Mitochondria-Targeted Human Catalase in the Mouse Longevity MCAT Model Mitigates Head-Tilt Bedrest-Induced Neuro-Inflammation in the Hippocampus
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Strains
2.2. Hindlimb Unloading to Produce Head-Down Tilt
2.3. Sample Collection and Immunohistochemistry
2.4. Mouse Behavioral Analysis
- Exploratory Behavior: burrowing in bedding, climbing, exploratory sniffing, and exploratory ambulation;
- Social Interaction: chasing, sniffing another mouse, allogrooming, nestlet social engagement, mounting, socializing with another mouse, and pulling another mouse.
- Active Behavior: active behavior contains exploratory behavior, eating, drinking, sniffing, nestlet manipulation, self-grooming, ambulation, and all social interaction behaviors;
- Rest/Sleep: rest/Sleep behavior contains sleep and non-directed movement.
2.5. Behavioral Categories Definitions
2.6. Statistical Analysis
3. Results
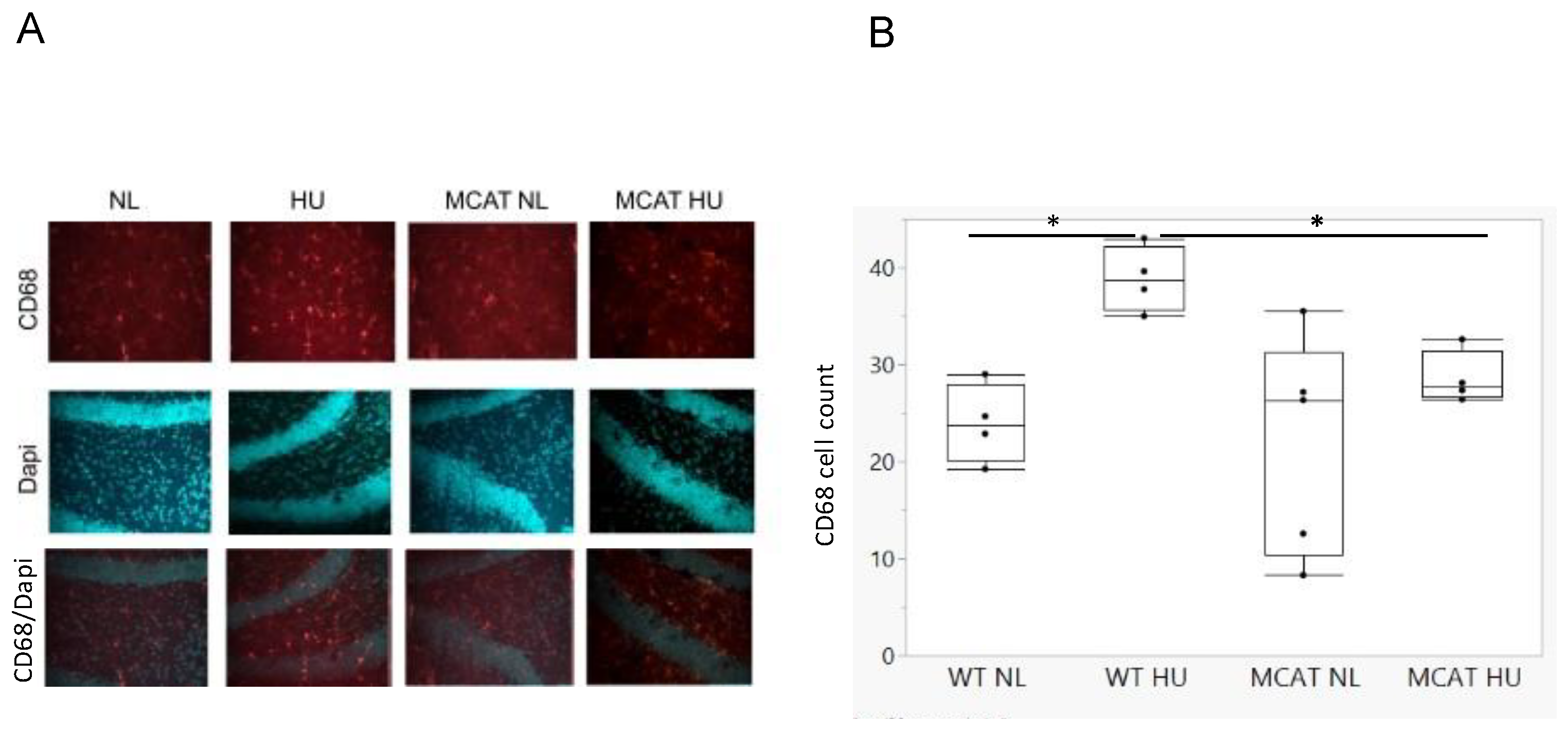
3.1. HU Increased Activated Microglia in the Hippocampus
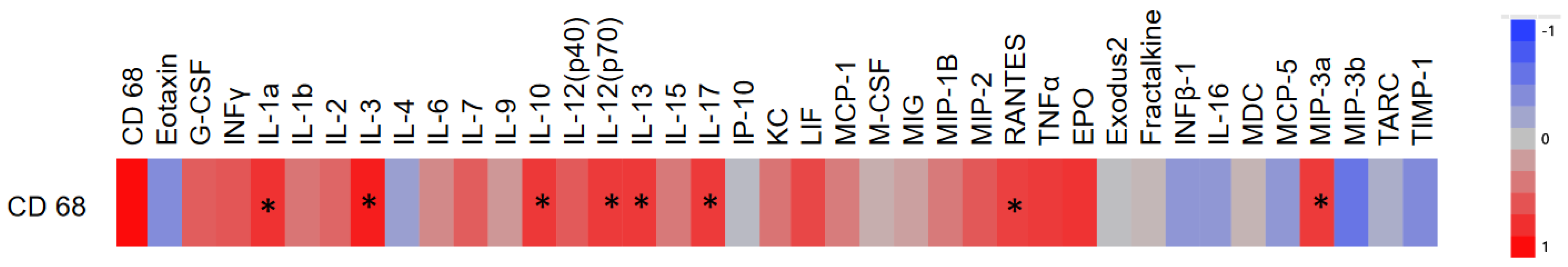
3.2. Correlation of CD68 Count and Hippocampal Cytokines
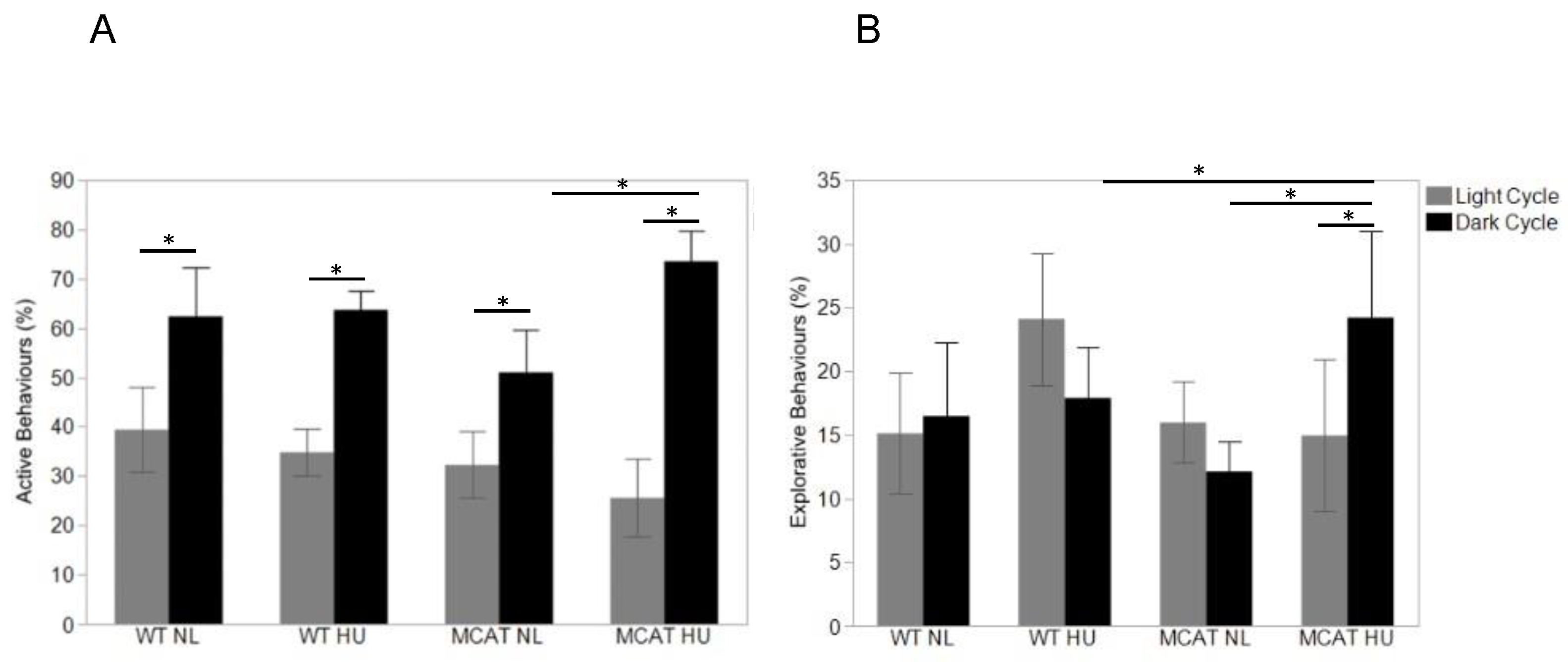
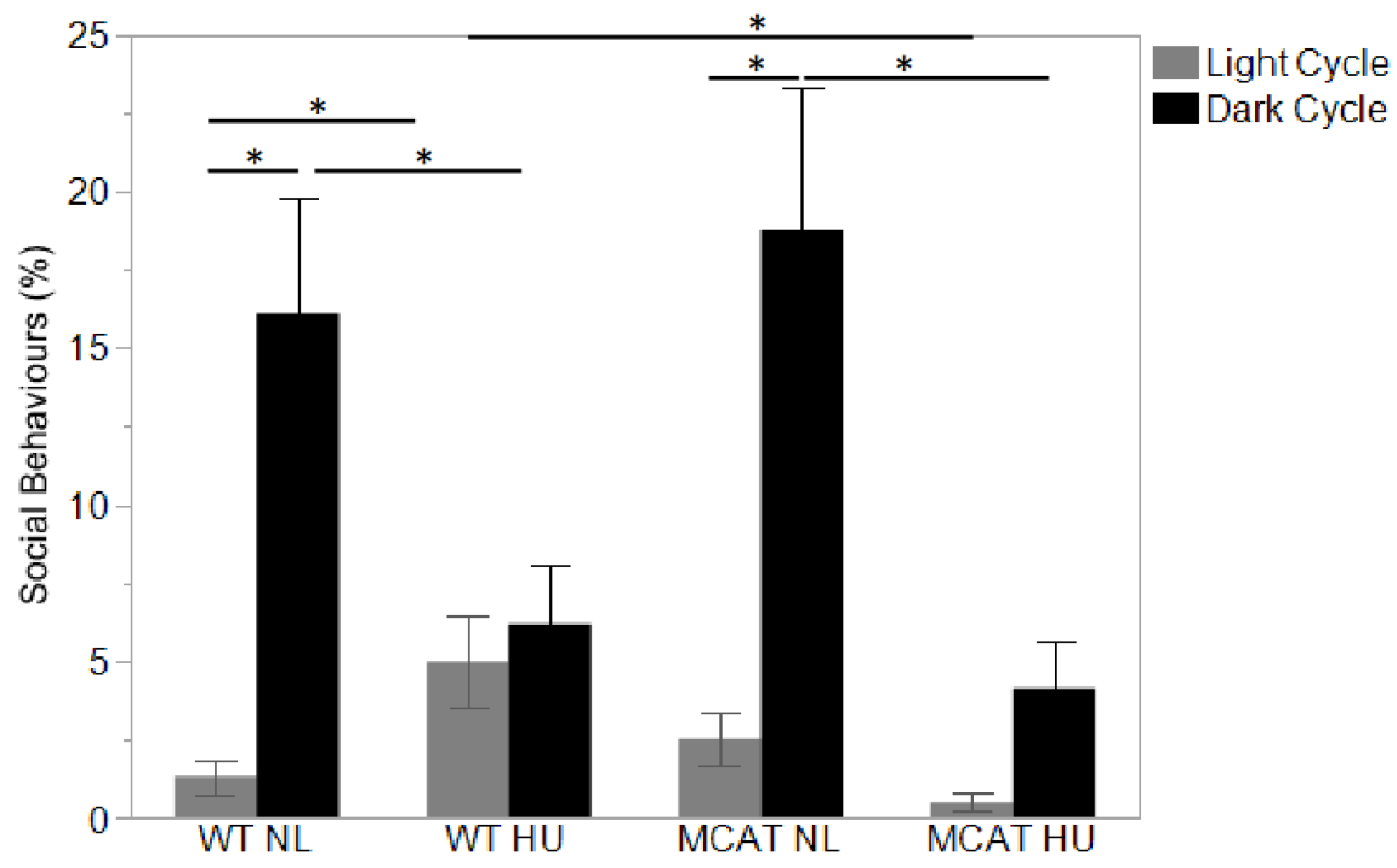
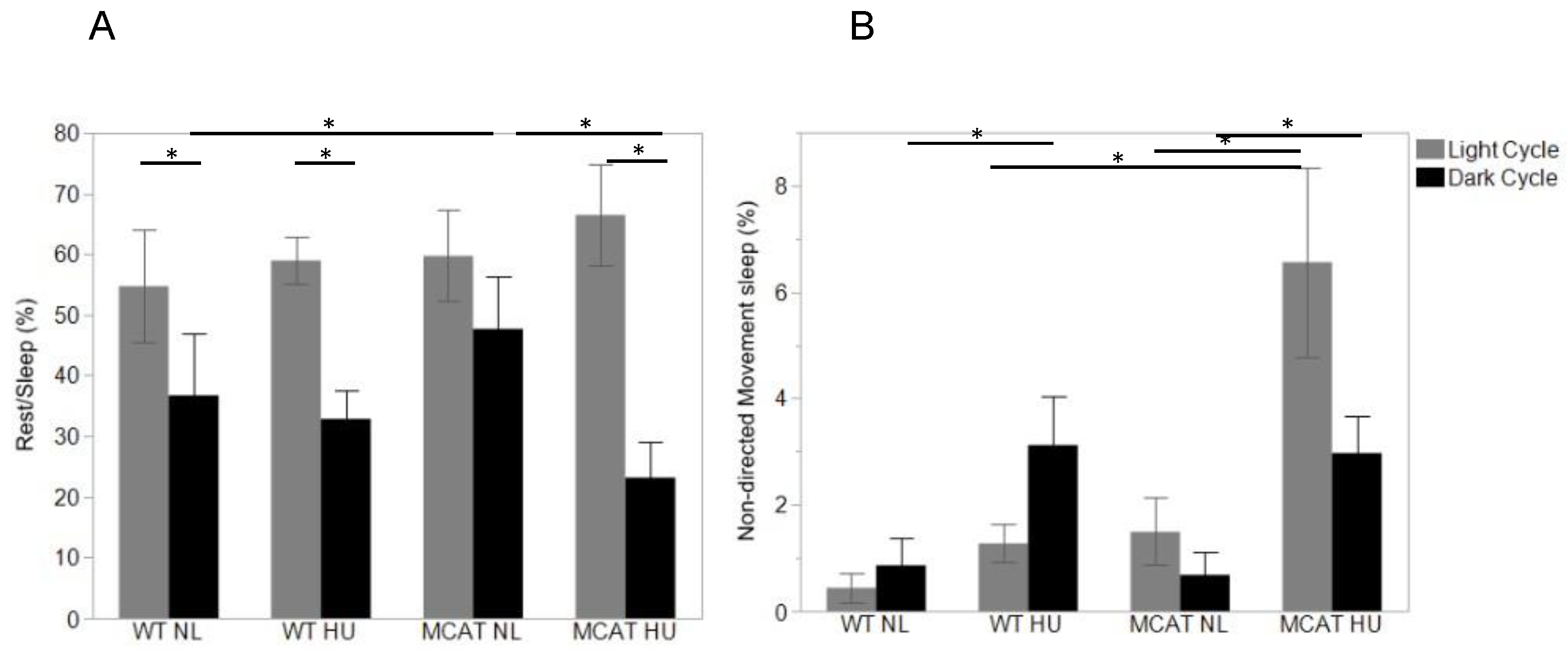
3.3. Changes in Behavior Observed during Light and Dark Cycles
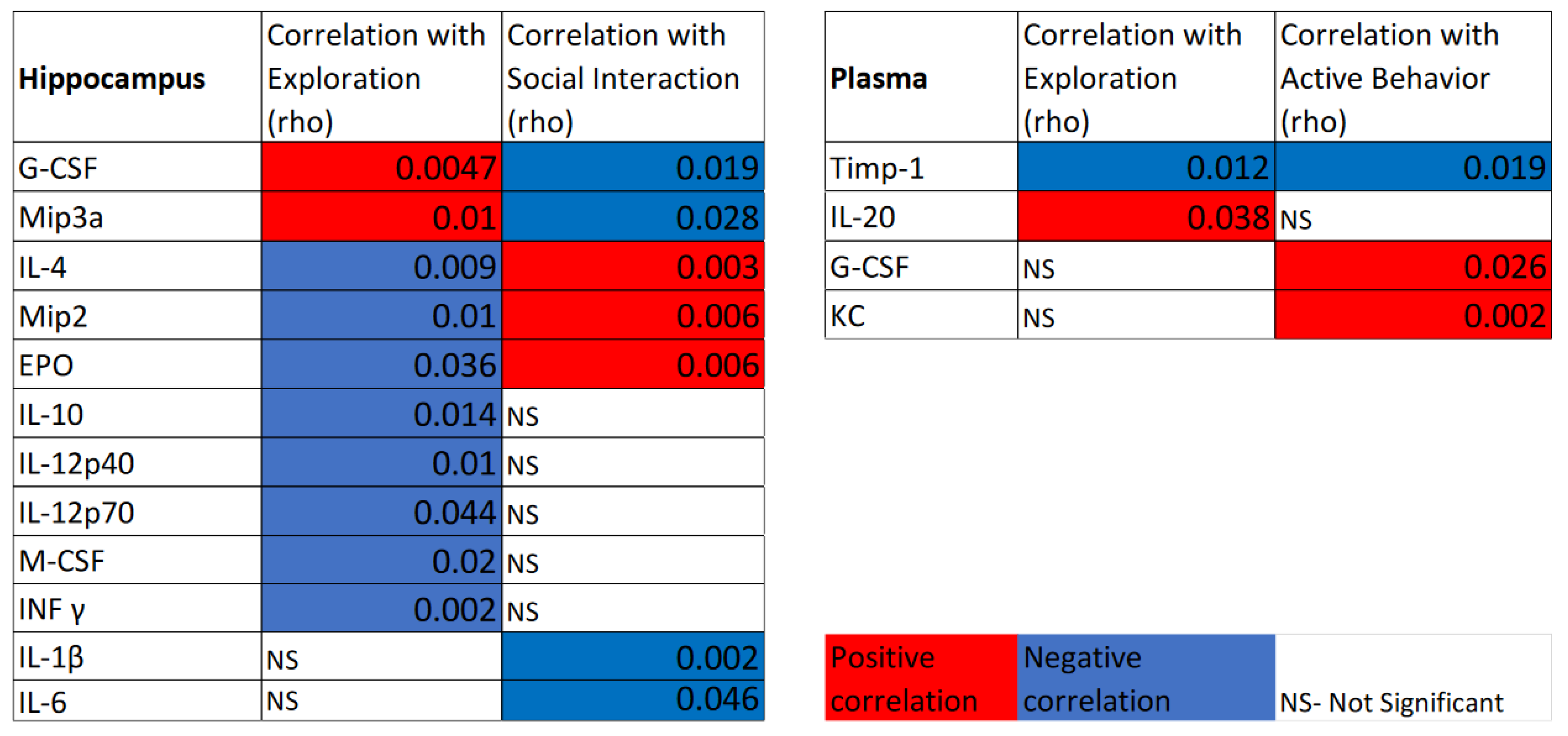
3.4. Correlations of Categorized Behaviors with Plasma and Hippocampal Cytokines
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Koppelmans, V.; Bloomberg, J.J.; Mulavara, A.P.; Seidler, R.D. Brain structural plasticity with spaceflight. NPJ Microgravity 2016, 2, 2. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, A.D.; Yamauchi, K.; Sundaresan, A.; Ramesh, G.T.; Pellis, N.R. Countermeasure for space flight effects on immune system: Nutritional nucleotides. Gravit. Space Biol. Bull. Publ. Am. Soc. Gravit. Space Biol. 2005, 18, 101–102. [Google Scholar]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in Physiology and Disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Izquierdo, P.; Attwell, D.; Madry, C. Ion Channels and Receptors as Determinants of Microglial Function. Trends Neurosci. 2019, 42, 278–292. [Google Scholar] [CrossRef]
- Kreutzberg, G.W. Microglia: A sensor for pathological events in the CNS. Trends Neurosci. 1996, 19, 312–318. [Google Scholar] [CrossRef]
- Xu, X.; Tan, C.; Li, P.; Zhang, S.; Pang, X.; Liu, H.; Li, L.; Sun, X.; Zhang, Y.; Wu, H.; et al. Changes of cytokines during a spaceflight analog--a 45-day head-down bed rest. PLoS ONE 2013, 8, e77401. [Google Scholar] [CrossRef] [PubMed]
- Lee, M. Neurotransmitters and microglial-mediated neuroinflammation. Curr. Protein Pept. Sci. 2013, 14, 21–32. [Google Scholar] [CrossRef]
- Hanisch, U.K. Microglia as a source and target of cytokines. Glia 2002, 40, 140–155. [Google Scholar] [CrossRef]
- Boche, D.; Perry, V.H.; Nicoll, J.A. Review: Activation patterns of microglia and their identification in the human brain. Neuropathol. Appl. Neurobiol. 2013, 39, 3–18. [Google Scholar] [CrossRef]
- Walker, F.R.; Nilsson, M.; Jones, K. Acute and chronic stress-induced disturbances of microglial plasticity, phenotype and function. Curr. Drug Targets 2013, 14, 1262–1276. [Google Scholar] [CrossRef]
- Liu, B.; Hinshaw, R.G.; Le, K.X.; Park, M.A.; Wang, S.; Belanger, A.P.; Dubey, S.; Frost, J.L.; Shi, Q.; Holton, P.; et al. Space-like (56)Fe irradiation manifests mild, early sex-specific behavioral and neuropathological changes in wildtype and Alzheimer's-like transgenic mice. Sci. Rep. 2019, 9, 12118. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, O.; Huber, K.; Lang, K. Signal transduction in cells of the immune system in microgravity. Cell Commun. Signal. CCS 2008, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.E.; Zwart, S.R.; Mehta, S.; Uchakin, P.; Quiriarte, H.D.; Pierson, D.; Sams, C.F.; Smith, S.M. Plasma cytokine concentrations indicate that in vivo hormonal regulation of immunity is altered during long-duration spaceflight. J. Interferon Cytokine Res. Off. J. Int. Soc. Interferon Cytokine Res. 2014, 34, 778–786. [Google Scholar] [CrossRef]
- Crucian, B.; Stowe, R.; Quiriarte, H.; Pierson, D.; Sams, C. Monocyte phenotype and cytokine production profiles are dysregulated by short-duration spaceflight. Aviat. Space Environ. Med. 2011, 82, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Crucian, B.; Stowe, R.P.; Mehta, S.; Quiriarte, H.; Pierson, D.; Sams, C. Alterations in adaptive immunity persist during long-duration spaceflight. NPJ Microgravity 2015, 1, 15013. [Google Scholar] [CrossRef] [PubMed]
- Hoff, P.; Belavy, D.L.; Huscher, D.; Lang, A.; Hahne, M.; Kuhlmey, A.K.; Maschmeyer, P.; Armbrecht, G.; Fitzner, R.; Perschel, F.H.; et al. Effects of 60-day bed rest with and without exercise on cellular and humoral immunological parameters. Cell. Mol. Immunol. 2015, 12, 483–492. [Google Scholar] [CrossRef]
- Frigeri, A.; Iacobas, D.A.; Iacobas, S.; Nicchia, G.P.; Desaphy, J.F.; Camerino, D.C.; Svelto, M.; Spray, D.C. Effect of microgravity on gene expression in mouse brain. Exp. Brain Res. 2008, 191, 289–300. [Google Scholar] [CrossRef]
- Chelyshev, Y.A.; Muhamedshina, Y.O.; Povysheva, T.V.; Shaymardanova, G.F.; Rizvanov, A.A.; Nigmetzyanova, M.V.; Tiapkina, O.V.; Bondarenko, N.I.; Nikolskiy, E.E.; Islamov, R.R. Characterization of spinal cord glial cells in a model of hindlimb unloading in mice. Neuroscience 2014, 280, 328–339. [Google Scholar] [CrossRef]
- Lin, T.; Du, J.; Liu, L.; Wu, Z.; Kong, X.; Liu, Y.; Cai, Y. Treatment with Minocycline Suppresses Microglia Activation and Reverses Neural Stem Cells Loss after Simulated Microgravity. BioMed Res. Int. 2020, 2020, 7348745. [Google Scholar] [CrossRef]
- Rubinstein, L.; Schreurs, A.S.; Torres, S.M.; Steczina, S.; Lowe, M.G.; Kiffer, F.; Allen, A.R.; Ronca, A.E.; Sowa, M.B.; Globus, R.K.; et al. Overexpression of catalase in mitochondria mitigates changes in hippocampal cytokine expression following simulated microgravity and isolation. NPJ Microgravity 2021, 7, 24. [Google Scholar] [CrossRef]
- Kawanokuchi, J.; Shimizu, K.; Nitta, A.; Yamada, K.; Mizuno, T.; Takeuchi, H.; Suzumura, A. Production and functions of IL-17 in microglia. J. Neuroimmunol. 2008, 194, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, R.P.; Koledova, V.V.; Schneider, K.; Sambandan, T.G.; Grayson, A.; Zeidman, G.; Artamonova, A.; Sambanthamurthi, R.; Fairus, S.; Sinskey, A.J.; et al. Palm Fruit Bioactives modulate human astrocyte activity in vitro altering the cytokine secretome reducing levels of TNFalpha, RANTES and IP-10. Sci. Rep. 2018, 8, 16423. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.S.; Lee, H.J.; Lim, I.; Satoh, J.; Kim, S.U. Human astrocytes: Secretome profiles of cytokines and chemokines. PLoS ONE 2014, 9, e92325. [Google Scholar] [CrossRef]
- Paulsen, K.; Tauber, S.; Dumrese, C.; Bradacs, G.; Simmet, D.M.; Golz, N.; Hauschild, S.; Raig, C.; Engeli, S.; Gutewort, A.; et al. Regulation of ICAM-1 in cells of the monocyte/macrophage system in microgravity. BioMed Res. Int. 2015, 2015, 538786. [Google Scholar] [CrossRef] [PubMed]
- Wise, K.C.; Manna, S.K.; Yamauchi, K.; Ramesh, V.; Wilson, B.L.; Thomas, R.L.; Sarkar, S.; Kulkarni, A.D.; Pellis, N.R.; Ramesh, G.T. Activation of nuclear transcription factor-kappaB in mouse brain induced by a simulated microgravity environment. In Vitro Cell. Dev. Biol. Anim. 2005, 41, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Olsen, R.H.; Johnson, L.A.; Zuloaga, D.G.; Limoli, C.L.; Raber, J. Enhanced hippocampus-dependent memory and reduced anxiety in mice over-expressing human catalase in mitochondria. J. Neurochem. 2013, 125, 303–313. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, Z.; Chen, X.; Liu, M.; Li, J.; Liu, N. The role of autophagy in pro-inflammatory responses of microglia activation via mitochondrial reactive oxygen species in vitro. J. Neurochem. 2017, 142, 215–230. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, Z.; Chen, X.; Liu, M.; Li, J.; Liu, N. Electron transport chain inhibitors induce microglia activation through enhancing mitochondrial reactive oxygen species production. Exp. Cell Res. 2016, 340, 315–326. [Google Scholar] [CrossRef]
- Bordt, E.A.; Polster, B.M. NADPH oxidase- and mitochondria-derived reactive oxygen species in proinflammatory microglial activation: A bipartisan affair? Free Radic. Biol. Med. 2014, 76, 34–46. [Google Scholar] [CrossRef]
- Park, J.; Min, J.S.; Kim, B.; Chae, U.B.; Yun, J.W.; Choi, M.S.; Kong, I.K.; Chang, K.T.; Lee, D.S. Mitochondrial ROS govern the LPS-induced pro-inflammatory response in microglia cells by regulating MAPK and NF-kappaB pathways. Neurosci. Lett. 2015, 584, 191–196. [Google Scholar] [CrossRef]
- Park, J.; Choi, H.; Min, J.S.; Park, S.J.; Kim, J.H.; Park, H.J.; Kim, B.; Chae, J.I.; Yim, M.; Lee, D.S. Mitochondrial dynamics modulate the expression of pro-inflammatory mediators in microglial cells. J. Neurochem. 2013, 127, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Schriner, S.E.; Linford, N.J.; Martin, G.M.; Treuting, P.; Ogburn, C.E.; Emond, M.; Coskun, P.E.; Ladiges, W.; Wolf, N.; Van Remmen, H.; et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 2005, 308, 1909–1911. [Google Scholar] [CrossRef] [PubMed]
- Csiszar, A.; Yabluchanskiy, A.; Ungvari, A.; Ungvari, Z.; Tarantini, S. Overexpression of catalase targeted to mitochondria improves neurovascular coupling responses in aged mice. GeroScience 2019, 41, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Liao, A.C.; Craver, B.M.; Tseng, B.P.; Tran, K.K.; Parihar, V.K.; Acharya, M.M.; Limoli, C.L. Mitochondrial-targeted human catalase affords neuroprotection from proton irradiation. Radiat. Res. 2013, 180, 1–6. [Google Scholar] [CrossRef] [PubMed]
- D'Souza, A.D.; Parish, I.A.; Krause, D.S.; Kaech, S.M.; Shadel, G.S. Reducing mitochondrial ROS improves disease-related pathology in a mouse model of ataxia-telangiectasia. Mol. Ther. J. Am. Soc. Gene Ther. 2013, 21, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Pehar, M.; Beeson, G.; Beeson, C.C.; Johnson, J.A.; Vargas, M.R. Mitochondria-targeted catalase reverts the neurotoxicity of hSOD1G(9)(3)A astrocytes without extending the survival of ALS-linked mutant hSOD1 mice. PLoS ONE 2014, 9, e103438. [Google Scholar] [CrossRef] [PubMed]
- Mao, P.; Manczak, M.; Calkins, M.J.; Truong, Q.; Reddy, T.P.; Reddy, A.P.; Shirendeb, U.; Lo, H.H.; Rabinovitch, P.S.; Reddy, P.H. Mitochondria-targeted catalase reduces abnormal APP processing, amyloid beta production and BACE1 in a mouse model of Alzheimer's disease: Implications for neuroprotection and lifespan extension. Hum. Mol. Genet. 2012, 21, 2973–2990. [Google Scholar] [CrossRef]
- Lekander, M.; Elofsson, S.; Neve, I.M.; Hansson, L.O.; Unden, A.L. Self-rated health is related to levels of circulating cytokines. Psychosom. Med. 2004, 66, 559–563. [Google Scholar] [CrossRef]
- Unden, A.L.; Andreasson, A.; Elofsson, S.; Brismar, K.; Mathsson, L.; Ronnelid, J.; Lekander, M. Inflammatory cytokines, behaviour and age as determinants of self-rated health in women. Clin. Sci. 2007, 112, 363–373. [Google Scholar] [CrossRef][Green Version]
- Andreasson, A.; Arborelius, L.; Erlanson-Albertsson, C.; Lekander, M. A putative role for cytokines in the impaired appetite in depression. Brain Behav. Immun. 2007, 21, 147–152. [Google Scholar] [CrossRef]
- Miller, A.H. Norman Cousins Lecture. Mechanisms of cytokine-induced behavioral changes: Psychoneuroimmunology at the translational interface. Brain Behav. Immun. 2009, 23, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Bortolato, B.; Carvalho, A.F.; Soczynska, J.K.; Perini, G.I.; McIntyre, R.S. The Involvement of TNF-alpha in Cognitive Dysfunction Associated with Major Depressive Disorder: An Opportunity for Domain Specific Treatments. Curr. Neuropharmacol. 2015, 13, 558–576. [Google Scholar] [CrossRef] [PubMed]
- Shang, X.; Xu, B.; Li, Q.; Zhai, B.; Xu, X.; Zhang, T. Neural oscillations as a bridge between glutamatergic system and emotional behaviors in simulated microgravity-induced mice. Behav. Brain Res. 2017, 317, 286–291. [Google Scholar] [CrossRef]
- Zhai, B.; Shang, X.; Fu, J.; Li, F.; Zhang, T. Rapamycin relieves anxious emotion and synaptic plasticity deficits induced by hindlimb unloading in mice. Neurosci. Lett. 2018, 677, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Tahimic, C.G.T.; Paul, A.M.; Schreurs, A.S.; Torres, S.M.; Rubinstein, L.; Steczina, S.; Lowe, M.; Bhattacharya, S.; Alwood, J.S.; Ronca, A.E.; et al. Influence of Social Isolation During Prolonged Simulated Weightlessness by Hindlimb Unloading. Front. Physiol. 2019, 10, 1147. [Google Scholar] [CrossRef]
- Palkovits, M. Punch sampling biopsy technique. Methods Enzymol. 1983, 103, 368–376. [Google Scholar] [CrossRef]
- Rubinstein, L.; Paul, A.M.; Houseman, C.; Abegaz, M.; Tabares Ruiz, S.; O'Neil, N.; Kunis, G.; Ofir, R.; Cohen, J.; Ronca, A.E.; et al. Placenta-Expanded Stromal Cell Therapy in a Rodent Model of Simulated Weightlessness. Cells 2021, 10, 940. [Google Scholar] [CrossRef]
- Mhatre, S.D.; Iyer, J.; Puukila, S.; Paul, A.M.; Tahimic, C.G.T.; Rubinstein, L.; Lowe, M.; Alwood, J.S.; Sowa, M.B.; Bhattacharya, S.; et al. Neuro-consequences of the spaceflight environment. Neurosci. Biobehav. Rev. 2021, 132, 908–935. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb unloading rodent model: Technical aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Sarkar, P.; Sarkar, S.; Ramesh, V.; Hayes, B.E.; Thomas, R.L.; Wilson, B.L.; Kim, H.; Barnes, S.; Kulkarni, A.; Pellis, N.; et al. Proteomic analysis of mice hippocampus in simulated microgravity environment. J. Proteome Res. 2006, 5, 548–553. [Google Scholar] [CrossRef][Green Version]
- Deng, B.; Wehling-Henricks, M.; Villalta, S.A.; Wang, Y.; Tidball, J.G. IL-10 triggers changes in macrophage phenotype that promote muscle growth and regeneration. J. Immunol. 2012, 189, 3669–3680. [Google Scholar] [CrossRef]
- Kiffer, F.; Boerma, M.; Allen, A. Behavioral effects of space radiation: A comprehensive review of animal studies. Life Sci. Space Res. 2019, 21, 1–21. [Google Scholar] [CrossRef]
- Raber, J.; Yamazaki, J.; Torres, E.R.S.; Kirchoff, N.; Stagaman, K.; Sharpton, T.; Turker, M.S.; Kronenberg, A. Combined Effects of Three High-Energy Charged Particle Beams Important for Space Flight on Brain, Behavioral and Cognitive Endpoints in B6D2F1 Female and Male Mice. Front. Physiol. 2019, 10, 179. [Google Scholar] [CrossRef] [PubMed]
- Kiffer, F.; Alexander, T.; Anderson, J.E.; Groves, T.; Wang, J.; Sridharan, V.; Boerma, M.; Allen, A.R. Late Effects of (16)O-Particle Radiation on Female Social and Cognitive Behavior and Hippocampal Physiology. Radiat. Res. 2019, 191, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Soler, I.; Yun, S.; Reynolds, R.P.; Whoolery, C.W.; Tran, F.H.; Kumar, P.L.; Rong, Y.; DeSalle, M.J.; Gibson, A.D.; Stowe, A.M.; et al. Multi-Domain Touchscreen-Based Cognitive Assessment of C57BL/6J Female Mice Shows Whole-Body Exposure to (56)Fe Particle Space Radiation in Maturity Improves Discrimination Learning Yet Impairs Stimulus-Response Rule-Based Habit Learning. Front. Behav. Neurosci. 2021, 15, 722780. [Google Scholar] [CrossRef] [PubMed]
- Globus, R.K.; Morey-Holton, E. Hindlimb unloading: Rodent analog for microgravity. J. Appl. Physiol. 2016, 120, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Vaccaro, A.; Kaplan Dor, Y.; Nambara, K.; Pollina, E.A.; Lin, C.; Greenberg, M.E.; Rogulja, D. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell 2020, 181, 1307–1328.e15. [Google Scholar] [CrossRef]
- Reddy, P.H. Mitochondrial oxidative damage in aging and Alzheimer's disease: Implications for mitochondrially targeted antioxidant therapeutics. J. Biomed. Biotechnol. 2006, 2006, 31372. [Google Scholar] [CrossRef]
- Sugama, S.; Kakinuma, Y. Stress and brain immunity: Microglial homeostasis through hypothalamus-pituitary-adrenal gland axis and sympathetic nervous system. Brain Behav. Immun.-Health 2020, 7, 100111. [Google Scholar] [CrossRef]
- Paul, A.M.; Mhatre, S.D.; Cekanaviciute, E.; Schreurs, A.S.; Tahimic, C.G.T.; Globus, R.K.; Anand, S.; Crucian, B.E.; Bhattacharya, S. Neutrophil-to-Lymphocyte Ratio: A Biomarker to Monitor the Immune Status of Astronauts. Front. Immunol. 2020, 11, 564950. [Google Scholar] [CrossRef]
- McEwen, B.S. Neurobiological and Systemic Effects of Chronic Stress. Chronic Stress 2017, 1, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R. Cytokine, sickness behavior, and depression. Immunol. Allergy Clin. N. Am. 2009, 29, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Roth, S.; Yang, J.; Cramer, J.V.; Malik, R.; Liesz, A. Detection of cytokine-induced sickness behavior after ischemic stroke by an optimized behavioral assessment battery. Brain Behav. Immun. 2021, 91, 668–672. [Google Scholar] [CrossRef]
- Shin, W.H.; Lee, D.Y.; Park, K.W.; Kim, S.U.; Yang, M.S.; Joe, E.H.; Jin, B.K. Microglia expressing interleukin-13 undergo cell death and contribute to neuronal survival in vivo. Glia 2004, 46, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, A.L.; Reis, H.J.; Nicolato, R.; Brito-Melo, G.; Correa, H.; Teixeira, M.M.; Romano-Silva, M.A. Increased serum levels of CCL11/eotaxin in schizophrenia. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2008, 32, 710–714. [Google Scholar] [CrossRef] [PubMed]
- Moll-Bernardes, R.; de Sousa, A.S.; Macedo, A.V.S.; Lopes, R.D.; Vera, N.; Maia, L.C.R.; Feldman, A.; Arruda, G.; Castro, M.J.C.; Pimentel-Coelho, P.M.; et al. IL-10 and IL-12 (P70) Levels Predict the Risk of Covid-19 Progression in Hypertensive Patients: Insights From the BRACE-CORONA Trial. Front. Cardiovasc. Med. 2021, 8, 702507. [Google Scholar] [CrossRef] [PubMed]
- Bellone, J.A.; Gifford, P.S.; Nishiyama, N.C.; Hartman, R.E.; Mao, X.W. Long-term effects of simulated microgravity and/or chronic exposure to low-dose gamma radiation on behavior and blood-brain barrier integrity. NPJ Microgravity 2016, 2, 16019. [Google Scholar] [CrossRef]
- Accattato, F.; Greco, M.; Pullano, S.A.; Care, I.; Fiorillo, A.S.; Pujia, A.; Montalcini, T.; Foti, D.P.; Brunetti, A.; Gulletta, E. Effects of acute physical exercise on oxidative stress and inflammatory status in young, sedentary obese subjects. PLoS ONE 2017, 12, e0178900. [Google Scholar] [CrossRef]
- Garrido, A.; Cruces, J.; Ceprian, N.; Vara, E.; de la Fuente, M. Oxidative-Inflammatory Stress in Immune Cells from Adult Mice with Premature Aging. Int. J. Mol. Sci. 2019, 20, 769. [Google Scholar] [CrossRef]
- Kokhan, V.S.; Matveeva, M.I.; Mukhametov, A.; Shtemberg, A.S. Risk of defeats in the central nervous system during deep space missions. Neurosci. Biobehav. Rev. 2016, 71, 621–632. [Google Scholar] [CrossRef]
- Lehmann, M.L.; Weigel, T.K.; Poffenberger, C.N.; Herkenham, M. The Behavioral Sequelae of Social Defeat Require Microglia and Are Driven by Oxidative Stress in Mice. J. Neurosci. Off. J. Soc. Neurosci. 2019, 39, 5594–5605. [Google Scholar] [CrossRef] [PubMed]
- Biber, K.; Neumann, H.; Inoue, K.; Boddeke, H.W. Neuronal 'On' and 'Off' signals control microglia. Trends Neurosci. 2007, 30, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Szatmari, T.; Safrany, G. Ionizing Radiation-Induced Immune and Inflammatory Reactions in the Brain. Front. Immunol. 2017, 8, 517. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubinstein, L.; Kiffer, F.; Puukila, S.; Lowe, M.G.; Goo, B.; Luthens, A.; Schreurs, A.-S.; Torres, S.M.; Steczina, S.; Tahimic, C.G.T.; et al. Mitochondria-Targeted Human Catalase in the Mouse Longevity MCAT Model Mitigates Head-Tilt Bedrest-Induced Neuro-Inflammation in the Hippocampus. Life 2022, 12, 1838. https://doi.org/10.3390/life12111838
Rubinstein L, Kiffer F, Puukila S, Lowe MG, Goo B, Luthens A, Schreurs A-S, Torres SM, Steczina S, Tahimic CGT, et al. Mitochondria-Targeted Human Catalase in the Mouse Longevity MCAT Model Mitigates Head-Tilt Bedrest-Induced Neuro-Inflammation in the Hippocampus. Life. 2022; 12(11):1838. https://doi.org/10.3390/life12111838
Chicago/Turabian StyleRubinstein, Linda, Frederico Kiffer, Stephanie Puukila, Moniece G. Lowe, Brie Goo, Amalia Luthens, Ann-Sofie Schreurs, Samantha M. Torres, Sonette Steczina, Candice G. T. Tahimic, and et al. 2022. "Mitochondria-Targeted Human Catalase in the Mouse Longevity MCAT Model Mitigates Head-Tilt Bedrest-Induced Neuro-Inflammation in the Hippocampus" Life 12, no. 11: 1838. https://doi.org/10.3390/life12111838
APA StyleRubinstein, L., Kiffer, F., Puukila, S., Lowe, M. G., Goo, B., Luthens, A., Schreurs, A.-S., Torres, S. M., Steczina, S., Tahimic, C. G. T., & Allen, A. R. (2022). Mitochondria-Targeted Human Catalase in the Mouse Longevity MCAT Model Mitigates Head-Tilt Bedrest-Induced Neuro-Inflammation in the Hippocampus. Life, 12(11), 1838. https://doi.org/10.3390/life12111838









