as Autophagy Inducers for Cancer Therapy. Int. J. Mol. Sci. 2017, 18, 1412. [Google Scholar] [CrossRef]
- Iqbal, H.; Menaa, F.; Khan, N.U.; Razzaq, A.; Khan, Z.U.; Ullah, K.; Kamal, R.; Sohail, M.; Thiripuranathar, G.; Uzair, B.; et al. Two Promising Anti-Cancer Compounds, 2-Hydroxycinnaldehyde and 2-Benzoyloxycinnamaldehyde: Where Do We Stand? Comb. Chem. High Throughput Screen. 2022, 25, 808–818. [Google Scholar] [CrossRef] [PubMed]
- Batista-Duharte, A.; Jorge Murillo, G.; Pérez, U.M.; Tur, E.N.; Portuondo, D.F.; Martínez, B.T.; Téllez-Martínez, D.; Betancourt, J.E.; Pérez, O. The Hen’s Egg Test on Chorioallantoic Membrane: An Alternative Assay for the Assessment of the Irritating Effect of Vaccine Adjuvants. Int. J. Toxicol. 2016, 35, 627–633. [Google Scholar] [CrossRef] [PubMed]
- Kis, A.M.; Macasoi, I.; Paul, C.; Radulescu, M.; Buzatu, R.; Watz, C.G.; Cheveresan, A.; Berceanu, D.; Pinzaru, I.; Dinu, S.; et al. Methotrexate and Cetuximab-Biological Impact on Non-Tumorigenic Models: In Vitro and In Ovo Assessments. Medicina 2022, 58, 167. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Salzillo, A.; Ragone, A.; Spina, A.; Naviglio, S.; Sapio, L. Chlorogenic Acid Enhances Doxorubicin-Mediated Cytotoxic Effect in Osteosarcoma Cells. Int. J. Mol. Sci. 2021, 22, 8586. [Google Scholar] [CrossRef]
- Stenkvist, B.; Bengtsson, E.; Eriksson, O.; Holmquist, J.; Nordin, B.; Westman-Naeser, S.; Eklund, G. Cardiac Glycosides and Breast Cancer. Lancet 1979, 313, 563. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Q.; Zhang, S.; Liu, H.; Zhao, B.; Du, B.; Wang, W.; Lin, P.; Zhang, Z.; Zhong, Y.; et al. Digoxin Enhances the Anticancer Effect on Non-Small Cell Lung Cancer While Reducing the Cardiotoxicity of Adriamycin. Front. Pharmacol. 2020, 11, 186. [Google Scholar] [CrossRef]
- Zhao, S.; Li, X.; Wu, W.; Liu, S.; Shen, M.; Zhang, Z.; He, J. Digoxin Reduces the Incidence of Prostate Cancer but Increases the Cancer-Specific Mortality: A Systematic Review and Pooled Analysis. Andrologia 2021, 53, e14217. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Yan, J.Y.; Han, X.C.; Niu, F.L.; Zhang, J.H.; Hu, W.N. Anti-Proliferative Effect of Digoxin on Breast Cancer Cells via Inducing Apoptosis. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 5837–5842. [Google Scholar] [CrossRef]
- Tan, Y.; Yu, R.; Pezzuto, J.M. Betulinic Acid-Induced Programmed Cell Death in Human Melanoma Cells Involves Mitogen-Activated Protein Kinase Activation. Clin. Cancer Res. 2003, 9, 2866–2875. [Google Scholar]
- Potze, L.; Mullauer, F.B.; Colak, S.; Kessler, J.H.; Medema, J.P. Betulinic Acid-Induced Mitochondria-Dependent Cell Death Is Counterbalanced by an Autophagic Salvage Response. Cell Death Dis. 2014, 5, e1169. [Google Scholar] [CrossRef] [PubMed]
- Castañeda, A.M.; Meléndez, C.M.; Uribe, D.; Pedroza-Díaz, J. Synergistic Effects of Natural Compounds and Conventional Chemotherapeutic Agents: Recent Insights for the Development of Cancer Treatment Strategies. Heliyon 2022, 8, e09519. [Google Scholar] [CrossRef] [PubMed]
- Rampersad, S.N. Multiple Applications of Alamar Blue as an Indicator of Metabolic Function and Cellular Health in Cell Viability Bioassays. Sensors 2012, 12, 12347–12360. [Google Scholar] [CrossRef] [PubMed]
- Hamid, R.; Rotshteyn, Y.; Rabadi, L.; Parikh, R.; Bullock, P. Comparison of Alamar Blue and MTT Assays for High Through-Put Screening. Toxicol. Vitr. 2004, 18, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Shankar, E.; Zhang, A.; Franco, D.; Gupta, S. Betulinic Acid-Mediated Apoptosis in Human Prostate Cancer Cells Involves P53 and Nuclear Factor-Kappa B (NF-ΚB) Pathways. Molecules 2017, 22, 264. [Google Scholar] [CrossRef]
- Zeng, A.-Q.; Yu, Y.; Yao, Y.-Q.; Yang, F.-F.; Liao, M.; Song, L.-J.; Li, Y.-L.; Yu, Y.; Li, Y.-J.; Deng, Y.-L.; et al. Betulinic Acid Impairs Metastasis and Reduces Immunosuppressive Cells in Breast Cancer Models. Oncotarget 2018, 9, 3794–3804. [Google Scholar] [CrossRef]
- Zeng, A.; Hua, H.; Liu, L.; Zhao, J. Betulinic Acid Induces Apoptosis and Inhibits Metastasis of Human Colorectal Cancer Cells in Vitro and in Vivo. Bioorg. Med. Chem. 2019, 27, 2546–2552. [Google Scholar] [CrossRef]
- Pfarr, K.; Danciu, C.; Arlt, O.; Neske, C.; Dehelean, C.; Pfeilschifter, J.M.; Radeke, H.H. Simultaneous and Dose Dependent Melanoma Cytotoxic and Immune Stimulatory Activity of Betulin. PLoS ONE 2015, 10, e0118802. [Google Scholar] [CrossRef]
- Gheorgheosu, D.; Jung, M.; Bilge, Ö.; Schmid, T.; Dehelean, C. Betulinic Acid Suppresses NGAL-Induced Epithelial-to-Mesenchymal Transition in Melanoma. Biol. Chem. 2013, 394, 773–781. [Google Scholar] [CrossRef]
- Coricovac, D.; Dehelean, C.A.; Pinzaru, I.; Mioc, A.; Aburel, O.-M.; Macasoi, I.; Draghici, G.A.; Petean, C.; Soica, C.; Boruga, M.; et al. Assessment of Betulinic Acid Cytotoxicity and Mitochondrial Metabolism Impairment in a Human Melanoma Cell Line. Int. J. Mol. Sci. 2021, 22, 4870. [Google Scholar] [CrossRef]
- Selzer, E.; Pimentel, E.; Wacheck, V.; Schlegel, W.; Pehamberger, H.; Jansen, B.; Kodym, R. Effects of Betulinic Acid Alone and in Combination with Irradiation in Human Melanoma Cells. J. Investig. Dermatol. 2000, 114, 935–940. [Google Scholar] [CrossRef] [PubMed]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective Cytotoxicity of Betulinic Acid on Tumor Cell Lines, but Not on Normal Cells. Cancer Lett. 2002, 175, 17–25. [Google Scholar] [CrossRef]
- Fulda, S.; Jeremias, I.; Pietsch, T.; Debatin, K.M. Betulinic Acid: A New Chemotherapeutic Agent in the Treatment of Neuroectodermal Tumors. Klin. Padiatr. 1999, 211, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Fulda, S.; Jeremias, I.; Steiner, H.H.; Pietsch, T.; Debatin, K.M. Betulinic Acid: A New Cytotoxic Agent against Malignant Brain-Tumor Cells. Int. J. Cancer 1999, 82, 435–441. [Google Scholar] [CrossRef]
- Lines, C.; Wr, P.; Cabaj, J.; Weronika, B. Additive Interactions between Betulinic Acid and Two Taxanes in In Vitro Tests against Four Human Malignant Melanoma. Int. J. Mol. Sci. 2022, 23, 9641. [Google Scholar]
- Adams, K.F.J.; Patterson, J.H.; Gattis, W.A.; O’Connor, C.M.; Lee, C.R.; Schwartz, T.A.; Gheorghiade, M. Relationship of Serum Digoxin Concentration to Mortality and Morbidity in Women in the Digitalis Investigation Group Trial: A Retrospective Analysis. J. Am. Coll. Cardiol. 2005, 46, 497–504. [Google Scholar] [CrossRef]
- Chou, J.-C.; Li, J.-H.; Chen, C.-C.; Chen, C.-W.; Lin, H.; Wang, P.S. Inhibitory Effects of Digoxin and Digitoxin on Cell Growth in Human Ovarian Cancer Cell Line SKOV-3. Integr. Cancer Ther. 2021, 20, 15347354211002662. [Google Scholar] [CrossRef]
- Syahputra, R.A.; Harahap, U.; Dalimunthe, A.; Nasution, M.P.; Satria, D. Drug Therapy Monitoring (TDM) of Digoxin: Safety and Efficacy Review. Pharmacia 2022, 69, 261–264. [Google Scholar] [CrossRef]
- Levy, A. Correlation between In-Vitro and In-Vivo Studies Based on Pharmacokinetic Considerations. Am. J. Biomed. Sci. Res. 2020, 8, 48–50. [Google Scholar] [CrossRef]
- Clarke, P.G.; Clarke, S. Nineteenth Century Research on Naturally Occurring Cell Death and Related Phenomena. Anat. Embryol. 1996, 193, 81–99. [Google Scholar] [CrossRef]
- Thuret, G.; Chiquet, C.; Herrag, S.; Dumollard, J.M.; Boudard, D.; Bednarz, J.; Campos, L.; Gain, P. Mechanisms of Staurosporine Induced Apoptosis in a Human Corneal Endothelial Cell Line. Br. J. Ophthalmol. 2003, 87, 346–352. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. News Physiol. Sci. 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Eidet, J.R.; Pasovic, L.; Maria, R.; Jackson, C.J.; Utheim, T.P. Objective Assessment of Changes in Nuclear Morphology and Cell Distribution Following Induction of Apoptosis. Diagn. Pathol. 2014, 9, 92. [Google Scholar] [CrossRef] [PubMed]
- Toné, S.; Sugimoto, K.; Tanda, K.; Suda, T.; Uehira, K.; Kanouchi, H.; Samejima, K.; Minatogawa, Y.; Earnshaw, W.C. Three Distinct Stages of Apoptotic Nuclear Condensation Revealed by Time-Lapse Imaging, Biochemical and Electron Microscopy Analysis of Cell-Free Apoptosis. Exp. Cell Res. 2007, 313, 3635–3644. [Google Scholar] [CrossRef]
- Ren, W.; Zhao, W.; Cao, L.; Huang, J. Involvement of the Actin Machinery in Programmed Cell Death. Front. Cell Dev. Biol. 2021, 8, 634849. [Google Scholar] [CrossRef] [PubMed]
- Budai, P.; Kormos, É.; Buda, I.; Somody, G.; Lehel, J. Comparative Evaluation of HET-CAM and ICE Methods for Objective Assessment of Ocular Irritation Caused by Selected Pesticide Products. Toxicol. Vitr. 2021, 74, 105150. [Google Scholar] [CrossRef]
- Qi, X.; Gao, C.; Yin, C.; Fan, J.; Wu, X.; Guo, C. Improved Anticancer Activity of Betulinic Acid on Breast Cancer through a Grafted Copolymer-Based Micelles System. Drug Deliv. 2021, 28, 1962–1971. [Google Scholar] [CrossRef]
- Dehelean, C.A.; Feflea, S.; Ganta, S.; Amiji, M. Anti-Angiogenic Effects of Betulinic Acid Administered in Nanoemulsion Formulation Using Chorioallantoic Membrane Assay. J. Biomed. Nanotechnol. 2011, 7, 317–324. [Google Scholar] [CrossRef]
- Coricovac, D.; Pînzaru, I.; Avram, Ș.; Macașoi, I.; Șoica, C.; Dehelean, C. In Vitro and In Ovo Assessment of Betulinic Acid Antimelanoma Effect. Timisoara Med. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Articles, O. Implants Composed of Digoxin and Poly (ε-Caprolactone): Development, Characterization, Anti-Proliferative and Anti-Angiogenic Activities. Die Pharm. Int. J. Pharm. Sci. 2017, 72, 383–388. [Google Scholar] [CrossRef]
- Svensson, A.; Azarbayjani, F.; Bäckman, U.; Matsumoto, T.; Christofferson, R. Digoxin Inhibits Neuroblastoma Tumor Growth in Mice. Anticancer Res. 2005, 25, 207–212. [Google Scholar] [PubMed]


| H2O | SDS 1% | BA 10 μM | DG 50 nM | DG 50 nM + BA 10 μM | |
|---|---|---|---|---|---|
| IS | 0.10 | 19.86 | 0.75 | 1.09 | 0.52 |
| tH | 300 | 20 s | 300 | 296 | 300 |
| tL | 300 | 18 s | 290 | 285 | 295 |
| tC | 299 | 15 s | 285 | 280 | 289 |
| Inhibitori Effect (Fa) | Combination Index (CI) | Dose BA | Dose DG | Dose Reduction Index (DRI) BA | Dose Reduction Index (DRI) DG |
|---|---|---|---|---|---|
| 0.65 | 11.9064 | 10 μM | 5 nM | 0.08454 | 12.7600 |
| 0.64 | 10.0020 | 10 μM | 10 nM | 0.10151 | 6.62483 |
| 0.4 | 0.32406 | 10 μM | 25 nM | 6.17001 | 6.17336 |
| 0.17 | 0.11826 | 10 μM | 50 nM | 864.114 | 8.53929 |
| Inhibitori Effect (Fa) | Combination Index (CI) | Dose BA | Dose DG | Dose Reduction Index (DRI) BA | Dose Reduction Index (DRI) DG |
|---|---|---|---|---|---|
| 0.74 | 0.24457 | 10 μM | 5 nM | 5.77905 | 13.9796 |
| 0.68 | 0.20759 | 10 μM | 10 nM | 9.93477 | 9.35133 |
| 0.55 | 0.19019 | 10 μM | 25 nM | 27.7052 | 6.48955 |
| 0.23 | 0.07838 | 10 μM | 50 nM | 377.738 | 13.2049 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rednic, R.; Macasoi, I.; Pinzaru, I.; Dehelean, C.A.; Tomescu, M.-C.; Susan, M.; Feier, H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life 2022, 12, 1855. https://doi.org/10.3390/life12111855
Rednic R, Macasoi I, Pinzaru I, Dehelean CA, Tomescu M-C, Susan M, Feier H. Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life. 2022; 12(11):1855. https://doi.org/10.3390/life12111855
Chicago/Turabian StyleRednic, Robert, Ioana Macasoi, Iulia Pinzaru, Cristina Adriana Dehelean, Mirela-Cleopatra Tomescu, Monica Susan, and Horea Feier. 2022. "Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells" Life 12, no. 11: 1855. https://doi.org/10.3390/life12111855
APA StyleRednic, R., Macasoi, I., Pinzaru, I., Dehelean, C. A., Tomescu, M.-C., Susan, M., & Feier, H. (2022). Pharmaco-Toxicological Assessment of the Combined Cytotoxic Effects of Digoxin and Betulinic Acid in Melanoma Cells. Life, 12(11), 1855. https://doi.org/10.3390/life12111855








