Are Skeletal Muscle Changes during Prolonged Space Flights Similar to Those Experienced by Frail and Sarcopenic Older Adults?
Abstract
1. Introduction
2. Frailty and Sarcopenia during Aging
3. Biological Mechanisms Underlying Sarcopenia and Frailty
3.1. Immune Activation and Inflammation
3.2. Role of Myokines
3.2.1. Insulin-Like Growth Factor 1 (IGF-1)
3.2.2. Myostatin
3.2.3. Irisin
3.2.4. Follistatin
3.3. Vitamin D
3.4. Oxidative Stress
4. Impact of Space Flight on Astronauts’ Skeletal Muscle Health
4.1. Clinical Manifestation of Sarcopenia/Frailty-like Phenotype in Astronauts
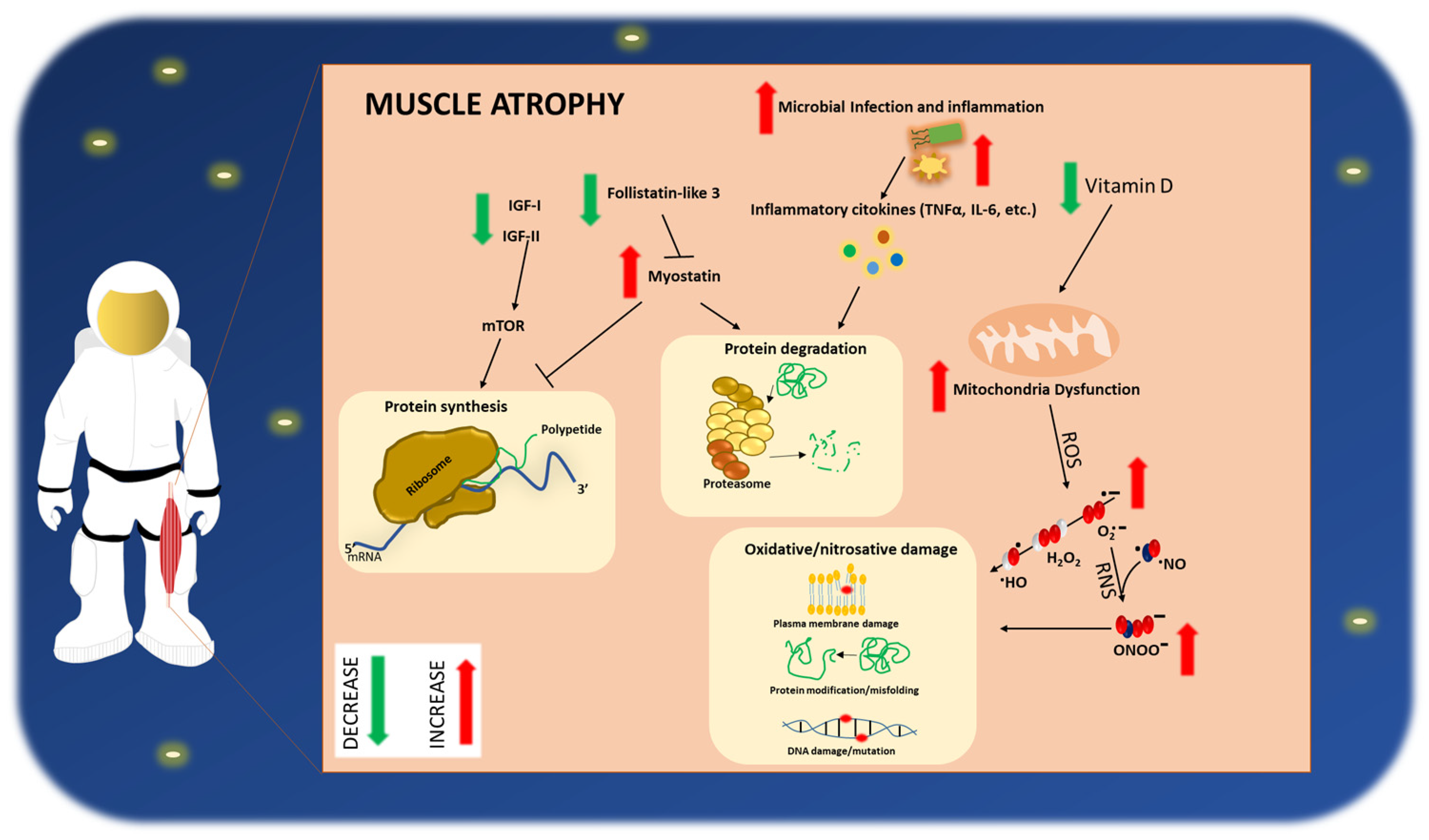
4.2. Pathophysiological Mechanisms Activated in the Muscle by Prolonged Space Missions
5. Potential Countermeasures
6. Perspectives and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Strollo, F. Chapter 4 Hormonal Changes in Humans During Spaceflight. In Advances in Space Biology and Medicine; Elsevier: Amsterdam, The Netherlands, 1999; Volume 7, pp. 99–129. ISBN 978-0-7623-0393-9. [Google Scholar]
- Strollo, F.; Gentile, S.; Strollo, G.; Mambro, A.; Vernikos, J. Recent Progress in Space Physiology and Aging. Front. Physiol. 2018, 9, 1551. [Google Scholar] [CrossRef]
- Vernikos, J.; Schneider, V.S. Space, Gravity and the Physiology of Aging: Parallel or Convergent Disciplines? A Mini-Review. Gerontology 2010, 56, 157–166. [Google Scholar] [CrossRef]
- Vandenburgh, H.; Chromiak, J.; Shansky, J.; Del Tatto, M.; Lemaire, J. Space travel directly induces skeletal muscle atrophy. FASEB J. 1999, 13, 1031–1038. [Google Scholar] [CrossRef]
- Williams, D.; Kuipers, A.; Mukai, C.; Thirsk, R. Acclimation during space flight: Effects on human physiology. Can. Med. Assoc. J. 2009, 180, 1317–1323. [Google Scholar] [CrossRef]
- Janssen, I. Influence of Sarcopenia on the Development of Physical Disability: The Cardiovascular Health Study. J. Am. Geriatr. Soc. 2006, 54, 56–62. [Google Scholar] [CrossRef]
- Zizola, C.; Schulze, P.C. Metabolic and structural impairment of skeletal muscle in heart failure. Heart Fail. Rev. 2013, 18, 623–630. [Google Scholar] [CrossRef]
- Goswami, N. Falls and Fall-Prevention in Older Persons: Geriatrics Meets Spaceflight! Front. Physiol. 2017, 8, 603. [Google Scholar] [CrossRef]
- Furukawa, S.; Chatani, M.; Higashitani, A.; Higashibata, A.; Kawano, F.; Nikawa, T.; Numaga-Tomita, T.; Ogura, T.; Sato, F.; Sehara-Fujisawa, A.; et al. Findings from recent studies by the Japan Aerospace Exploration Agency examining musculoskeletal atrophy in space and on Earth. NPJ Microgravity 2021, 7, 18. [Google Scholar] [CrossRef]
- Goswami, N.; Blaber, A.P.; Hinghofer-Szalkay, H.; Montani, J.-P. Orthostatic Intolerance in Older Persons: Etiology and Countermeasures. Front. Physiol. 2017, 8, 803. [Google Scholar] [CrossRef]
- Keskin, K. Orthostatic hypotension and age-related sarcopenia. Turk. J. Phys. Med. Rehabilit. 2021, 67, 25–31. [Google Scholar] [CrossRef]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Umegaki, H. Sarcopenia and frailty in older patients with diabetes mellitus: Sarcopenia and frailty in DM. Geriatr. Gerontol. Int. 2016, 16, 293–299. [Google Scholar] [CrossRef]
- Cesari, M.; Landi, F.; Vellas, B.; Bernabei, R.; Marzetti, E. Sarcopenia and Physical Frailty: Two Sides of the Same Coin. Front. Aging Neurosci. 2014, 6, 192. [Google Scholar] [CrossRef]
- Rahimi Foroushani, A.; Estebsari, F.; Mostafaei, D.; Eftekhar Ardebili, H.; Shojaeizadeh, D.; Dastoorpour, M.; Jamshidi, E.; Taghdisi, M.H. The effect of health promoting intervention on healthy lifestyle and social support in elders: A clinical trial study. Iran. Red Crescent Med. J. 2014, 16, e18399. [Google Scholar] [CrossRef]
- Brown, G.C. Living too long: The current focus of medical research on increasing the quantity, rather than the quality, of life is damaging our health and harming the economy. EMBO Rep. 2015, 16, 137–141. [Google Scholar] [CrossRef]
- Dominguez, L.J.; Veronese, N.; Baiamonte, E.; Guarrera, M.; Parisi, A.; Ruffolo, C.; Tagliaferri, F.; Barbagallo, M. Healthy Aging and Dietary Patterns. Nutrients 2022, 14, 889. [Google Scholar] [CrossRef]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef]
- Capri, M.; Salvioli, S.; Sevini, F.; Valensin, S.; Celani, L.; Monti, D.; Pawelec, G.; De Benedictis, G.; Gonos, E.S.; Franceschi, C. The genetics of human longevity. Ann. N. Y. Acad. Sci. 2006, 1067, 252–263. [Google Scholar] [CrossRef]
- Salvioli, S.; Olivieri, F.; Marchegiani, F.; Cardelli, M.; Santoro, A.; Bellavista, E.; Mishto, M.; Invidia, L.; Capri, M.; Valensin, S.; et al. Genes, ageing and longevity in humans: Problems, advantages and perspectives. Free Radic. Res. 2006, 40, 1303–1323. [Google Scholar] [CrossRef]
- Franceschi, C.; Valensin, S.; Bonafè, M.; Paolisso, G.; Yashin, A.I.; Monti, D.; De Benedictis, G. The network and the remodeling theories of aging: Historical background and new perspectives. Exp. Gerontol. 2000, 35, 879–896. [Google Scholar] [CrossRef]
- Calvani, R.; Marini, F.; Cesari, M.; Tosato, M.; Anker, S.D.; von Haehling, S.; Miller, R.R.; Bernabei, R.; Landi, F.; Marzetti, E.; et al. Biomarkers for physical frailty and sarcopenia: State of the science and future developments. J. Cachexia Sarcopenia Muscle 2015, 6, 278–286. [Google Scholar] [CrossRef]
- Clegg, A.; Young, J.; Iliffe, S.; Rikkert, M.O.; Rockwood, K. Frailty in elderly people. Lancet 2013, 381, 752–762. [Google Scholar] [CrossRef]
- Collard, R.M.; Boter, H.; Schoevers, R.A.; Oude Voshaar, R.C. Prevalence of frailty in community-dwelling older persons: A systematic review. J. Am. Geriatr. Soc. 2012, 60, 1487–1492. [Google Scholar] [CrossRef]
- Mitnitski, A.B.; Mogilner, A.J.; Rockwood, K. Accumulation of deficits as a proxy measure of aging. Sci. World J. 2001, 1, 323–336. [Google Scholar] [CrossRef]
- Cesari, M.; Calvani, R.; Marzetti, E. Frailty in Older Persons. Clin. Geriatr. Med. 2017, 33, 293–303. [Google Scholar] [CrossRef]
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433–441. [Google Scholar] [CrossRef]
- Looman, W.M.; Fabbricotti, I.N.; Blom, J.W.; Jansen, A.P.D.; Lutomski, J.E.; Metzelthin, S.F.; Huijsman, R.; TOPICS-MDS Research Consortium. The frail older person does not exist: Development of frailty profiles with latent class analysis. BMC Geriatr. 2018, 18, 84. [Google Scholar] [CrossRef]
- Stolz, E.; Mayerl, H.; Freidl, W. Fluctuations in frailty among older adults. Age Ageing 2019, 48, 547–552. [Google Scholar] [CrossRef]
- Cesari, M.; Marzetti, E.; Calvani, R.; Vellas, B.; Bernabei, R.; Bordes, P.; Roubenoff, R.; Landi, F.; Cherubini, A.; SPRINTT consortium. The need of operational paradigms for frailty in older persons: The SPRINTT project. Aging Clin. Exp. Res. 2017, 29, 3–10. [Google Scholar] [CrossRef]
- Morley, J.E.; Vellas, B.; van Kan, G.A.; Anker, S.D.; Bauer, J.M.; Bernabei, R.; Cesari, M.; Chumlea, W.C.; Doehner, W.; Evans, J.; et al. Frailty consensus: A call to action. J. Am. Med. Dir. Assoc. 2013, 14, 392–397. [Google Scholar] [CrossRef]
- Coelho-Júnior, H.J.; Calvani, R.; Picca, A.; Gonçalves, I.O.; Landi, F.; Bernabei, R.; Cesari, M.; Uchida, M.C.; Marzetti, E. Protein-Related Dietary Parameters and Frailty Status in Older Community-Dwellers across Different Frailty Instruments. Nutrients 2020, 12, 508. [Google Scholar] [CrossRef]
- Lucas, M.; Goblet, C.; Keller, A.; Lamandé, N.; Gros, F.; Whalen, R.G.; Lazar, M. Modulation of embryonic and muscle-specific enolase gene products in the developing mouse hindlimb. Differentiation 1992, 51, 1–7. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2001, 56, M146–M157. [Google Scholar] [CrossRef]
- Rockwood, K.; Mitnitski, A. Frailty in relation to the accumulation of deficits. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2007, 62, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Arosio, B.; Ferri, E.; Casati, M.; Mari, D.; Vitale, G.; Cesari, M. The Frailty Index in centenarians and their offspring. Aging Clin. Exp. Res. 2019, 31, 1685–1688. [Google Scholar] [CrossRef]
- Arosio, B.; Geraci, A.; Ferri, E.; Mari, D.; Cesari, M. Biological Frailty Index in centenarians. Aging Clin. Exp. Res. 2022, 34, 687–690. [Google Scholar] [CrossRef]
- Oksuzyan, A.; Juel, K.; Vaupel, J.W.; Christensen, K. Men: Good health and high mortality. Sex differences in health and aging. Aging Clin. Exp. Res. 2008, 20, 91–102. [Google Scholar] [CrossRef]
- Thorslund, M.; Wastesson, J.W.; Agahi, N.; Lagergren, M.; Parker, M.G. The rise and fall of women’s advantage: A comparison of national trends in life expectancy at age 65 years. Eur. J. Ageing 2013, 10, 271–277. [Google Scholar] [CrossRef]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Siparsky, P.N.; Kirkendall, D.T.; Garrett, W.E. Muscle changes in aging: Understanding sarcopenia. Sports Health 2014, 6, 36–40. [Google Scholar] [CrossRef]
- Vandervoot, A.A.; Symons, T.B. Functional and metabolic consequences of sarcopenia. Can. J. Appl. Physiol. 2001, 26, 90–101. [Google Scholar] [CrossRef]
- Clark, B.C. Neuromuscular Changes with Aging and Sarcopenia. J. Frailty Aging 2019, 8, 7–9. [Google Scholar] [CrossRef]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. J. Nutr. 1997, 127, 990S–991S. [Google Scholar] [CrossRef]
- Baker, B.A. Efficacy of Age-Specific High-Intensity Stretch-Shortening Contractions in Reversing Dynapenia, Sarcopenia, and Loss of Skeletal Muscle Quality. J. Funct. Morphol. Kinesiol. 2018, 3, 36. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M. Sarcopenia =/= dynapenia. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2008, 63, 829–834. [Google Scholar] [CrossRef]
- Clark, B.C.; Manini, T.M. What is dynapenia? Nutrition 2012, 28, 495–503. [Google Scholar] [CrossRef]
- Morley, J.E.; Abbatecola, A.M.; Argiles, J.M.; Baracos, V.; Bauer, J.; Bhasin, S.; Cederholm, T.; Coats, A.J.S.; Cummings, S.R.; Evans, W.J.; et al. Sarcopenia with limited mobility: An international consensus. J. Am. Med. Dir. Assoc. 2011, 12, 403–409. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Anker, S.D.; Morley, J.E.; von Haehling, S. Welcome to the ICD-10 code for sarcopenia. J. Cachexia Sarcopenia Muscle 2016, 7, 512–514. [Google Scholar] [CrossRef]
- Vellas, B.; Fielding, R.A.; Bens, C.; Bernabei, R.; Cawthon, P.M.; Cederholm, T.; Cruz-Jentoft, A.J.; Del Signore, S.; Donahue, S.; Morley, J.; et al. Implications of ICD-10 for Sarcopenia Clinical Practice and Clinical Trials: Report by the International Conference on Frailty and Sarcopenia Research Task Force. J. Frailty Aging 2018, 7, 2–9. [Google Scholar] [CrossRef]
- Malafarina, V.; Uriz-Otano, F.; Iniesta, R.; Gil-Guerrero, L. Sarcopenia in the elderly: Diagnosis, physiopathology and treatment. Maturitas 2012, 71, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, L.; Hojman, P. Muscle-to-organ cross talk mediated by myokines. Adipocyte 2012, 1, 164–167. [Google Scholar] [CrossRef] [PubMed]
- Pagano, A.F.; Brioche, T.; Arc-Chagnaud, C.; Demangel, R.; Chopard, A.; Py, G. Short-term disuse promotes fatty acid infiltration into skeletal muscle. J. Cachexia Sarcopenia Muscle 2018, 9, 335–347. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2017, 8, 1960. [Google Scholar] [CrossRef]
- Sui, S.X.; Williams, L.J.; Holloway-Kew, K.L.; Hyde, N.K.; Pasco, J.A. Skeletal Muscle Health and Cognitive Function: A Narrative Review. Int. J. Mol. Sci. 2020, 22, 255. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Bernabei, R.; Onder, G.; Marzetti, E. Sarcopenia as the Biological Substrate of Physical Frailty. Clin. Geriatr. Med. 2015, 31, 367–374. [Google Scholar] [CrossRef]
- Landi, F.; Calvani, R.; Cesari, M.; Tosato, M.; Martone, A.M.; Ortolani, E.; Savera, G.; Salini, S.; Sisto, A.; Picca, A.; et al. Sarcopenia: An Overview on Current Definitions, Diagnosis and Treatment. Curr. Protein Pept. Sci. 2018, 19, 633–638. [Google Scholar] [CrossRef]
- Marzetti, E.; Calvani, R.; Cesari, M.; Tosato, M.; Cherubini, A.; Di Bari, M.; Pahor, M.; Savera, G.; Collamati, A.; D’Angelo, E.; et al. Operationalization of the physical frailty & sarcopenia syndrome: Rationale and clinical implementation. Transl. Med. UniSa 2015, 13, 29–32. [Google Scholar] [PubMed]
- Guerville, F.; De Souto Barreto, P.; Ader, I.; Andrieu, S.; Casteilla, L.; Dray, C.; Fazilleau, N.; Guyonnet, S.; Langin, D.; Liblau, R.; et al. Revisiting the Hallmarks of Aging to Identify Markers of Biological Age. J. Prev. Alzheimers Dis. 2020, 7, 56–64. [Google Scholar] [CrossRef]
- Tagliaferri, C.; Wittrant, Y.; Davicco, M.-J.; Walrand, S.; Coxam, V. Muscle and bone, two interconnected tissues. Ageing Res. Rev. 2015, 21, 55–70. [Google Scholar] [CrossRef]
- Florin, A.; Lambert, C.; Sanchez, C.; Zappia, J.; Durieux, N.; Tieppo, A.M.; Mobasheri, A.; Henrotin, Y. The secretome of skeletal muscle cells: A systematic review. Osteoarthr. Cartil. Open 2020, 2, 100019. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Xue, Q.-L.; Cappola, A.R.; Ferrucci, L.; Chaves, P.; Varadhan, R.; Guralnik, J.M.; Leng, S.X.; Semba, R.D.; Walston, J.D.; et al. Nonlinear multisystem physiological dysregulation associated with frailty in older women: Implications for etiology and treatment. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2009, 64, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.C.; Walston, J. CMV Infection and Frailty: Immunologic Consequences and Disease Pathogenesis. In Handbook on Immunosenescence: Basic Understanding and Clinical Applications; Springer: Dordrecht, The Netherlands, 2009; Volume 9781402090639, pp. 1305–1326. ISBN 978-1-4020-9062-2. [Google Scholar]
- Yu, Z.; Ruan, Q.; D’Onofrio, G.; Greco, A. From Sarcopenia to Frailty: The Pathophysiological Basis and Potential Target Molecules of Intervention. Frailty Sarcopenia-Onset Dev. Clin. Chall 2017, 3, 55–69. [Google Scholar]
- Fulop, T.; Larbi, A.; Witkowski, J.M.; McElhaney, J.; Loeb, M.; Mitnitski, A.; Pawelec, G. Aging, frailty and age-related diseases. Biogerontology 2010, 11, 547–563. [Google Scholar] [CrossRef]
- Ruan, Q.; D’Onofrio, G.; Sancarlo, D.; Greco, A.; Lozupone, M.; Seripa, D.; Panza, F.; Yu, Z. Emerging biomarkers and screening for cognitive frailty. Aging Clin. Exp. Res. 2017, 29, 1075–1086. [Google Scholar] [CrossRef]
- Borras, C.; Ingles, M.; Mas-Bargues, C.; Dromant, M.; Sanz-Ros, J.; Román-Domínguez, A.; Gimeno-Mallench, L.; Gambini, J.; Viña, J. Centenarians: An excellent example of resilience for successful ageing. Mech. Ageing Dev. 2020, 186, 111199. [Google Scholar] [CrossRef]
- Yao, X.; Li, H.; Leng, S.X. Inflammation and immune system alterations in frailty. Clin. Geriatr. Med. 2011, 27, 79–87. [Google Scholar] [CrossRef]
- Wertheimer, A.M.; Bennett, M.S.; Park, B.; Uhrlaub, J.L.; Martinez, C.; Pulko, V.; Currier, N.L.; Nikolich-Žugich, D.; Kaye, J.; Nikolich-Žugich, J. Aging and cytomegalovirus infection differentially and jointly affect distinct circulating T cell subsets in humans. J. Immunol. 2014, 192, 2143–2155. [Google Scholar] [CrossRef]
- Pawelec, G.; Derhovanessian, E. Role of CMV in immune senescence. Virus Res. 2011, 157, 175–179. [Google Scholar] [CrossRef]
- Ying, L.; Zhang, Q.; Yang, Y.-M.; Zhou, J.-Y. A Combination of Serum Biomarkers in Elderly Patients with Sarcopenia: A Cross-Sectional Observational Study. Int. J. Endocrinol. 2022, 2022, 4026940. [Google Scholar] [CrossRef]
- Rong, Y.-D.; Bian, A.-L.; Hu, H.-Y.; Ma, Y.; Zhou, X.-Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef] [PubMed]
- Bian, A.-L.; Hu, H.-Y.; Rong, Y.-D.; Wang, J.; Wang, J.-X.; Zhou, X.-Z. A study on relationship between elderly sarcopenia and inflammatory factors IL-6 and TNF-α. Eur. J. Med. Res. 2017, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Reuben, D.B.; Cheh, A.I.; Harris, T.B.; Ferrucci, L.; Rowe, J.W.; Tracy, R.P.; Seeman, T.E. Peripheral blood markers of inflammation predict mortality and functional decline in high-functioning community-dwelling older persons. J. Am. Geriatr. Soc. 2002, 50, 638–644. [Google Scholar] [CrossRef] [PubMed]
- Visser, M.; Pahor, M.; Taaffe, D.R.; Goodpaster, B.H.; Simonsick, E.M.; Newman, A.B.; Nevitt, M.; Harris, T.B. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: The Health ABC Study. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2002, 57, M326–M332. [Google Scholar] [CrossRef]
- Ershler, W.B. Interleukin-6: A cytokine for gerontologists. J. Am. Geriatr. Soc. 1993, 41, 176–181. [Google Scholar] [CrossRef]
- Maggio, M.; Guralnik, J.M.; Longo, D.L.; Ferrucci, L. Interleukin-6 in aging and chronic disease: A magnificent pathway. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2006, 61, 575–584. [Google Scholar] [CrossRef]
- Harris, T.B.; Ferrucci, L.; Tracy, R.P.; Corti, M.C.; Wacholder, S.; Ettinger, W.H.; Heimovitz, H.; Cohen, H.J.; Wallace, R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am. J. Med. 1999, 106, 506–512. [Google Scholar] [CrossRef]
- Ferrucci, L.; Penninx, B.W.J.H.; Volpato, S.; Harris, T.B.; Bandeen-Roche, K.; Balfour, J.; Leveille, S.G.; Fried, L.P.; Md, J.M.G. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J. Am. Geriatr. Soc. 2002, 50, 1947–1954. [Google Scholar] [CrossRef]
- Leng, S.; Chaves, P.; Koenig, K.; Walston, J. Serum interleukin-6 and hemoglobin as physiological correlates in the geriatric syndrome of frailty: A pilot study. J. Am. Geriatr. Soc. 2002, 50, 1268–1271. [Google Scholar] [CrossRef]
- Heinze-Milne, S.D.; Banga, S.; Howlett, S.E. Frailty and cytokines in preclinical models: Comparisons with humans. Mech. Ageing Dev. 2022, 206, 111706. [Google Scholar] [CrossRef]
- Tembo, M.C.; Holloway-Kew, K.L.; Bortolasci, C.C.; Brennan-Olsen, S.L.; Williams, L.J.; Kotowicz, M.A.; Pasco, J.A. Association between serum interleukin-6 and frailty in older men: Cross-sectional data. Eur. Geriatr. Med. 2021, 12, 887–892. [Google Scholar] [CrossRef]
- Semmarath, W.; Seesen, M.; Yodkeeree, S.; Sapbamrer, R.; Ayood, P.; Malasao, R.; Siviroj, P.; Limtrakul Dejkriengkraikul, P. The Association between Frailty Indicators and Blood-Based Biomarkers in Early-Old Community Dwellers of Thailand. Int. J. Environ. Res. Public Health 2019, 16, 3457. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, N.; Kadambi, S.; Lei, L.; Loh, K.P.; Mohamed, M.; Magnuson, A.; Cole, S.; Esparaz, B.T.; Giguere, J.K.; Mohile, S.; et al. Associations of inflammation with frailty in patients with breast cancer aged 50 and over receiving chemotherapy. J. Geriatr. Oncol. 2020, 11, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Boxer, R.S.; Dauser, D.A.; Walsh, S.J.; Hager, W.D.; Kenny, A.M. The association between vitamin D and inflammation with the 6-minute walk and frailty in patients with heart failure. J. Am. Geriatr. Soc. 2008, 56, 454–461. [Google Scholar] [CrossRef]
- Samson, L.D.; Buisman, A.-M.; Ferreira, J.A.; Picavet, H.S.J.; Verschuren, W.M.M.; Boots, A.M.; Engelfriet, P. Inflammatory marker trajectories associated with frailty and ageing in a 20-year longitudinal study. Clin. Transl. Immunol. 2022, 11, e1374. [Google Scholar] [CrossRef]
- Leng, S.X.; Yang, H.; Walston, J.D. Decreased cell proliferation and altered cytokine production in frail older adults. Aging Clin. Exp. Res. 2004, 16, 249–252. [Google Scholar] [CrossRef]
- Qu, T.; Walston, J.D.; Yang, H.; Fedarko, N.S.; Xue, Q.-L.; Beamer, B.A.; Ferrucci, L.; Rose, N.R.; Leng, S.X. Upregulated ex vivo expression of stress-responsive inflammatory pathway genes by LPS-challenged CD14(+) monocytes in frail older adults. Mech. Ageing Dev. 2009, 130, 161–166. [Google Scholar] [CrossRef]
- Schmaltz, H.N.; Fried, L.P.; Xue, Q.-L.; Walston, J.; Leng, S.X.; Semba, R.D. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J. Am. Geriatr. Soc. 2005, 53, 747–754. [Google Scholar] [CrossRef]
- Kawamura, N.; Ohnuki, Y.; Matsuo, I.; Suita, K.; Ishikawa, M.; Mototani, Y.; Shiozawa, K.; Ito, A.; Yagisawa, Y.; Hayakawa, Y.; et al. Effects of chronic Porphyromonas gingivalis lipopolysaccharide infusion on skeletal muscles in mice. J. Physiol. Sci. 2019, 69, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Liccardo, D.; Cannavo, A.; Spagnuolo, G.; Ferrara, N.; Cittadini, A.; Rengo, C.; Rengo, G. Periodontal Disease: A Risk Factor for Diabetes and Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 1414. [Google Scholar] [CrossRef] [PubMed]
- Del Giudice, C.; Vaia, E.; Liccardo, D.; Marzano, F.; Valletta, A.; Spagnuolo, G.; Ferrara, N.; Rengo, C.; Cannavo, A.; Rengo, G. Infective Endocarditis: A Focus on Oral Microbiota. Microorganisms 2021, 9, 1218. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.; Jackson, T.; Sapey, E.; Lord, J.M. Frailty and sarcopenia: The potential role of an aged immune system. Ageing Res. Rev. 2017, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Murton, A.J.; Maddocks, M.; Stephens, F.B.; Marimuthu, K.; England, R.; Wilcock, A. Consequences of Late-Stage Non-Small-Cell Lung Cancer Cachexia on Muscle Metabolic Processes. Clin. Lung Cancer 2017, 18, e1–e11. [Google Scholar] [CrossRef] [PubMed]
- Crossland, H.; Constantin-Teodosiu, D.; Gardiner, S.M.; Constantin, D.; Greenhaff, P.L. A potential role for Akt/FOXO signalling in both protein loss and the impairment of muscle carbohydrate oxidation during sepsis in rodent skeletal muscle. J. Physiol. 2008, 586, 5589–5600. [Google Scholar] [CrossRef] [PubMed]
- Kamper, R.S.; Alcazar, J.; Andersen, L.L.; Haddock, B.; Jørgensen, N.R.; Hovind, P.; Suetta, C. Associations between inflammatory markers, body composition, and physical function: The Copenhagen Sarcopenia Study. J. Cachexia Sarcopenia Muscle 2021, 12, 1641–1652. [Google Scholar] [CrossRef]
- Suetta, C.; Haddock, B.; Alcazar, J.; Noerst, T.; Hansen, O.M.; Ludvig, H.; Kamper, R.S.; Schnohr, P.; Prescott, E.; Andersen, L.L.; et al. The Copenhagen Sarcopenia Study: Lean mass, strength, power, and physical function in a Danish cohort aged 20–93 years. J. Cachexia Sarcopenia Muscle 2019, 10, 1316–1329. [Google Scholar] [CrossRef]
- Pijet, B.; Pijet, M.; Litwiniuk, A.; Gajewska, M.; Pająk, B.; Orzechowski, A. TNF- α and IFN-s-dependent muscle decay is linked to NF-κB- and STAT-1α-stimulated Atrogin1 and MuRF1 genes in C2C12 myotubes. Mediat. Inflamm. 2013, 2013, 171437. [Google Scholar] [CrossRef]
- Ladner, K.J.; Caligiuri, M.A.; Guttridge, D.C. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J. Biol. Chem. 2003, 278, 2294–2303. [Google Scholar] [CrossRef]
- Li, H.; Malhotra, S.; Kumar, A. Nuclear factor-kappa B signaling in skeletal muscle atrophy. J. Mol. Med. 2008, 86, 1113–1126. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Hydrogen Sulfide Regulates Irisin and Glucose Metabolism in Myotubes and Muscle of HFD-Fed Diabetic Mice. Antioxidants 2022, 11, 1369. [Google Scholar] [CrossRef]
- Zhu, W.; Sahar, N.E.; Javaid, H.M.A.; Pak, E.S.; Liang, G.; Wang, Y.; Ha, H.; Huh, J.Y. Exercise-Induced Irisin Decreases Inflammation and Improves NAFLD by Competitive Binding with MD2. Cells 2021, 10, 3306. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gil, A.M.; Elizondo-Montemayor, L. The Role of Exercise in the Interplay between Myokines, Hepatokines, Osteokines, Adipokines, and Modulation of Inflammation for Energy Substrate Redistribution and Fat Mass Loss: A Review. Nutrients 2020, 12, 1899. [Google Scholar] [CrossRef]
- Doi, T.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Makino, K.; Suzuki, T.; Shimada, H. Association between Insulin-Like Growth Factor-1 and Frailty among Older Adults. J. Nutr. Health Aging 2018, 22, 68–72. [Google Scholar] [CrossRef]
- Bian, A.; Ma, Y.; Zhou, X.; Guo, Y.; Wang, W.; Zhang, Y.; Wang, X. Association between sarcopenia and levels of growth hormone and insulin-like growth factor-1 in the elderly. BMC Musculoskelet. Disord. 2020, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, M.I.; Khater, M.S. Evaluation of insulin like growth factor-1 (IGF-1) level and its impact on muscle and bone mineral density in frail elderly male. Arch. Gerontol. Geriatr. 2015, 60, 124–127. [Google Scholar] [CrossRef] [PubMed]
- van Nieuwpoort, I.C.; Vlot, M.C.; Schaap, L.A.; Lips, P.; Drent, M.L. The relationship between serum IGF-1, handgrip strength, physical performance and falls in elderly men and women. Eur. J. Endocrinol. 2018, 179, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Cappola, A.R.; Xue, Q.-L.; Ferrucci, L.; Guralnik, J.M.; Volpato, S.; Fried, L.P. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J. Clin. Endocrinol. Metab. 2003, 88, 2019–2025. [Google Scholar] [CrossRef]
- Doi, T.; Shimada, H.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Nakakubo, S.; Suzuki, T. Insulin-Like Growth Factor-1 Related to Disability Among Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 797–802. [Google Scholar] [CrossRef]
- Carnac, G.; Vernus, B.; Bonnieu, A. Myostatin in the pathophysiology of skeletal muscle. Curr. Genom. 2007, 8, 415–422. [Google Scholar] [CrossRef]
- Scharf, G.; Heineke, J. Finding good biomarkers for sarcopenia. J. Cachexia Sarcopenia Muscle 2012, 3, 145–148. [Google Scholar] [CrossRef]
- White, T.A.; LeBrasseur, N.K. Myostatin and sarcopenia: Opportunities and challenges—A mini-review. Gerontology 2014, 60, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Léger, B.; Derave, W.; De Bock, K.; Hespel, P.; Russell, A.P. Human sarcopenia reveals an increase in SOCS-3 and myostatin and a reduced efficiency of Akt phosphorylation. Rejuvenation Res. 2008, 11, 163–175B. [Google Scholar] [CrossRef]
- Yarasheski, K.E.; Bhasin, S.; Sinha-Hikim, I.; Pak-Loduca, J.; Gonzalez-Cadavid, N.F. Serum myostatin-immunoreactive protein is increased in 60-92 year old women and men with muscle wasting. J. Nutr. Health Aging 2002, 6, 343–348. [Google Scholar] [PubMed]
- Arrieta, H.; Hervás, G.; Rezola-Pardo, C.; Ruiz-Litago, F.; Iturburu, M.; Yanguas, J.J.; Gil, S.M.; Rodriguez-Larrad, A.; Irazusta, J. Serum Myostatin Levels Are Higher in Fitter, More Active, and Non-Frail Long-Term Nursing Home Residents and Increase after a Physical Exercise Intervention. Gerontology 2019, 65, 229–239. [Google Scholar] [CrossRef]
- Peng, L.-N.; Lee, W.-J.; Liu, L.-K.; Lin, M.-H.; Chen, L.-K. Healthy community-living older men differ from women in associations between myostatin levels and skeletal muscle mass. J. Cachexia Sarcopenia Muscle 2018, 9, 635–642. [Google Scholar] [CrossRef]
- Fife, E.; Kostka, J.; Kroc, Ł.; Guligowska, A.; Pigłowska, M.; Sołtysik, B.; Kaufman-Szymczyk, A.; Fabianowska-Majewska, K.; Kostka, T. Relationship of muscle function to circulating myostatin, follistatin and GDF11 in older women and men. BMC Geriatr. 2018, 18, 200. [Google Scholar] [CrossRef]
- Ratkevicius, A.; Joyson, A.; Selmer, I.; Dhanani, T.; Grierson, C.; Tommasi, A.M.; DeVries, A.; Rauchhaus, P.; Crowther, D.; Alesci, S.; et al. Serum concentrations of myostatin and myostatin-interacting proteins do not differ between young and sarcopenic elderly men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2011, 66, 620–626. [Google Scholar] [CrossRef]
- Bergen, H.R.; Farr, J.N.; Vanderboom, P.M.; Atkinson, E.J.; White, T.A.; Singh, R.J.; Khosla, S.; LeBrasseur, N.K. Myostatin as a mediator of sarcopenia versus homeostatic regulator of muscle mass: Insights using a new mass spectrometry-based assay. Skelet. Muscle 2015, 5, 21. [Google Scholar] [CrossRef]
- Chew, J.; Tay, L.; Lim, J.P.; Leung, B.P.; Yeo, A.; Yew, S.; Ding, Y.Y.; Lim, W.S. Serum Myostatin and IGF-1 as Gender-Specific Biomarkers of Frailty and Low Muscle Mass in Community-Dwelling Older Adults. J. Nutr. Health Aging 2019, 23, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Furihata, T.; Kinugawa, S.; Fukushima, A.; Takada, S.; Homma, T.; Masaki, Y.; Abe, T.; Yokota, T.; Oba, K.; Okita, K.; et al. Serum myostatin levels are independently associated with skeletal muscle wasting in patients with heart failure. Int. J. Cardiol. 2016, 220, 483–487. [Google Scholar] [CrossRef]
- Huh, J.Y.; Panagiotou, G.; Mougios, V.; Brinkoetter, M.; Vamvini, M.T.; Schneider, B.E.; Mantzoros, C.S. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 2012, 61, 1725–1738. [Google Scholar] [CrossRef] [PubMed]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovic, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef] [PubMed]
- Paris, M.T.; Bell, K.E.; Mourtzakis, M. Myokines and adipokines in sarcopenia: Understanding cross-talk between skeletal muscle and adipose tissue and the role of exercise. Curr. Opin. Pharmacol. 2020, 52, 61–66. [Google Scholar] [CrossRef]
- Reza, M.M.; Subramaniyam, N.; Sim, C.M.; Ge, X.; Sathiakumar, D.; McFarlane, C.; Sharma, M.; Kambadur, R. Irisin is a pro-myogenic factor that induces skeletal muscle hypertrophy and rescues denervation-induced atrophy. Nat. Commun. 2017, 8, 1104. [Google Scholar] [CrossRef]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.-S.; Kim, N.; Kong, I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef]
- Fossati, C.; Papalia, R.; Torre, G.; Vadalà, G.; Borrione, P.; Grazioli, E.; Mazzola, C.; Parisi, A.; Pigozzi, F.; Denaro, V. Frailty of the elderly in orthopaedic surgery and body composition changes: The musculoskeletal crosstalk through irisin. J. Biol. Regul. Homeost. Agents 2020, 34, 327–335. [Google Scholar]
- Nakatani, M.; Takehara, Y.; Sugino, H.; Matsumoto, M.; Hashimoto, O.; Hasegawa, Y.; Murakami, T.; Uezumi, A.; Takeda, S.; Noji, S.; et al. Transgenic expression of a myostatin inhibitor derived from follistatin increases skeletal muscle mass and ameliorates dystrophic pathology in mdx mice. FASEB J. 2008, 22, 477–487. [Google Scholar] [CrossRef]
- Kota, J.; Handy, C.R.; Haidet, A.M.; Montgomery, C.L.; Eagle, A.; Rodino-Klapac, L.R.; Tucker, D.; Shilling, C.J.; Therlfall, W.R.; Walker, C.M.; et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci. Transl. Med. 2009, 1, 6ra15. [Google Scholar] [CrossRef]
- Amthor, H.; Nicholas, G.; McKinnell, I.; Kemp, C.F.; Sharma, M.; Kambadur, R.; Patel, K. Follistatin complexes Myostatin and antagonises Myostatin-mediated inhibition of myogenesis. Dev. Biol. 2004, 270, 19–30. [Google Scholar] [CrossRef]
- Yaden, B.C.; Croy, J.E.; Wang, Y.; Wilson, J.M.; Datta-Mannan, A.; Shetler, P.; Milner, A.; Bryant, H.U.; Andrews, J.; Dai, G.; et al. Follistatin: A novel therapeutic for the improvement of muscle regeneration. J. Pharmacol. Exp. Ther. 2014, 349, 355–371. [Google Scholar] [CrossRef] [PubMed]
- Sepulveda, P.V.; Lamon, S.; Hagg, A.; Thomson, R.E.; Winbanks, C.E.; Qian, H.; Bruce, C.R.; Russell, A.P.; Gregorevic, P. Evaluation of follistatin as a therapeutic in models of skeletal muscle atrophy associated with denervation and tenotomy. Sci. Rep. 2015, 5, 17535. [Google Scholar] [CrossRef] [PubMed]
- Mendell, J.R.; Sahenk, Z.; Al-Zaidy, S.; Rodino-Klapac, L.R.; Lowes, L.P.; Alfano, L.N.; Berry, K.; Miller, N.; Yalvac, M.; Dvorchik, I.; et al. Follistatin Gene Therapy for Sporadic Inclusion Body Myositis Improves Functional Outcomes. Mol. Ther. 2017, 25, 870–879. [Google Scholar] [CrossRef] [PubMed]
- Negaresh, R.; Ranjbar, R.; Baker, J.S.; Habibi, A.; Mokhtarzade, M.; Gharibvand, M.M.; Fokin, A. Skeletal Muscle Hypertrophy, Insulin-like Growth Factor 1, Myostatin and Follistatin in Healthy and Sarcopenic Elderly Men: The Effect of Whole-body Resistance Training. Int. J. Prev. Med. 2019, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Echeverria, I.; Besga, A.; Sanz, B.; Amasene, M.; Hervás, G.; Barroso, J.; Rodriguez-Larrad, A.; Irazusta, J. Identification of frailty and sarcopenia in hospitalised older people. Eur. J. Clin. Investig. 2021, 51, e13420. [Google Scholar] [CrossRef] [PubMed]
- Bollen, S.E.; Bass, J.J.; Fujita, S.; Wilkinson, D.; Hewison, M.; Atherton, P.J. The Vitamin D/Vitamin D receptor (VDR) axis in muscle atrophy and sarcopenia. Cell Signal 2022, 96, 110355. [Google Scholar] [CrossRef]
- Köller, M. Sarcopenia-a geriatric pandemic: A narrative review. Wien. Med. Wochenschr. 2022. [Google Scholar] [CrossRef]
- Bischoff-Ferrari, H.A.; Borchers, M.; Gudat, F.; Dürmüller, U.; Stähelin, H.B.; Dick, W. Vitamin D receptor expression in human muscle tissue decreases with age. J. Bone Miner. Res. 2004, 19, 265–269. [Google Scholar] [CrossRef]
- Bollen, S.E.; Atherton, P.J. Myogenic, genomic and non-genomic influences of the vitamin D axis in skeletal muscle. Cell Biochem. Funct. 2021, 39, 48–59. [Google Scholar] [CrossRef]
- Wicherts, I.S.; van Schoor, N.M.; Boeke, A.J.P.; Visser, M.; Deeg, D.J.H.; Smit, J.; Knol, D.L.; Lips, P. Vitamin D status predicts physical performance and its decline in older persons. J. Clin. Endocrinol. Metab. 2007, 92, 2058–2065. [Google Scholar] [CrossRef]
- Yu, S.; Ren, B.; Chen, H.; Goltzman, D.; Yan, J.; Miao, D. 1,25-Dihydroxyvitamin D deficiency induces sarcopenia by inducing skeletal muscle cell senescence. Am. J. Transl. Res. 2021, 13, 12638–12649. [Google Scholar] [PubMed]
- Parsanathan, R.; Achari, A.E.; Manna, P.; Jain, S.K. l-Cysteine and Vitamin D Co-Supplementation Alleviates Markers of Musculoskeletal Disorders in Vitamin D-Deficient High-Fat Diet-Fed Mice. Nutrients 2020, 12, 3406. [Google Scholar] [CrossRef] [PubMed]
- Glendenning, P.; Zhu, K.; Inderjeeth, C.; Howat, P.; Lewis, J.R.; Prince, R.L. Effects of three-monthly oral 150,000 IU cholecalciferol supplementation on falls, mobility, and muscle strength in older postmenopausal women: A randomized controlled trial. J. Bone Miner. Res. 2012, 27, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Austin, N.; Devine, A.; Bruce, D.; Prince, R.L. A randomized controlled trial of the effects of vitamin D on muscle strength and mobility in older women with vitamin D insufficiency. J. Am. Geriatr. Soc. 2010, 58, 2063–2068. [Google Scholar] [CrossRef]
- Gao, L.; Zhu, W.; Liu, Y.; Gu, J.; Zhang, Z.; Wang, O.; Xing, X.; Xu, L. Physical performance and life quality in postmenopausal women supplemented with vitamin D: A two-year prospective study. Acta Pharmacol. Sin. 2015, 36, 1065–1073. [Google Scholar] [CrossRef]
- Gkekas, N.K.; Anagnostis, P.; Paraschou, V.; Stamiris, D.; Dellis, S.; Kenanidis, E.; Potoupnis, M.; Tsiridis, E.; Goulis, D.G. The effect of vitamin D plus protein supplementation on sarcopenia: A systematic review and meta-analysis of randomized controlled trials. Maturitas 2021, 145, 56–63. [Google Scholar] [CrossRef]
- Angulo, J.; El Assar, M.; Rodríguez-Mañas, L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol. Asp. Med. 2016, 50, 1–32. [Google Scholar] [CrossRef]
- Meng, S.-J.; Yu, L.-J. Oxidative stress, molecular inflammation and sarcopenia. Int. J. Mol. Sci. 2010, 11, 1509–1526. [Google Scholar] [CrossRef]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef]
- Mecocci, P.; Fanó, G.; Fulle, S.; MacGarvey, U.; Shinobu, L.; Polidori, M.C.; Cherubini, A.; Vecchiet, J.; Senin, U.; Beal, M.F. Age-dependent increases in oxidative damage to DNA, lipids, and proteins in human skeletal muscle. Free Radic. Biol. Med. 1999, 26, 303–308. [Google Scholar] [CrossRef]
- Scherz-Shouval, R.; Shvets, E.; Fass, E.; Shorer, H.; Gil, L.; Elazar, Z. Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J. 2007, 26, 1749–1760. [Google Scholar] [CrossRef]
- Sandiford, S.D.E.; Kennedy, K.A.M.; Xie, X.; Pickering, J.G.; Li, S.S.C. Dual oxidase maturation factor 1 (DUOXA1) overexpression increases reactive oxygen species production and inhibits murine muscle satellite cell differentiation. Cell Commun. Signal 2014, 12, 5. [Google Scholar] [CrossRef]
- Sakellariou, G.K.; Pearson, T.; Lightfoot, A.P.; Nye, G.A.; Wells, N.; Giakoumaki, I.I.; Vasilaki, A.; Griffiths, R.D.; Jackson, M.J.; McArdle, A. Mitochondrial ROS regulate oxidative damage and mitophagy but not age-related muscle fiber atrophy. Sci. Rep. 2016, 6, 33944. [Google Scholar] [CrossRef]
- Cannavo, A.; Liccardo, D.; Eguchi, A.; Elliott, K.J.; Traynham, C.J.; Ibetti, J.; Eguchi, S.; Leosco, D.; Ferrara, N.; Rengo, G.; et al. Myocardial pathology induced by aldosterone is dependent on non-canonical activities of G protein-coupled receptor kinases. Nat. Commun. 2016, 7, 10877. [Google Scholar] [CrossRef]
- Barbieri, E.; Sestili, P. Reactive oxygen species in skeletal muscle signaling. J. Signal Transduct. 2012, 2012, 982794. [Google Scholar] [CrossRef]
- Braga, M.; Sinha Hikim, A.P.; Datta, S.; Ferrini, M.G.; Brown, D.; Kovacheva, E.L.; Gonzalez-Cadavid, N.F.; Sinha-Hikim, I. Involvement of oxidative stress and caspase 2-mediated intrinsic pathway signaling in age-related increase in muscle cell apoptosis in mice. Apoptosis 2008, 13, 822–832. [Google Scholar] [CrossRef]
- Marzetti, E.; Wohlgemuth, S.E.; Lees, H.A.; Chung, H.-Y.; Giovannini, S.; Leeuwenburgh, C. Age-related activation of mitochondrial caspase-independent apoptotic signaling in rat gastrocnemius muscle. Mech. Ageing Dev. 2008, 129, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Yakes, F.M.; Van Houten, B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc. Natl. Acad. Sci. USA 1997, 94, 514–519. [Google Scholar] [CrossRef]
- Melov, S.; Shoffner, J.M.; Kaufman, A.; Wallace, D.C. Marked increase in the number and variety of mitochondrial DNA rearrangements in aging human skeletal muscle. Nucleic Acids Res. 1995, 23, 4122–4126. [Google Scholar] [CrossRef]
- Kovalenko, S.A.; Kopsidas, G.; Kelso, J.M.; Linnane, A.W. Deltoid human muscle mtDNA is extensively rearranged in old age subjects. Biochem. Biophys. Res. Commun. 1997, 232, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Liu, V.W.; Addessi, C.L.; Sheffield, D.A.; Linnane, A.W.; Nagley, P. Differential occurrence of mutations in mitochondrial DNA of human skeletal muscle during aging. Hum. Mutat. 1998, 11, 360–371. [Google Scholar] [CrossRef]
- Wanagat, J.; Cao, Z.; Pathare, P.; Aiken, J.M. Mitochondrial DNA deletion mutations colocalize with segmental electron transport system abnormalities, muscle fiber atrophy, fiber splitting, and oxidative damage in sarcopenia. FASEB J. 2001, 15, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Fayet, G.; Jansson, M.; Sternberg, D.; Moslemi, A.R.; Blondy, P.; Lombès, A.; Fardeau, M.; Oldfors, A. Ageing muscle: Clonal expansions of mitochondrial DNA point mutations and deletions cause focal impairment of mitochondrial function. Neuromuscul. Disord. 2002, 12, 484–493. [Google Scholar] [CrossRef]
- Yarovaya, N.O.; Kramarova, L.; Borg, J.; Kovalenko, S.A.; Caragounis, A.; Linnane, A.W. Age-related atrophy of rat soleus muscle is accompanied by changes in fibre type composition, bioenergy decline and mtDNA rearrangements. Biogerontology 2002, 3, 25–27. [Google Scholar] [CrossRef]
- Bua, E.A.; McKiernan, S.H.; Wanagat, J.; McKenzie, D.; Aiken, J.M. Mitochondrial abnormalities are more frequent in muscles undergoing sarcopenia. J. Appl. Physiol. 2002, 92, 2617–2624. [Google Scholar] [CrossRef] [PubMed]
- Herbst, A.; Prior, S.J.; Lee, C.C.; Aiken, J.M.; McKenzie, D.; Hoang, A.; Liu, N.; Chen, X.; Xun, P.; Allison, D.B.; et al. Skeletal muscle mitochondrial DNA copy number and mitochondrial DNA deletion mutation frequency as predictors of physical performance in older men and women. Geroscience 2021, 43, 1253–1264. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. G6PD deficiency shifts polarization of monocytes/macrophages towards a proinflammatory and profibrotic phenotype. Cell Mol. Immunol. 2021, 18, 770–772. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Li, S.; Hong, M.; Wang, X.; Feng, Y. The Reactive Oxygen Species in Macrophage Polarization: Reflecting Its Dual Role in Progression and Treatment of Human Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 2795090. [Google Scholar] [CrossRef]
- Kwon, D.H.; Lee, H.; Park, C.; Hong, S.-H.; Hong, S.H.; Kim, G.-Y.; Cha, H.-J.; Kim, S.; Kim, H.-S.; Hwang, H.-J.; et al. Glutathione Induced Immune-Stimulatory Activity by Promoting M1-Like Macrophages Polarization via Potential ROS Scavenging Capacity. Antioxidants 2019, 8, 413. [Google Scholar] [CrossRef]
- Pérez, S.; Rius-Pérez, S. Macrophage Polarization and Reprogramming in Acute Inflammation: A Redox Perspective. Antioxidants 2022, 11, 1394. [Google Scholar] [CrossRef]
- Arnold, L.; Henry, A.; Poron, F.; Baba-Amer, Y.; van Rooijen, N.; Plonquet, A.; Gherardi, R.K.; Chazaud, B. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007, 204, 1057–1069. [Google Scholar] [CrossRef] [PubMed]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef] [PubMed]
- Siu, P.M.; Pistilli, E.E.; Alway, S.E. Age-dependent increase in oxidative stress in gastrocnemius muscle with unloading. J. Appl. Physiol. 2008, 105, 1695–1705. [Google Scholar] [CrossRef]
- Inglés, M.; Gambini, J.; Carnicero, J.A.; García-García, F.J.; Rodríguez-Mañas, L.; Olaso-González, G.; Dromant, M.; Borrás, C.; Viña, J. Oxidative stress is related to frailty, not to age or sex, in a geriatric population: Lipid and protein oxidation as biomarkers of frailty. J. Am. Geriatr. Soc. 2014, 62, 1324–1328. [Google Scholar] [CrossRef]
- El Assar, M.; Angulo, J.; Rodríguez-Mañas, L. Frailty as a phenotypic manifestation of underlying oxidative stress. Free Radic. Biol. Med. 2020, 149, 72–77. [Google Scholar] [CrossRef]
- Saum, K.-U.; Dieffenbach, A.K.; Jansen, E.H.J.M.; Schöttker, B.; Holleczek, B.; Hauer, K.; Brenner, H. Association between Oxidative Stress and Frailty in an Elderly German Population: Results from the ESTHER Cohort Study. Gerontology 2015, 61, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Howard, C.; Ferrucci, L.; Sun, K.; Fried, L.P.; Walston, J.; Varadhan, R.; Guralnik, J.M.; Semba, R.D. Oxidative protein damage is associated with poor grip strength among older women living in the community. J. Appl. Physiol. 2007, 103, 17–20. [Google Scholar] [CrossRef]
- Serviddio, G.; Romano, A.D.; Greco, A.; Rollo, T.; Bellanti, F.; Altomare, E.; Vendemiale, G. Frailty syndrome is associated with altered circulating redox balance and increased markers of oxidative stress. Int. J. Immunopathol. Pharmacol. 2009, 22, 819–827. [Google Scholar] [CrossRef]
- Bellanti, F.; Romano, A.D.; Lo Buglio, A.; Castriotta, V.; Guglielmi, G.; Greco, A.; Serviddio, G.; Vendemiale, G. Oxidative stress is increased in sarcopenia and associated with cardiovascular disease risk in sarcopenic obesity. Maturitas 2018, 109, 6–12. [Google Scholar] [CrossRef]
- Bernabeu-Wittel, M.; Gómez-Díaz, R.; González-Molina, Á.; Vidal-Serrano, S.; Díez-Manglano, J.; Salgado, F.; Soto-Martín, M.; Ollero-Baturone, M.; On Behalf Of The Proteo Researchers. Oxidative Stress, Telomere Shortening, and Apoptosis Associated to Sarcopenia and Frailty in Patients with Multimorbidity. J. Clin. Med. 2020, 9, 2669. [Google Scholar] [CrossRef]
- Sandonà, D.; Desaphy, J.-F.; Camerino, G.M.; Bianchini, E.; Ciciliot, S.; Danieli-Betto, D.; Dobrowolny, G.; Furlan, S.; Germinario, E.; Goto, K.; et al. Adaptation of mouse skeletal muscle to long-term microgravity in the MDS mission. PLoS ONE 2012, 7, e33232. [Google Scholar] [CrossRef] [PubMed]
- Comfort, P.; McMahon, J.J.; Jones, P.A.; Cuthbert, M.; Kendall, K.; Lake, J.P.; Haff, G.G. Effects of Spaceflight on Musculoskeletal Health: A Systematic Review and Meta-analysis, Considerations for Interplanetary Travel. Sports Med. 2021, 51, 2097–2114. [Google Scholar] [CrossRef] [PubMed]
- Stein, T.P.; Gaprindashvili, T. Spaceflight and protein metabolism, with special reference to humans. Am. J. Clin. Nutr. 1994, 60, 806S–819S. [Google Scholar] [CrossRef]
- Tanaka, K.; Nishimura, N.; Kawai, Y. Adaptation to microgravity, deconditioning, and countermeasures. J. Physiol. Sci. 2017, 67, 271–281. [Google Scholar] [CrossRef]
- LeBlanc, A.D.; Spector, E.R.; Evans, H.J.; Sibonga, J.D. Skeletal responses to space flight and the bed rest analog: A review. J. Musculoskelet. Neuronal Interact. 2007, 7, 33–47. [Google Scholar]
- Narici, M.V.; de Boer, M.D. Disuse of the musculo-skeletal system in space and on earth. Eur. J. Appl. Physiol. 2011, 111, 403–420. [Google Scholar] [CrossRef]
- Laurens, C.; Simon, C.; Vernikos, J.; Gauquelin-Koch, G.; Blanc, S.; Bergouignan, A. Revisiting the Role of Exercise Countermeasure on the Regulation of Energy Balance During Space Flight. Front. Physiol. 2019, 10, 321. [Google Scholar] [CrossRef]
- Stein, T.P. The relationship between dietary intake, exercise, energy balance and the space craft environment. Pflügers Arch. Eur. J. Physiol. 2000, 441, R21–R31. [Google Scholar] [CrossRef]
- Stein, T.P.; Leskiw, M.J.; Schluter, M.D.; Hoyt, R.W.; Lane, H.W.; Gretebeck, R.E.; LeBlanc, A.D. Energy expenditure and balance during spaceflight on the space shuttle. Am. J. Physiol. 1999, 276, R1739–R1748. [Google Scholar] [CrossRef]
- Xia, Z.; Cholewa, J.; Zhao, Y.; Shang, H.-Y.; Yang, Y.-Q.; Araújo Pessôa, K.; Su, Q.-S.; Lima-Soares, F.; Zanchi, N.E. Targeting Inflammation and Downstream Protein Metabolism in Sarcopenia: A Brief Up-Dated Description of Concurrent Exercise and Leucine-Based Multimodal Intervention. Front. Physiol. 2017, 8, 434. [Google Scholar] [CrossRef] [PubMed]
- Hargens, A.R.; Vico, L. Long-duration bed rest as an analog to microgravity. J. Appl. Physiol. 2016, 120, 891–903. [Google Scholar] [CrossRef] [PubMed]
- Rundfeldt, L.C.; Gunga, H.C.; Steinach, M. Anabolic signaling and response in sarcopenia as a model for microgravity induced muscle deconditioning: A systematic review. REACH 2019, 13, 100025. [Google Scholar] [CrossRef]
- LeBlanc, A.; Lin, C.; Shackelford, L.; Sinitsyn, V.; Evans, H.; Belichenko, O.; Schenkman, B.; Kozlovskaya, I.; Oganov, V.; Bakulin, A.; et al. Muscle volume, MRI relaxation times (T2), and body composition after spaceflight. J. Appl. Physiol. 2000, 89, 2158–2164. [Google Scholar] [CrossRef] [PubMed]
- Fitts, R.H.; Trappe, S.W.; Costill, D.L.; Gallagher, P.M.; Creer, A.C.; Colloton, P.A.; Peters, J.R.; Romatowski, J.G.; Bain, J.L.; Riley, D.A. Prolonged space flight-induced alterations in the structure and function of human skeletal muscle fibres. J. Physiol. 2010, 588, 3567–3592. [Google Scholar] [CrossRef] [PubMed]
- Kozlovskaya, I.B.; Kreidich, Y.V.; Oganov, V.S.; Koserenko, O.P. Pathophysiology of motor functions in prolonged manned space flights. Acta Astronaut. 1981, 8, 1059–1072. [Google Scholar] [CrossRef]
- Koryak, Y.U. Electrically evoked and voluntary properties of the human triceps surae muscle: Effects of long-term spaceflights. Acta Physiol. Pharmacol. Bulg. 2001, 26, 21–27. [Google Scholar]
- Akima, H.; Kawakami, Y.; Kubo, K.; Sekiguchi, C.; Ohshima, H.; Miyamoto, A.; Fukunaga, T. Effect of short-duration spaceflight on thigh and leg muscle volume. Med. Sci. Sports Exerc. 2000, 32, 1743–1747. [Google Scholar] [CrossRef]
- Tesch, P.A.; Berg, H.E.; Bring, D.; Evans, H.J.; LeBlanc, A.D. Effects of 17-day spaceflight on knee extensor muscle function and size. Eur. J. Appl. Physiol. 2005, 93, 463–468. [Google Scholar] [CrossRef]
- Lee, P.H.U.; Chung, M.; Ren, Z.; Mair, D.B.; Kim, D.-H. Factors mediating spaceflight-induced skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 2022, 322, C567–C580. [Google Scholar] [CrossRef]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures. Front. Physiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Campbell, M.J.; McComas, A.J.; Petito, F. Physiological changes in ageing muscles. J. Neurol. Neurosurg. Psychiatry 1973, 36, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Scicchitano, B.M.; Rizzuto, E.; Musarò, A. Counteracting muscle wasting in aging and neuromuscular diseases: The critical role of IGF-1. Aging 2009, 1, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Robbins, H.A.; Callister, M.; Sasieni, P.; Quaife, S.L.; Cheung, L.C.; Brennan, P.; Katki, H.A.; Berg, C.D.; Baldwin, D.; Johansson, M. Benefits and harms in the National Lung Screening Trial: Eexpected outcomes with a modern management protocol. Lancet Respir. Med. 2019, 7, 655–656. [Google Scholar] [CrossRef] [PubMed]
- Metter, E.J.; Conwit, R.; Tobin, J.; Fozard, J.L. Age-associated loss of power and strength in the upper extremities in women and men. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 1997, 52, B267–B276. [Google Scholar] [CrossRef] [PubMed]
- Denison, H.J.; Cooper, C.; Sayer, A.A.; Robinson, S.M. Prevention and optimal management of sarcopenia: A review of combined exercise and nutrition interventions to improve muscle outcomes in older people. Clin. Interv. Aging 2015, 10, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.L.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J.C. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492–498. [Google Scholar] [CrossRef]
- Monti, E.; Reggiani, C.; Franchi, M.V.; Toniolo, L.; Sandri, M.; Armani, A.; Zampieri, S.; Giacomello, E.; Sarto, F.; Sirago, G.; et al. Neuromuscular junction instability and altered intracellular calcium handling as early determinants of force loss during unloading in humans. J. Physiol. 2021, 599, 3037–3061. [Google Scholar] [CrossRef] [PubMed]
- Inns, T.B.; Bass, J.J.; Hardy, E.J.O.; Wilkinson, D.J.; Stashuk, D.W.; Atherton, P.J.; Phillips, B.E.; Piasecki, M. Motor unit dysregulation following 15 days of unilateral lower limb immobilisation. J. Physiol. 2022, 600, 4753–4769. [Google Scholar] [CrossRef]
- Prior, S.J.; Ryan, A.S.; Blumenthal, J.B.; Watson, J.M.; Katzel, L.I.; Goldberg, A.P. Sarcopenia Is Associated With Lower Skeletal Muscle Capillarization and Exercise Capacity in Older Adults. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2016, 71, 1096–1101. [Google Scholar] [CrossRef]
- Betz, M.W.; Aussieker, T.; Kruger, C.Q.; Gorissen, S.H.M.; van Loon, L.J.C.; Snijders, T. Muscle fiber capillarization is associated with various indices of skeletal muscle mass in healthy, older men. Exp. Gerontol. 2021, 143, 111161. [Google Scholar] [CrossRef]
- Barnouin, Y.; McPhee, J.S.; Butler-Browne, G.; Bosutti, A.; De Vito, G.; Jones, D.A.; Narici, M.; Behin, A.; Hogrel, J.-Y.; Degens, H. Coupling between skeletal muscle fiber size and capillarization is maintained during healthy aging. J. Cachexia Sarcopenia Muscle 2017, 8, 647–659. [Google Scholar] [CrossRef] [PubMed]
- Buoite Stella, A.; Ajčević, M.; Furlanis, G.; Manganotti, P. Neurophysiological Adaptations to Space-flight and Simulated Microgravity. Clin. Neurophysiol. 2021, 132, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Manganotti, P.; Buoite Stella, A.; Ajcevic, M.; di Girolamo, F.G.; Biolo, G.; Franchi, M.V.; Monti, E.; Sirago, G.; Marusic, U.; Simunic, B.; et al. Peripheral Nerve Adaptations to 10 Days of Horizontal Bed Rest in Healthy Young Adult Males. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2021, 321, R495–R503. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Koike, W.; Takenouchi, T.; Sugama, S.; Wei, J.; Waragai, M.; Sekiyama, K.; Hashimoto, M. Protection against Neurodegenerative Disease on Earth and in Space. NPJ Microgravity 2016, 2, 16013. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Zhou, M.Y.; Ohira, Y.; Klitgaard, H.; Jiang, B.; Bell, G.; Harris, B.; Saltin, B.; Gollnick, P.D.; Roy, R.R. Human Fiber Size and Enzymatic Properties after 5 and 11 Days of Spaceflight. J. Appl. Physiol. 1995, 78, 1733–1739. [Google Scholar] [CrossRef]
- Gabel, L.; Liphardt, A.-M.; Hulme, P.A.; Heer, M.; Zwart, S.R.; Sibonga, J.D.; Smith, S.M.; Boyd, S.K. Pre-Flight Exercise and Bone Metabolism Predict Unloading-Induced Bone Loss Due to Spaceflight. Br. J. Sports Med. 2022, 56, 196–203. [Google Scholar] [CrossRef]
- Stroud, J.E.; Gale, M.S.; Zwart, S.R.; Heer, M.; Smith, S.M.; Montina, T.; Metz, G.A.S. Longitudinal metabolomic profiles reveal sex-specific adjustments to long-duration spaceflight and return to Earth. Cell. Mol. Life Sci. 2022, 79, 578. [Google Scholar] [CrossRef]
- Ronca, A.E.; Baker, E.S.; Bavendam, T.G.; Beck, K.D.; Miller, V.M.; Tash, J.S.; Jenkins, M. Effects of sex and gender on adaptations to space: Reproductive health. J. Womens Health 2014, 23, 967–974. [Google Scholar] [CrossRef]
- Geraci, A.; Calvani, R.; Ferri, E.; Marzetti, E.; Arosio, B.; Cesari, M. Sarcopenia and Menopause: The Role of Estradiol. Front. Endocrinol. 2021, 12, 682012. [Google Scholar] [CrossRef]
- Wade, C.E.; Keil, L.C. Reduction of pituitary AVP and OT contents in rats following spaceflight. Aviat. Space Environ. Med. 1998, 69, A53–A57. [Google Scholar]
- Berio, E.; Divari, S.; Starvaggi Cucuzza, L.; Biolatti, B.; Cannizzo, F.T. 17β-estradiol upregulates oxytocin and the oxytocin receptor in C2C12 myotubes. PeerJ 2017, 5, e3124. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Greene, K.A.; Tooze, J.A.; Lenchik, L.; Weaver, A.A. Change in Lumbar Muscle Size and Composition on MRI with Long-Duration Spaceflight. Ann. Biomed. Eng. 2022, 50, 816–824. [Google Scholar] [CrossRef] [PubMed]
- Qaisar, R.; Karim, A.; Elmoselhi, A.B. Muscle unloading: A comparison between spaceflight and ground-based models. Acta Physiol. 2020, 228, e13431. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Berger, S.L.; Brunet, A.; Campisi, J.; Cuervo, A.M.; Epel, E.S.; Franceschi, C.; Lithgow, G.J.; Morimoto, R.I.; Pessin, J.E.; et al. Geroscience: Linking aging to chronic disease. Cell 2014, 159, 709–713. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef]
- Martone, A.M.; Onder, G.; Vetrano, D.L.; Ortolani, E.; Tosato, M.; Marzetti, E.; Landi, F. Anorexia of aging: A modifiable risk factor for frailty. Nutrients 2013, 5, 4126–4133. [Google Scholar] [CrossRef]
- Azzolino, D.; Arosio, B.; Marzetti, E.; Calvani, R.; Cesari, M. Nutritional Status as a Mediator of Fatigue and Its Underlying Mechanisms in Older People. Nutrients 2020, 12, 444. [Google Scholar] [CrossRef]
- Jyväkorpi, S.K.; Ramel, A.; Strandberg, T.E.; Piotrowicz, K.; Błaszczyk-Bębenek, E.; Urtamo, A.; Rempe, H.M.; Geirsdóttir, Ó.; Vágnerová, T.; Billot, M.; et al. The sarcopenia and physical frailty in older people: Multi-component treatment strategies (SPRINTT) project: Description and feasibility of a nutrition intervention in community-dwelling older Europeans. Eur. Geriatr. Med. 2021, 12, 303–312. [Google Scholar] [CrossRef]
- Lane, H.W.; Bourland, C.; Barrett, A.; Heer, M.; Smith, S.M. The role of nutritional research in the success of human space flight. Adv. Nutr. 2013, 4, 521–523. [Google Scholar] [CrossRef]
- Ohira, Y.; Jiang, B.; Roy, R.R.; Oganov, V.; Ilyina-Kakueva, E.; Marini, J.F.; Edgerton, V.R. Rat soleus muscle fiber responses to 14 days of spaceflight and hindlimb suspension. J. Appl. Physiol. 1992, 73, S51–S57. [Google Scholar] [CrossRef]
- Desplanches, D.; Mayet, M.H.; Ilyina-Kakueva, E.I.; Sempore, B.; Flandrois, R. Skeletal muscle adaptation in rats flown on Cosmos 1667. J. Appl. Physiol. 1990, 68, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Harrison, B.C.; Allen, D.L.; Girten, B.; Stodieck, L.S.; Kostenuik, P.J.; Bateman, T.A.; Morony, S.; Lacey, D.; Leinwand, L.A. Skeletal muscle adaptations to microgravity exposure in the mouse. J. Appl. Physiol. 2003, 95, 2462–2470. [Google Scholar] [CrossRef] [PubMed]
- Yamashita-Goto, K.; Okuyama, R.; Honda, M.; Kawasaki, K.; Fujita, K.; Yamada, T.; Nonaka, I.; Ohira, Y.; Yoshioka, T. Maximal and submaximal forces of slow fibers in human soleus after bed rest. J. Appl. Physiol. 2001, 91, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Morey-Holton, E.R.; Hill, E.L.; Souza, K.A. Animals and spaceflight: From survival to understanding. J. Musculoskelet. Neuronal Interact. 2007, 7, 17–25. [Google Scholar] [PubMed]
- Allen, D.L.; Bandstra, E.R.; Harrison, B.C.; Thorng, S.; Stodieck, L.S.; Kostenuik, P.J.; Morony, S.; Lacey, D.L.; Hammond, T.G.; Leinwand, L.L.; et al. Effects of spaceflight on murine skeletal muscle gene expression. J. Appl. Physiol. 2009, 106, 582–595. [Google Scholar] [CrossRef]
- Lalani, R.; Bhasin, S.; Byhower, F.; Tarnuzzer, R.; Grant, M.; Shen, R.; Asa, S.; Ezzat, S.; Gonzalez-Cadavid, N.F. Myostatin and insulin-like growth factor-I and -II expression in the muscle of rats exposed to the microgravity environment of the NeuroLab space shuttle flight. J. Endocrinol. 2000, 167, 417–428. [Google Scholar] [CrossRef]
- Buchheim, J.-I.; Matzel, S.; Rykova, M.; Vassilieva, G.; Ponomarev, S.; Nichiporuk, I.; Hörl, M.; Moser, D.; Biere, K.; Feuerecker, M.; et al. Stress Related Shift Toward Inflammaging in Cosmonauts After Long-Duration Space Flight. Front. Physiol. 2019, 10, 85. [Google Scholar] [CrossRef]
- Nogami, M.; Huang, J.T.; James, S.J.; Lubinski, J.M.; Nakamura, L.T.; Makinodan, T. Mice chronically exposed to low dose ionizing radiation possess splenocytes with elevated levels of HSP70 mRNA, HSC70 and HSP72 and with an increased capacity to proliferate. Int. J. Radiat. Biol. 1993, 63, 775–783. [Google Scholar] [CrossRef]
- Guéguinou, N.; Huin-Schohn, C.; Bascove, M.; Bueb, J.-L.; Tschirhart, E.; Legrand-Frossi, C.; Frippiat, J.-P. Could spaceflight-associated immune system weakening preclude the expansion of human presence beyond Earth’s orbit? J. Leukoc. Biol. 2009, 86, 1027–1038. [Google Scholar] [CrossRef]
- Felix, K.; Wise, K.; Manna, S.; Yamauchi, K.; Wilson, B.L.; Thomas, R.L.; Kulkarni, A.; Pellis, N.R.; Ramesh, G.T. Altered cytokine expression in tissues of mice subjected to simulated microgravity. Mol. Cell. Biochem. 2004, 266, 79–85. [Google Scholar] [CrossRef]
- Belay, T.; Aviles, H.; Vance, M.; Fountain, K.; Sonnenfeld, G. Effects of the hindlimb-unloading model of spaceflight conditions on resistance of mice to infection with Klebsiella pneumoniae. J. Allergy Clin. Immunol. 2002, 110, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, J.L.; Hong, B.-Y. Dysbiosis and Immune Dysregulation in Outer Space. Int. Rev. Immunol. 2016, 35, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Orsini, S.S.; Lewis, A.M.; Rice, K.C. Investigation of simulated microgravity effects on Streptococcus mutans physiology and global gene expression. NPJ Microgravity 2017, 3, 4. [Google Scholar] [CrossRef] [PubMed]
- Agha, N.H.; Mehta, S.K.; Rooney, B.V.; Laughlin, M.S.; Markofski, M.M.; Pierson, D.L.; Katsanis, E.; Crucian, B.E.; Simpson, R.J. Exercise as a countermeasure for latent viral reactivation during long duration space flight. FASEB J. 2020, 34, 2869–2881. [Google Scholar] [CrossRef]
- Mehta, S.K.; Laudenslager, M.L.; Stowe, R.P.; Crucian, B.E.; Sams, C.F.; Pierson, D.L. Multiple latent viruses reactivate in astronauts during Space Shuttle missions. Brain Behav. Immun. 2014, 41, 210–217. [Google Scholar] [CrossRef]
- Tahimic, C.G.T.; Globus, R.K. Redox Signaling and Its Impact on Skeletal and Vascular Responses to Spaceflight. Int. J. Mol. Sci. 2017, 18, 2153. [Google Scholar] [CrossRef]
- Smith, R.C.; Cramer, M.S.; Mitchell, P.J.; Lucchesi, J.; Ortega, A.M.; Livingston, E.W.; Ballard, D.; Zhang, L.; Hanson, J.; Barton, K.; et al. Inhibition of myostatin prevents microgravity-induced loss of skeletal muscle mass and strength. PLoS ONE 2020, 15, e0230818. [Google Scholar] [CrossRef]
- Petersen, N.; Jaekel, P.; Rosenberger, A.; Weber, T.; Scott, J.; Castrucci, F.; Lambrecht, G.; Ploutz-Snyder, L.; Damann, V.; Kozlovskaya, I.; et al. Exercise in space: The European Space Agency approach to in-flight exercise countermeasures for long-duration missions on ISS. Extrem Physiol. Med. 2016, 5, 9. [Google Scholar] [CrossRef]
- Corbi, G.; Conti, V.; Filippelli, A.; Di Costanzo, A.; Ferrara, N. The Role of Physical Activity on the Prevention of Cognitive Impairment. Transl. Med. UniSa 2015, 13, 42–46. [Google Scholar]
- Mancinelli, R.; Checcaglini, F.; Coscia, F.; Gigliotti, P.; Fulle, S.; Fanò-Illic, G. Biological Aspects of Selected Myokines in Skeletal Muscle: Focus on Aging. Int. J. Mol. Sci. 2021, 22, 8520. [Google Scholar] [CrossRef]
- Kwon, J.H.; Moon, K.M.; Min, K.-W. Exercise-Induced Myokines can Explain the Importance of Physical Activity in the Elderly: An Overview. Healthcare 2020, 8, 378. [Google Scholar] [CrossRef] [PubMed]
- Hackney, K.J.; Scott, J.M.; Hanson, A.M.; English, K.L.; Downs, M.E.; Ploutz-Snyder, L.L. The Astronaut-Athlete: Optimizing Human Performance in Space. J. Strength Cond. Res. 2015, 29, 3531–3545. [Google Scholar] [CrossRef] [PubMed]
- Bernabei, R.; Landi, F.; Calvani, R.; Cesari, M.; Del Signore, S.; Anker, S.D.; Bejuit, R.; Bordes, P.; Cherubini, A.; Cruz-Jentoft, A.J.; et al. Multicomponent intervention to prevent mobility disability in frail older adults: Randomised controlled trial (SPRINTT project). BMJ 2022, 377, e068788. [Google Scholar] [CrossRef] [PubMed]
- Clément, G. International roadmap for artificial gravity research. NPJ Microgravity 2017, 3, 29. [Google Scholar] [CrossRef] [PubMed]
- Richter, C.; Braunstein, B.; Winnard, A.; Nasser, M.; Weber, T. Human Biomechanical and Cardiopulmonary Responses to Partial Gravity—A Systematic Review. Front. Physiol. 2017, 8, 583. [Google Scholar] [CrossRef]
- Diaz-Artiles, A.; Heldt, T.; Young, L.R. Computational model of cardiovascular response to centrifugation and lower body cycling exercise. J. Appl. Physiol. 2019, 127, 1453–1468. [Google Scholar] [CrossRef]
- Sibonga, J.; Matsumoto, T.; Jones, J.; Shapiro, J.; Lang, T.; Shackelford, L.; Smith, S.M.; Young, M.; Keyak, J.; Kohri, K.; et al. Resistive exercise in astronauts on prolonged spaceflights provides partial protection against spaceflight-induced bone loss. Bone 2019, 128, 112037. [Google Scholar] [CrossRef]
- Lambrecht, G.; Petersen, N.; Weerts, G.; Pruett, C.; Evetts, S.; Stokes, M.; Hides, J. The role of physiotherapy in the European Space Agency strategy for preparation and reconditioning of astronauts before and after long duration space flight. Musculoskelet. Sci. Pract. 2017, 27, S15–S22. [Google Scholar] [CrossRef]
- Petersen, N.; Lambrecht, G.; Scott, J.; Hirsch, N.; Stokes, M.; Mester, J. Postflight reconditioning for European Astronauts—A case report of recovery after six months in space. Musculoskelet. Sci. Pract. 2017, 27, S23–S31. [Google Scholar] [CrossRef]
- Lo, J.H.-T.; U, K.P.; Yiu, T.; Ong, M.T.-Y.; Lee, W.Y.-W. Sarcopenia: Current treatments and new regenerative therapeutic approaches. J. Orthop. Transl. 2020, 23, 38–52. [Google Scholar] [CrossRef]
- Snijders, T.; Nederveen, J.P.; Joanisse, S.; Leenders, M.; Verdijk, L.B.; van Loon, L.J.C.; Parise, G. Muscle fibre capillarization is a critical factor in muscle fibre hypertrophy during resistance exercise training in older men. J. Cachexia Sarcopenia Muscle 2017, 8, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Uchitomi, R.; Oyabu, M.; Kamei, Y. Vitamin D and Sarcopenia: Potential of Vitamin D Supplementation in Sarcopenia Prevention and Treatment. Nutrients 2020, 12, 3189. [Google Scholar] [CrossRef] [PubMed]
- Khoshvaghti, A. Vitamin D in Space. In Fads and Facts about Vitamin D; IntechOpen: London, UK, 2019; pp. 1–18. [Google Scholar]
- Cesari, M.; Bernabei, R.; Vellas, B.; Fielding, R.A.; Rooks, D.; Azzolino, D.; Mariani, J.; Oliva, A.A.; Bhasin, S.; Rolland, Y. Challenges in the Development of Drugs for Sarcopenia and Frailty—Report from the International Conference on Frailty and Sarcopenia Research (ICFSR) Task Force. J. Frailty Aging 2022, 11, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Gomes, M.J.; Martinez, P.F.; Pagan, L.U.; Damatto, R.L.; Cezar, M.D.M.; Lima, A.R.R.; Okoshi, K.; Okoshi, M.P. Skeletal muscle aging: Influence of oxidative stress and physical exercise. Oncotarget 2017, 8, 20428–20440. [Google Scholar] [CrossRef]
- Camporez, J.-P.G.; Petersen, M.C.; Abudukadier, A.; Moreira, G.V.; Jurczak, M.J.; Friedman, G.; Haqq, C.M.; Petersen, K.F.; Shulman, G.I. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl. Acad. Sci. USA 2016, 113, 2212–2217. [Google Scholar] [CrossRef]
- Becker, C.; Lord, S.R.; Studenski, S.A.; Warden, S.J.; Fielding, R.A.; Recknor, C.P.; Hochberg, M.C.; Ferrari, S.L.; Blain, H.; Binder, E.F.; et al. Myostatin antibody (LY2495655) in older weak fallers: A proof-of-concept, randomised, phase 2 trial. Lancet Diabetes Endocrinol. 2015, 3, 948–957. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cannavo, A.; Carandina, A.; Corbi, G.; Tobaldini, E.; Montano, N.; Arosio, B. Are Skeletal Muscle Changes during Prolonged Space Flights Similar to Those Experienced by Frail and Sarcopenic Older Adults? Life 2022, 12, 2139. https://doi.org/10.3390/life12122139
Cannavo A, Carandina A, Corbi G, Tobaldini E, Montano N, Arosio B. Are Skeletal Muscle Changes during Prolonged Space Flights Similar to Those Experienced by Frail and Sarcopenic Older Adults? Life. 2022; 12(12):2139. https://doi.org/10.3390/life12122139
Chicago/Turabian StyleCannavo, Alessandro, Angelica Carandina, Graziamaria Corbi, Eleonora Tobaldini, Nicola Montano, and Beatrice Arosio. 2022. "Are Skeletal Muscle Changes during Prolonged Space Flights Similar to Those Experienced by Frail and Sarcopenic Older Adults?" Life 12, no. 12: 2139. https://doi.org/10.3390/life12122139
APA StyleCannavo, A., Carandina, A., Corbi, G., Tobaldini, E., Montano, N., & Arosio, B. (2022). Are Skeletal Muscle Changes during Prolonged Space Flights Similar to Those Experienced by Frail and Sarcopenic Older Adults? Life, 12(12), 2139. https://doi.org/10.3390/life12122139










