Lipid Rafts and Plant Gravisensitivity
Abstract
1. Introduction
2. Lipid Rafts in Plants
3. Lipid Rafts under Clinorotation
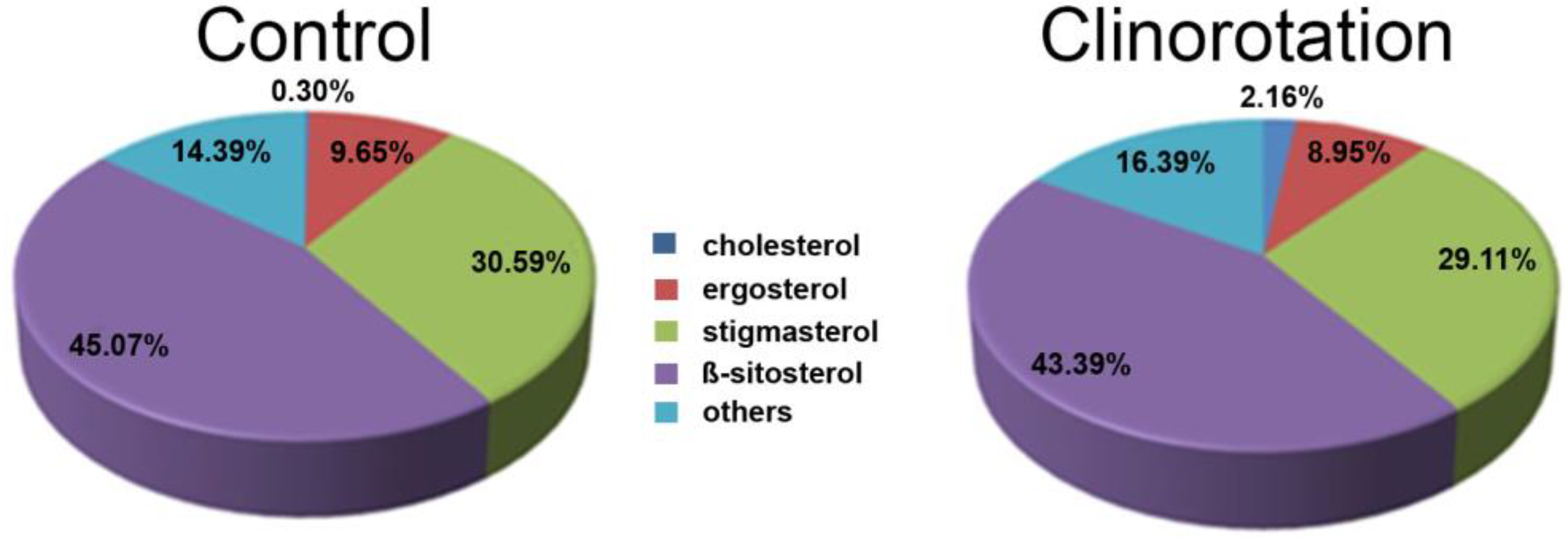
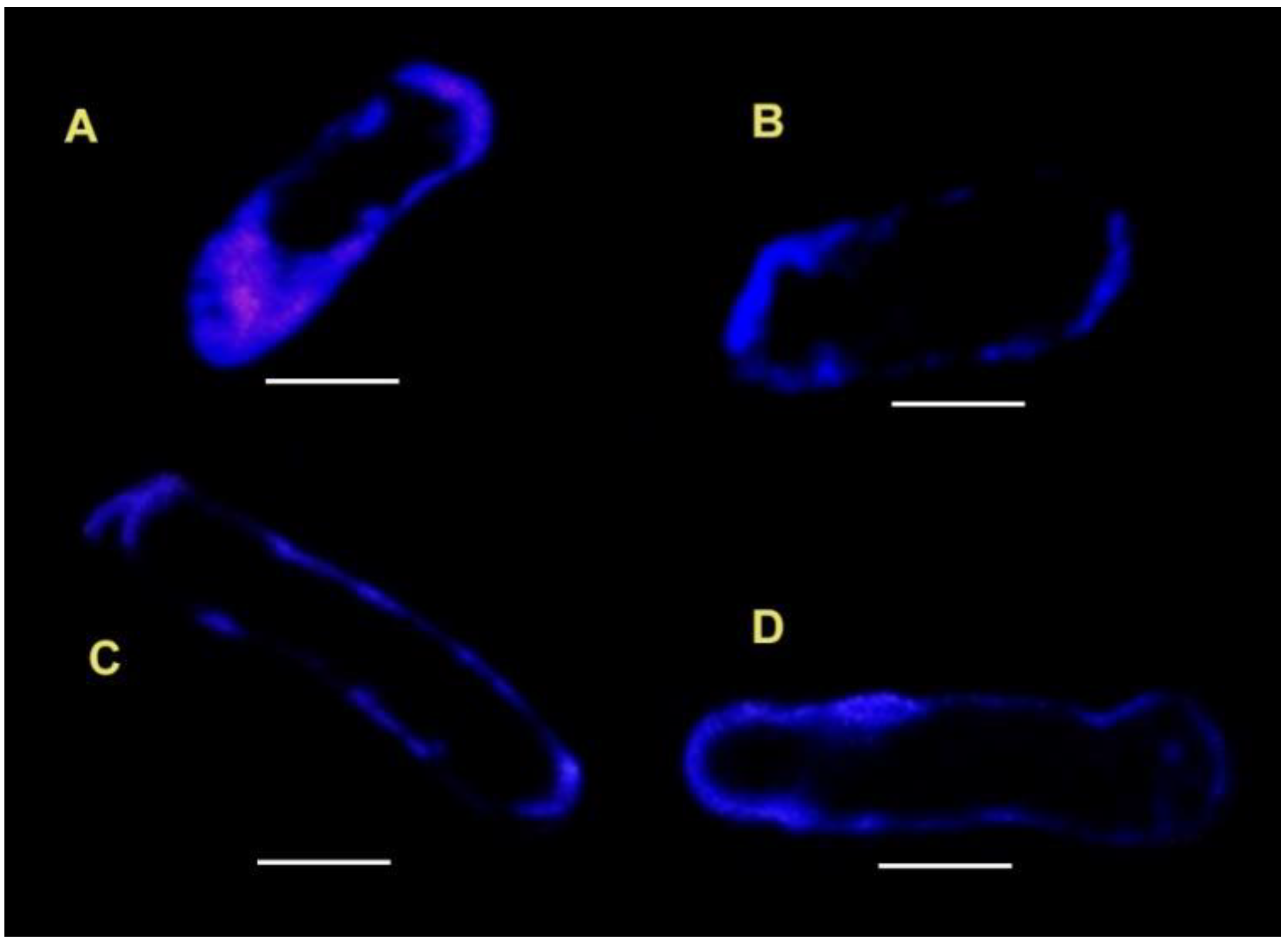
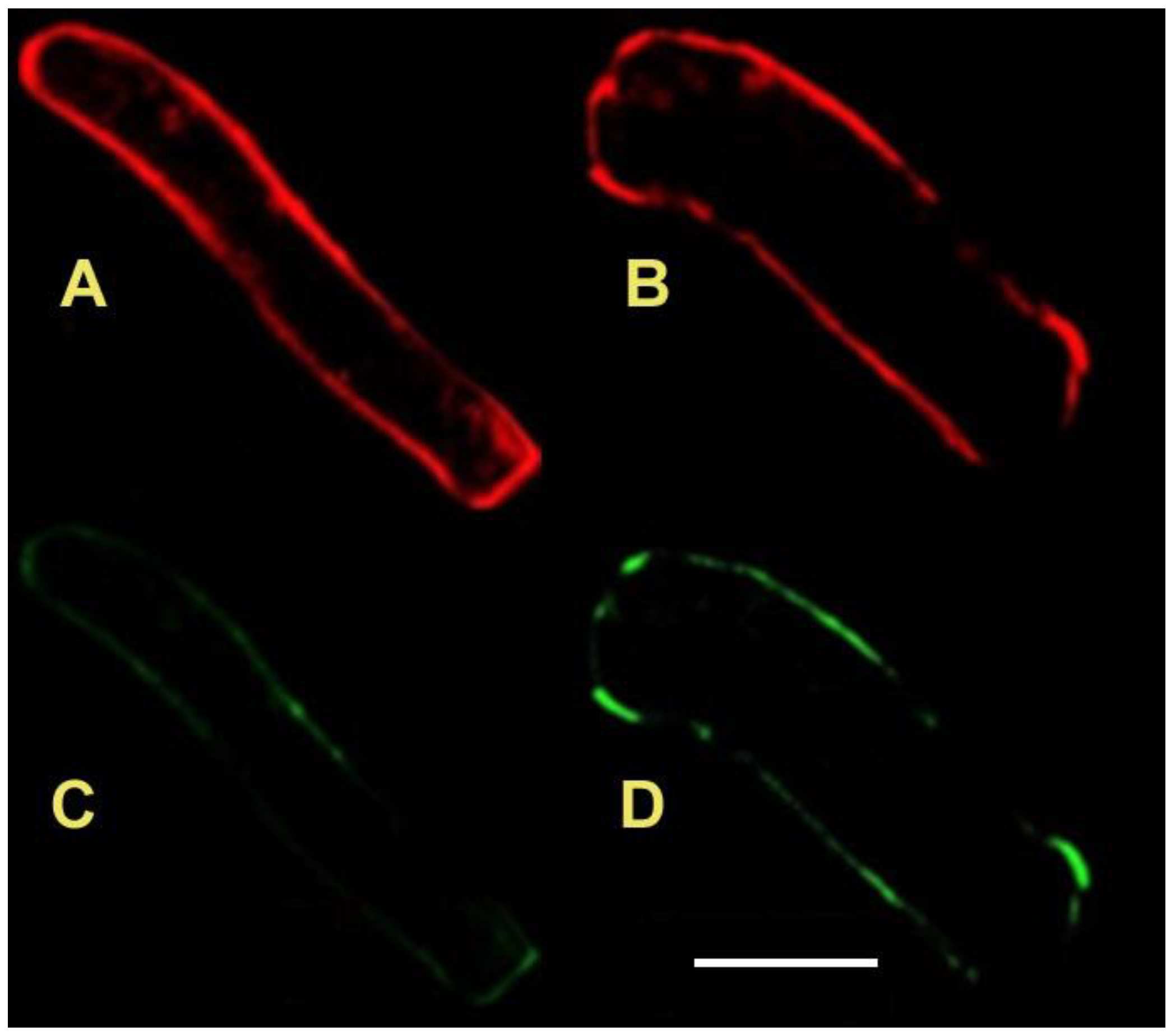
3.1. Filipin Staining
3.2. Laurdan Staining
4. How Do Lipid Raft Studies Advance the Understanding of the Gravisensitivity of Plant Cells?
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lehto, K.A.; Lehto, H.J.; Kanervo, E.A. Suitability of different photosynthetic organisms for an extraterrestrial biological life support system. Res. Microbiol. 2006, 157, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, C.A. Bioregenerative life-support-systems. Am. J. Clin. Nutr. 1994, 60, 820S–824S. [Google Scholar] [CrossRef] [PubMed]
- Ferl, R.; Wheeler, R.; Levine, H.G.; Paul, A.L. Plants in Space. Curr. Opin. Plant Biol. 2002, 5, 258–263. [Google Scholar] [CrossRef]
- Ferl, R.J.; Koh, J.; Denison, F.; Paul, A.L. Spaceflight Induces Specific Alterations in the Proteomes of Arabidopsis. Astrobiology 2015, 15, 32–56. [Google Scholar] [CrossRef]
- Wheeler, R.M. Plants for human life support in space: From Myers to Mars. Gravit. Space Res. 2010, 23, 25–35. [Google Scholar]
- Fu, Y.M.; Li, L.Y.; Xie, B.Z.; Dong, C.; Wang, M.J.; Jia, B.Y.; Shao, L.Z.; Dong, Y.Y.; Deng, S.D.; Liu, H.; et al. How to establish a bioregenerative life support system for long-term crewed missions to the Moon or Mars. Astrobiology 2016, 16, 925–936. [Google Scholar] [CrossRef]
- Halstead, T.W.; Dutcher, F.R. Plants in Space. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1987, 38, 317–345. [Google Scholar] [CrossRef]
- Claassen, D.; Spooner, B. International Review of Cytology—A Survey of Cell Biology; Jeon, K.W., Berezney, R., Eds.; Academic Press: Cambridge, MA, USA, 1994; Volume 156, pp. 301–373. [Google Scholar]
- Kordyum, E.L. Plant cell gravisensitivity and adaptation to microgravity. Plant Biol. 2014, 16, 79–90. [Google Scholar] [CrossRef]
- Wolverton, C.; Kiss, J.Z. An update on plant space biology. Gravit. Space Biol. 2009, 22, 13–20. [Google Scholar]
- Paul, A.L.; Wheeler, R.M.; Levine, H.G.; Ferl, R.J. Fundamental plant biology enabled by the Space Shuttle. Am. J. Bot. 2013, 100, 226–234. [Google Scholar] [CrossRef]
- Kittang, A.I.; Iversen, T.H.; Fossum, K.R.; Mazars, C.; Carnero-Diaz, E.; Boucheron-Dubuisson, E.; Le Disquet, I.; Legue, V.; Herranz, R.; Pereda-Loth, V.; et al. Exploration of plant growth and development using the European Modular Cultivation System facility on the International Space Station. Plant Biol. 2014, 16, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Hoson, T. Plant growth and morphogenesis under different gravity conditions: Relevance to plant life in space. Life 2014, 4, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.L.; Ferl, R.J. Spaceflight exploration in plant gravitational biology. Methods Mol. Biol. 2015, 1309, 285–305. [Google Scholar] [PubMed]
- Manzano, A.I.; Herranz, R.; Manzano, A.; van Loon, J.; Medina, F.J. Early effects of altered gravity environments on plant cell growth and cell proliferation: Characterization of morphofunctional nucleolar types in an Arabidopsis cell culture system. Front. Astron. Space Sci. 2016, 3, 2. [Google Scholar] [CrossRef]
- Kordyum, E.; Hasenstein, K.H. Plant biology for space exploration—Building on the past, preparing for the future. Life Sci. Space Res. 2021, 29, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Slenska, K.; Kordyum, E.L. Gravity, cellular membranes and associated processes: An introduction. Adv. Space Res. 1995, 17, 141–142. [Google Scholar] [CrossRef]
- Masson, F.; Rossignol, M. Basic plasticity of protein expression in tobacco leaf plasma-membrane. Plant J. 1995, 8, 77–85. [Google Scholar] [CrossRef]
- Gronnier, J.; Gerbeau-Pissot, P.; Germain, V.; Mongrand, S.; Simon-Plas, F. Divide and Rule: Plant Plasma Membrane Organization. Trends Plant Sci. 2018, 23, 899–917. [Google Scholar] [CrossRef]
- Santos, A.L.; Preta, G. Lipids in the cell: Organisation regulates function. Cell. Mol. Life Sci. 2018, 75, 1909–1927. [Google Scholar] [CrossRef]
- Cassim, A.M.; Gouguet, P.; Gronnier, J.; Laurent, N.; Germain, V.; Grison, M.; Boutte, Y.; Gerbeau-Pissot, P.; Simon-Plas, F.; Mongrand, S. Plant lipids: Key players of plasma membrane organization and function. Prog. Lipid Res. 2019, 73, 1–27. [Google Scholar] [CrossRef]
- Rogowska, A.; Szakiel, A. The role of sterols in plant response to abiotic stress. Phytochem. Rev. 2020, 19, 1525–1538. [Google Scholar] [CrossRef]
- Jaillais, Y.; Ott, T. The Nanoscale Organization of the Plasma Membrane and Its Importance in Signaling: A Proteolipid Perspective(1) (OPEN). Plant Physiol. 2020, 182, 1682–1696. [Google Scholar] [CrossRef] [PubMed]
- Rawat, N.; Singla-Pareek, S.L.; Pareek, A. Membrane dynamics during individual and combined abiotic stresses in plants and tools to study the same. Physiol. Plant. 2021, 171, 653–676. [Google Scholar] [CrossRef]
- Popova, A.F.; Sytnik, K.M.; Kordyum, E.L.; Meleshko, G.I.; Sychev, V.N.; Levinskykh, M.A. Ultrastructural and growth indices of chlorella culture in multicomponent aquatic systems under space flight conditions. Adv. Space Res. 1989, 9, 79–82. [Google Scholar] [CrossRef]
- Hanke, W. Studies of the interaction of gravity with biological-membranes using alamethicin doped planar lipid bilayers as a model system. Adv. Space Res. 1994, 17, 143–150. [Google Scholar] [CrossRef]
- Goldermann, M.; Hanke, W. Ion channel are sensitive to gravity changes. Microgravity Sci. Technol. 2001, 13, 35–38. [Google Scholar] [CrossRef]
- Sieber, M.; Hanke, W.; Kohn, F.P.M. Modification of Membrane Fluidity by Gravity. Open J. Biophys. 2014, 04, 105–111. [Google Scholar] [CrossRef][Green Version]
- Polulyakh, Y.A. Phospholipid and fatty-acid composition of pea root cell plasma-membranes under clinostating. Acad. Sci. Ukr. Ser. B-Geol. Chem. Biol. Sci. 1988, 10, 67–69. [Google Scholar]
- Polulyakh, Y.; Zhadko, S.; Klimchuk, D.; Baraboy, V.; Alpatov, A.; Sytnik, K. Plant cell plasma membrane structure and properties under clinostatting. Adv. Space Res. 1989, 9, 71–74. [Google Scholar] [CrossRef]
- Kordyum, E.; Nedukha, O.; Grakhov, V.; Mel’nik, A.; Vorobyova, T.; Klimenko, O.; Zhupanov, I. Study of the influence of simulated microgravity on the cytoplasmic membrane lipid bilayer of plant cells. Kosm. Nauka Tehnol. 2015, 21, 40–47. [Google Scholar] [CrossRef]
- Kordyum, E.L. International Review of Cytology—A Survey of Cell Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 1997; Volume 171, pp. 1–78. [Google Scholar]
- Simons, K.; Toomre, D. Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 2000, 1, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, R.J.; Ahmed, S.N.; Zhu, Y.Z.; London, E.; Brown, D.A. Cholesterol and sphingolipid enhance the Triton X-100 insolubility of glycosylphosphatidylinositol-anchored proteins by promoting the formation of detergent-insoluble ordered membrane domains. J. Biol. Chem. 1998, 273, 1150–1157. [Google Scholar] [CrossRef]
- Simons, K.; Ikonen, E. Functional rafts in cell membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.A.; London, E. Functions of lipid rafts in biological membranes. Annu. Rev. Cell Dev. Biol. 1998, 14, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.L.; Bittman, R.; Duportail, G.; Heissler, D.; Vilcheze, C.; London, E. Effect of the structure of natural sterols and sphingolipids on the formation of ordered sphingolipid/sterol domains (rafts). J. Biol. Chem. 2001, 276, 33540–33546. [Google Scholar] [CrossRef]
- Mongrand, S.; Morel, J.; Laroche, J.; Claverol, S.; Carde, J.P.; Hartmann, M.A.; Bonneu, M.; Simon-Plas, F.; Lessire, R.; Bessoule, J.J. Lipid rafts in higher plant cells—Purification and characterization of triton X-100-insoluble microdomains from tobacco plasma membrane. J. Biol. Chem. 2004, 279, 36277–36286. [Google Scholar] [CrossRef]
- Mongrand, S.; Stanislas, T.; Bayer, E.M.F.; Lherminier, J.; Simon-Plas, F. Membrane rafts in plant cells. Trends Plant Sci. 2010, 15, 656–663. [Google Scholar] [CrossRef]
- Grennan, A.K. Lipid rafts in plants. Plant Physiol. 2007, 143, 1083–1085. [Google Scholar] [CrossRef]
- Cacas, J.L.; Furt, F.; Le Guedard, M.; Schmitter, J.M.; Bure, C.; Gerbeau-Pissot, P.; Moreau, P.; Bessoule, J.J.; Simon-Plas, F.; Mongrand, S. Lipids of plant membrane rafts. Prog. Lipid Res. 2012, 51, 272–299. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Moore, W.; Kim, H.; Budin, I. Bringing rafts to life: Lessons learned from lipid organization across diverse biological membranes. Chem. Phys. Lipids 2020, 233, 104984. [Google Scholar] [CrossRef]
- Carquin, M.; D’Auria, L.; Pollet, H.; Bongarzone, E.R.; Tyteca, D. Recent progress on lipid lateral heterogeneity in plasma membranes: From rafts to submicrometric domains. Prog. Lipid Res. 2016, 62, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Huby, E.; Napier, J.A.; Baillieul, F.; Michaelson, L.V.; Dhondt-Cordelier, S. Sphingolipids: Towards an integrated view of metabolism during the plant stress response. New Phytol. 2020, 225, 659–670. [Google Scholar] [CrossRef] [PubMed]
- Cassim, A.M.; Grison, M.; Ito, Y.; Simon-Plas, F.; Mongrand, S.; Boutte, Y. Sphingolipids in plants: A guidebook on their function in membrane architecture, cellular processes, and environmental or developmental responses. FEBS Lett. 2020, 594, 3719–3738. [Google Scholar] [CrossRef] [PubMed]
- Cassim, A.M.; Navon, Y.; Gao, Y.; Decossas, M.; Fouillen, L.; Grelard, A.; Nagano, M.; Lambert, O.; Bahammou, D.; Van Delft, P.; et al. Biophysical analysis of the plant-specific GIPC sphingolipids reveals multiple modes of membrane regulation. J. Biol. Chem. 2021, 296, 100602. [Google Scholar] [CrossRef] [PubMed]
- Kordyum, E.L.; Klymenko, O.; Bulavin, I.V.; Zhupanov, I.V.; Vorobyova, T.M.; Ruelland, E. Lipid rafts in plant cells are sensitive to the influence of simulated microgravity (clinorotation). Space Sci. Technol.-Kosm. Nauka Tehnol. 2018, 24, 48–58. [Google Scholar] [CrossRef]
- Peskan, T.; Westermann, M.; Oelmuller, R. Identification of low-density Triton X-100-insoluble plasma membrane microdomains in higher plants. Eur. J. Biochem. 2000, 267, 6989–6995. [Google Scholar] [CrossRef] [PubMed]
- Borner, G.H.H.; Sherrier, D.J.; Weimar, T.; Michaelson, L.V.; Hawkins, N.D.; MacAskill, A.; Napier, J.A.; Beale, M.H.; Lilley, K.S.; Dupree, P. Analysis of detergent-resistant membranes in Arabidopsis. Evidence for plasma membrane lipid rafts. Plant Physiol. 2005, 137, 104–116. [Google Scholar] [CrossRef]
- Bhat, R.A.; Panstruga, R. Lipid Rafts in Plants. Planta 2005, 223, 5–19. [Google Scholar] [CrossRef]
- Morel, J.; Claverol, S.; Mongrand, S.; Furt, F.; Fromentin, J.; Bessoule, J.J.; Blein, J.P.; Simon-Plas, F. Proteomics of plant detergent-resistant membranes. Mol. Cell. Proteom. 2006, 5, 1396–1411. [Google Scholar] [CrossRef]
- Lefebvre, B.; Furt, F.; Hartmann, M.A.; Michaelson, L.V.; Carde, J.P.; Sargueil-Boiron, F.; Rossignol, M.; Napier, J.A.; Cullimore, J.; Bessoule, J.J.; et al. Characterization of lipid rafts from Medicago truncatula root plasma membranes: A proteomic study reveals the presence of a raft-associated redox system. Plant Physiol. 2007, 144, 402–418. [Google Scholar] [CrossRef]
- Laloi, M.; Perret, A.M.; Chatre, L.; Melser, S.; Cantrel, C.; Vaultier, M.N.; Zachowski, A.; Bathany, K.; Schmitter, J.M.; Vallet, M.; et al. Insights into the role of specific lipids in the formation and delivery of lipid microdomains to the plasma membrane of plant cells. Plant Physiol. 2007, 143, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Lingwood, D.; Simons, K. Lipid Rafts As a Membrane-Organizing Principle. Science 2010, 327, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Kraft, M.L. Plasma membrane organization and function: Moving past lipid rafts. Mol. Biol. Cell 2013, 24, 2765–2768. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Cui, Y.N.; Zhang, X.; Li, R.L.; Lin, J.X. Organization and dynamics of functional plant membrane microdomains. Cell. Mol. Life Sci. 2020, 77, 275–287. [Google Scholar] [CrossRef]
- Ozolina, N.V.; Kapustina, I.S.; Gurina, V.V.; Bobkova, V.A.; Nurminsky, V.N. Role of Plasmalemma Microdomains (Rafts) in Protection of the Plant Cell Under Osmotic Stress. J. Membr. Biol. 2021, 254, 429–439. [Google Scholar] [CrossRef] [PubMed]
- Furt, F.; Konig, S.; Bessoule, J.J.; Sargueil, F.; Zallot, R.; Stanislas, T.; Noirot, E.; Lherminier, J.; Simon-Plas, F.; Heilmann, I.; et al. Polyphosphoinositides Are Enriched in Plant Membrane Rafts and Form Microdomains in the Plasma Membrane. Plant Physiol. 2010, 152, 2173–2187. [Google Scholar] [CrossRef] [PubMed]
- Grosjean, K.; Der, C.; Robert, F.; Thomas, D.; Mongrand, S.; Simon-Plas, F.; Gerbeau-Pissot, P. Interactions between lipids and proteins are critical for organization of plasma membrane-ordered domains in tobacco BY-2 cells. J. Exp. Bot. 2018, 69, 3545–3557. [Google Scholar] [CrossRef]
- Shahollari, B.; Peskan-Berghofer, T.; Oelmuller, R. Receptor kinases with leucine-rich repeats are enriched in Triton X-100 insoluble plasma membrane microdomains from plants. Physiol. Plant. 2004, 112, 397–403. [Google Scholar] [CrossRef]
- Demir, F.; Horntrich, C.; Blachutzik, J.O.; Scherzer, S.; Reinders, Y.; Kierszniowska, S.; Schulze, W.X.; Harms, G.S.; Hedrich, R.; Geiger, D.; et al. Arabidopsis nanodomain-delimited ABA signaling pathway regulates the anion channel SLAH3. Proc. Natl. Acad. Sci. USA 2013, 110, 8296–8301. [Google Scholar] [CrossRef]
- Seifert, G.J.; Xue, H.; Acet, T. The Arabidopsis thaliana FASCICLIN-LIKE ARABINOGALACTAN PROTEIN 4 gene acts synergistically with abscisic acid signalling to control root growth. Ann. Bot. 2014, 114, 1125–1133. [Google Scholar] [CrossRef]
- Furt, F.; Lefebvre, B.; Cullimore, J.; Bessoule, J.-J.; Mongrand, S. Plant Lipid Rafts. Plant Signal. Behav. 2007, 2, 508–511. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Aki, T.; Yanagisawa, S.; Uchimiya, H.; Kawai-Yamada, M. Overexpression of BAX INHIBITOR-1 Links Plasma Membrane Microdomain Proteins to Stress. Plant Physiol. 2015, 169, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, D.; Imai, H.; Kawamura, Y.; Uemura, M. Lipid profiles of detergent resistant fractions of the plasma membrane in oat and rye in association with cold acclimation and freezing tolerance. Cryobiology 2016, 72, 123–134. [Google Scholar] [CrossRef]
- Takahashi, D.; Kawamura, Y.; Yamashita, T.; Uemura, M. Detergent-resistant plasma membrane proteome in oat and rye: Similarities and dissimilarities between two monocotyledonous plants. J. Proteome Res. 2012, 11, 1654–1665. [Google Scholar] [CrossRef] [PubMed]
- Grison, M.S.; Brocard, L.; Fouillen, L.; Nicolas, W.; Wewer, V.; Dormann, P.; Nacir, H.; Benitez-Alfonso, Y.; Claverol, S.; Germain, V.; et al. Specific Membrane Lipid Composition Is Important for Plasmodesmata Function in Arabidopsis. Plant Cell 2015, 27, 1228–1250. [Google Scholar] [CrossRef] [PubMed]
- Iswanto, A.B.; Kim, J.Y. Lipid Raft, Regulator of Plasmodesmal Callose Homeostasis. Plants 2017, 6, 15. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, S.; Wang, L.; Chang, X.; Fan, Y.; He, M.; Yan, D. Sphingolipids at Plasmodesmata: Structural Components and Functional Modulators. Int. J. Mol. Sci. 2022, 23, 5677. [Google Scholar] [CrossRef]
- Srivastava, V.; Malm, E.; Sundqvist, G.; Bulone, V. Quantitative proteomics reveals that plasma membrane microdomains from poplar cell suspension cultures are enriched in markers of signal transduction, molecular transport, and callose biosynthesis. Mol. Cell. Proteom. 2013, 12, 3874–3885. [Google Scholar] [CrossRef]
- Zhukovsky, M.A.; Filograna, A.; Luini, A.; Corda, D.; Valente, C. Phosphatidic acid in membrane rearrangements. FEBS Lett. 2019, 593, 2428–2451. [Google Scholar] [CrossRef]
- Minami, A.; Fujiwara, M.; Furuto, A.; Fukao, Y.; Yamashita, T.; Kamo, M.; Kawamura, Y.; Uemura, M. Alterations in detergent-resistant plasma membrane microdomains in Arabidopsis thaliana during cold acclimation. Plant Cell Physiol. 2009, 50, 341–359. [Google Scholar] [CrossRef]
- Yepes-Molina, L.; Carvajal, M.; Martinez-Ballesta, M.C. Detergent Resistant Membrane Domains in Broccoli Plasma Membrane Associated to the Response to Salinity Stress. Int. J. Mol. Sci. 2020, 21, 7694. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Carbonell, E.; Takahashi, D.; Luethje, S.; Gonzalez-Reyes, J.; Mongrand, S.; Contreras-Moreira, B.; Abadia, A.; Uemura, M.; Abadia, J.; Lopez-Millan, A.F. Shotgun proteomic approach reveals that Fe deficiency causes marked changes in the protein profiles of plasma membrane and detergent-resistant microdomain preparations from Beta vulgaris roots. J. Proteome Res. 2016, 15, 2510–2524. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Suo, X.; Li, F.; Bao, C.; He, S.; Huang, L.; Luo, M. Membrane lipid raft organization during cotton fiber development. J. Cotton Res. 2020, 3, 13. [Google Scholar] [CrossRef]
- Rozentsvet, O.; Nesterkina, I.; Ozolina, N.; Nesterov, V. Detergent-resistant microdomains (lipid rafts) in endomembranes of the wild halophytes. Funct. Plant Biol. 2019, 46, 869–876. [Google Scholar] [CrossRef]
- Pike, L.J. The challenge of lipid rafts. J. Lipid Res. 2009, 50, S323–S328. [Google Scholar] [CrossRef]
- Nedukha, O.; Kordyum, E.; Vorobyova, T. Sensitivity of Plant Plasma Membrane to Simulated Microgravity. Microgravity Sci. Technol. 2021, 33, 10. [Google Scholar] [CrossRef]
- Bergy, M.E.; Eble, T.E. Filipin complex. Biochemistry 1968, 7, 653–659. [Google Scholar] [CrossRef]
- Grebe, M.; Xu, J.; Mobius, W.; Ueda, T.; Nakano, A.; Geuze, H.J.; Rook, M.B.; Scheres, B. Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 2003, 13, 1378–1387. [Google Scholar] [CrossRef]
- Boutte, Y.; Frescatada-Rosa, M.; Men, S.Z.; Chow, C.M.; Ebine, K.; Gustavsson, A.; Johansson, L.; Ueda, T.; Moore, I.; Jurgens, G.; et al. Endocytosis restricts Arabidopsis KNOLLE syntaxin to the cell division plane during late cytokinesis. EMBO J. 2010, 29, 546–558. [Google Scholar] [CrossRef]
- Tan, L.H.; Tan, A.J.; Ng, Y.Y.; Chua, J.J.; Chew, W.S.; Muralidharan, S.; Torta, F.; Dutta, B.; Sze, S.K.; Herr, D.R.; et al. Enriched Expression of Neutral Sphingomyelinase 2 in the Striatum is Essential for Regulation of Lipid Raft Content and Motor Coordination. Mol. Neurobiol. 2018, 55, 5741–5756. [Google Scholar] [CrossRef]
- Rochetti, V.P.; Rollin-Pinheiro, R.; de Oliveira, E.B.; Xisto, M.; Barreto-Bergter, E. Glucosylceramide Plays a Role in Fungal Germination, Lipid Raft Organization and Biofilm Adhesion of the Pathogenic Fungus Scedosporium aurantiacum. J. Fungi 2020, 6, 345. [Google Scholar] [CrossRef]
- Wang, H.Y.; Bharti, D.; Levental, I. Membrane Heterogeneity Beyond the Plasma Membrane. Front. Cell Dev. Biol. 2020, 8, 580814. [Google Scholar] [CrossRef] [PubMed]
- Grundner, M.; Panevska, A.; Sepcic, K.; Skocaj, M. What Can Mushroom Proteins Teach Us about Lipid Rafts? Membranes 2021, 11, 264. [Google Scholar] [CrossRef] [PubMed]
- Artemenko, O.A. The study of the functional state of lipid rafts in the cytoplasmic membrane of Pisum sativum seedlings under clinorotation. Space Sci. Technol.-Kosm. Nauka Tehnol. 2021, 27, 35–46. [Google Scholar] [CrossRef]
- Ovecka, M.; Berson, T.; Beck, M.; Derksen, J.; Samaj, J.; Baluska, F.; Lichtscheidl, I.K. Structural Sterols Are Involved in Both the Initiation and Tip Growth of Root Hairs in Arabidopsis thaliana. Plant Cell 2010, 22, 2999–3019. [Google Scholar] [CrossRef]
- Weber, G.; Farris, F.J. Synthesis and spectral properties of a hydrophobic fluorescent probe: 6-propionyl-2-(dimethylamino)naphthalene. Biochemistry 1979, 18, 3075–3078. [Google Scholar] [CrossRef]
- Collot, M.; Boutant, E.; Fam, K.T.; Danglot, L.; Klymchenko, A.S. Molecular Tuning of Styryl Dyes Leads to Versatile and Efficient Plasma Membrane Probes for Cell and Tissue Imaging. Bioconj. Chem. 2020, 31, 875–883. [Google Scholar] [CrossRef]
- Ghysels, A.; Kramer, A.; Venable, R.M.; Teague, W.E., Jr.; Lyman, E.; Gawrisch, K.; Pastor, R.W. Permeability of membranes in the liquid ordered and liquid disordered phases. Nat. Commun. 2019, 10, 5616. [Google Scholar] [CrossRef]
- Baoukina, S.; Rozmanov, D.; Tieleman, D.P. Simulation study of dynamic heterogeneity in lipid bilayers. Eur. Biophys. J. Biophys. Lett. 2015, 44, S109. [Google Scholar]
- M’Baye, G.; Mely, Y.; Duportail, G.; Klymchenko, A.S. Liquid ordered and gel phases of lipid bilayers: Fluorescent probes reveal close fluidity but different hydration. Biophys. J. 2008, 95, 1217–1225. [Google Scholar] [CrossRef]
- Quinn, P.J. Long N-acyl fatty acids on sphingolipids are responsible for miscibility with phospholipids to form liquid-ordered phase. Biochim. Biophys. Acta-Biomembr. 2009, 1788, 2267–2276. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chernyshov, M.Y.; Nurminsky, V.N.; Ozolina, N.V. Lipid-Protein Microinclusions in the Morphological Structures of Organelle Membranes Studied by Fluorescent Confocal Microscopy. Adv. Biol. Chem. 2017, 07, 42–59. [Google Scholar] [CrossRef][Green Version]
- Butler, C.E.; Wheeler, G.; Graham, J.; Tyler, K.M. Fluorescent Methods to Study Biological Membranes; Springer: Berlin/Heidelberg, Germany, 2012; Volume 13. [Google Scholar]
- Blachutzik, J.; Demir, F.; Kreuzer, I.; Hedrich, R.; Harms, G.S. Methods of staining and visualization of sphingolipid enriched and non-enriched plasma membrane regions of Arabidopsis thaliana with fluorescent dyes and lipid analogues. Plant Methods 2012, 8, 28. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, T.; Sakaki, T.; Usui, S.; Soga, K.; Wakabayashi, K.; Hoson, T. Changes in membrane lipid composition in azuki bean epicotyls under hypergravity conditions: Possible role of membrane sterols in gravity resistance. Adv. Space Res. 2007, 39, 1198–1203. [Google Scholar] [CrossRef]
- Hasenstein, K.H.; Park, M.R.; John, S.P.; Ajala, C. High-gradient magnetic fields and starch metabolism: Results from a space experiment. Sci. Rep. 2022, 12, 18256. [Google Scholar] [CrossRef]
- Edidin, M. The state of lipid rafts: From model membranes to cells. Annu. Rev. Biophys. Biomol. Struct. 2003, 32, 257–283. [Google Scholar] [CrossRef]
- Nebl, T.; Pestonjamasp, K.N.; Leszyk, J.D.; Crowley, J.L.; Oh, S.W.; Luna, E.J. Proteomic analysis of a detergent-resistant membrane skeleton from neutrophil plasma membranes. J. Biol. Chem. 2002, 277, 43399–43409. [Google Scholar] [CrossRef]
- Brown, D.A. Lipid rafts, detergent-resistant membranes, and raft targeting signals. Physiology 2006, 21, 430–439. [Google Scholar] [CrossRef]
- Baluska, F.; Hasenstein, K.H. Root cytoskeleton: Its role in perception of and response to gravity. Planta 1997, 203, S69–S78. [Google Scholar] [CrossRef]
- Vorselen, D.; Roos, W.H.; MacKintosh, F.C.; Wuite, G.J.L.; van Loon, J. The role of the cytoskeleton in sensing changes in gravity by nonspecialized cells. FASEB J. 2014, 28, 536–547. [Google Scholar] [CrossRef]
- Pozhvanov, G.; Sharova, E.; Medvedev, S. Microgravity modelling by two-axial clinorotation leads to scattered organisation of cytoskeleton in Arabidopsis seedlings. Funct. Plant Biol. 2021, 48, 1062–1073. [Google Scholar] [CrossRef] [PubMed]
- Shevchenko, G.V.; Kalinina, Y.M.; Kordyum, E.L. Interrelation between microtubules and microfilaments in the elongation zone of Arabidopsis root under clinorotation. Adv. Space Res. 2007, 39, 1171–1175. [Google Scholar] [CrossRef]
- Kalinina, I.; Shevchenko, G.; Kordyum, E. Tubulin Cytoskeleton in Arabidopsis thaliana Root Cells Under Clinorotation. Microgravity Sci. Technol. 2009, 21, 187–190. [Google Scholar] [CrossRef]
- Hasezawa, S.; Nozaki, H. Role of cortical microtubules in the orientation of cellulose microfibril deposition in higher-plant cells. Protoplasma 1999, 209, 98–104. [Google Scholar] [CrossRef] [PubMed]
- Baskin, T.I. On the alignment of cellulose microfibrils by cortical microtubules: A review and a model. Protoplasma 2001, 215, 150–171. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.; Chan, J. Microtubules and the shape of plants to come. Nat. Rev. Mol. Cell Biol. 2004, 5, 13–22. [Google Scholar] [CrossRef]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Stimulation of elongation growth and xyloglucan breakdown in Arabidopsis hypocotyls under microgravity conditions in space. Planta 2002, 215, 1040–1046. [Google Scholar] [CrossRef]
- Soga, K.; Wakabayashi, K.; Kamisaka, S.; Hoson, T. Effects of hypergravity on expression of XTH genes in azuki bean epicotyls. Physiol. Plant. 2007, 131, 332–340. [Google Scholar] [CrossRef]
- Ishikawa, H.; Evans, M. Specialized Zones of Development in Roots. Plant Physiol. 1995, 109, 725–727. [Google Scholar] [CrossRef]
- Baluška, F.; Jasik, J.; Edelmann, H.G.; Salajova, T.; Volkmann, D. Latrunculin B-induced plant dwarfism: Plant cell elongation is F-actin-dependent. Dev. Biol. 2001, 231, 113–124. [Google Scholar] [CrossRef]
- Baluska, F.; Mancuso, S.; Volkmann, D.; Barlow, P.W. Root apex transition zone: A signalling-response nexus in the root. Trends Plant Sci. 2010, 15, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Baluska, F.; Samaj, J.; Wojtaszek, P.; Volkmann, D.; Menzel, D. Cytoskeleton-plasma membrane-cell wall continuum in plants. Emerging links revisited. Plant Physiol. 2003, 133, 482–491. [Google Scholar] [CrossRef]
- Sedbrook, J.C. MAPs in plant cells: Delineating microtubule growth dynamics and organization. Curr. Opin. Plant Biol. 2004, 7, 632–640. [Google Scholar] [CrossRef] [PubMed]
- Nedukha, E.M. Effects of microgravity on the structure and function of plant cell walls. Int. Rev. Cytol. 1997, 170, 39–77. [Google Scholar] [CrossRef] [PubMed]
- Hoson, T.; Soga, K.; Mori, R.; Saiki, M.; Nakamura, Y.; Wakabayashi, K.; Kamisaka, S. Stimulation of elongation growth and cell wall loosening in rice coleoptiles under microgravity conditions in space. Plant Cell Physiol. 2002, 43, 1067–1071. [Google Scholar] [CrossRef]
- Hoson, T.; Soga, K.; Wakabayashi, K.; Kamisaka, S.; Tanimoto, E. Growth and cell wall changes in rice roots during spaceflight. Plant Soil 2003, 255, 19–26. [Google Scholar] [CrossRef]
- Levine, L.H.; Heyenga, A.G.; Levine, H.G.; Choi, J.W.; Davin, L.B.; Krikorian, A.D.; Lewis, N.G. Cell-wall architecture and lignin composition of wheat developed in a microgravity environment. Phytochemistry 2001, 57, 835–846. [Google Scholar] [CrossRef]
- Hilaire, E.; Paulsen, A.Q.; Brown, C.S.; Guikema, J.A. Microgravity and clinorotation cause redistribution of free calcium in sweet clover columella cells. Plant Cell Physiol. 1995, 36, 831–837. [Google Scholar] [CrossRef]
- Klymchuk, D.O.; Brown, C.S.; Chapman, D.K.; Vorobyova, T.V.; Martyn, G.M. Cytochemical localization of calcium in soybean root cap cells in microgravity. Adv. Space Res. 2001, 27, 967–972. [Google Scholar] [CrossRef]
- Kordyum, E.L. A role for the cytoskeleton in plant cell gravisensitivity and Ca2+-signaling in microgravity. Cell Biol. Int. 2003, 27, 219–221. [Google Scholar] [CrossRef]
- Hausmann, N.; Fengler, S.; Hennig, A.; Franz-Wachtel, M.; Hampp, R.; Neef, M. Cytosolic calcium, hydrogen peroxide and related gene expression and protein modulation in Arabidopsis thaliana cell cultures respond immediately to altered gravitation: Parabolic flight data. Plant Biol. 2014, 16, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Hepler, P.; Wayne, R. Calcium and plant development. Annu. Rev. Plant Physiol. 1985, 36, 397–439. [Google Scholar] [CrossRef]
- Lee, J.; Mulkey, T.; Evans, M. Gravity-induced polar transport of calcium across root-tips of maize. Plant Physiol. 1983, 73, 874–876. [Google Scholar] [CrossRef] [PubMed]
- Knight, H. International Review of Cytology—A Survey of Cell Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2000; Volume 195, pp. 269–324. [Google Scholar]
- Bose, J.; Pottosin, I.I.; Shabala, S.S.; Palmgren, M.G.; Shabala, S. Calcium efflux systems in stress signaling and adaptation in plants. Front. Plant Sci. 2011, 2, 85. [Google Scholar] [CrossRef]
- Demidchik, V.; Maathuis, F.; Voitsekhovskaja, O. Unravelling the plant signalling machinery: An update on the cellular and genetic basis of plant signal transduction. Funct. Plant Biol. 2018, 45, 1–8. [Google Scholar] [CrossRef]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Ward, S.G.; Mills, S.J.; Liu, C.S.; Westwick, J.; Potter, B.V.L. D-myo-inositol 1,4,5-trisphosphate analogs modified at the 3-position inhibit phosphatidylinositol 3-kinase. J. Biol. Chem. 1995, 270, 12075–12084. [Google Scholar] [CrossRef]
- Miyamoto, K.; Hoshino, T.; Yamashita, M.; Ueda, J. Automorphosis of etiolated pea seedlings in space is simulated by a three-dimensional clinostat and the application of inhibitors of auxin polar transport. Physiol. Plant. 2005, 123, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Haswell, E.S. MscS-Like Proteins in Plants. Curr. Top. Membr. 2007, 58, 329–359. [Google Scholar] [CrossRef]
- Pang, Y.H.; Zhu, H.; Wu, P.; Chen, J.W. The characterization of plasma membrane Ca2+-ATPase in rich sphingomyelin-cholesterol domains. FEBS Lett. 2005, 579, 2397–2403. [Google Scholar] [CrossRef]
- Fratini, M.; Krishnamoorthy, P.; Stenzel, I.; Riechmann, M.; Matzner, M.; Bacia, K.; Heilmann, M.; Heilmann, I. Plasma membrane nano-organization specifies phosphoinositide effects on Rho-GTPases and actin dynamics in tobacco pollen tubes. Plant Cell 2021, 33, 642–670. [Google Scholar] [CrossRef] [PubMed]
- Amaral, V.S.G.; Fernandes, C.M.; Felicio, M.R.; Valle, A.S.; Quintana, P.G.; Almeida, C.C.; Barreto-Bergter, E.; Goncalves, S.; Santos, N.C.; Kurtenbach, E. Psd2 pea defensin shows a preference for mimetic membrane rafts enriched with glucosylceramide and ergosterol. Biochim. Biophys. Acta-Biomembr. 2019, 1861, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Luttgeharm, K.D.; Kimberlin, A.N.; Cahoon, R.E.; Cerny, R.L.; Napier, J.A.; Markham, J.E.; Cahoon, E.B. Sphingolipid metabolism is strikingly different between pollen and leaf in Arabidopsis as revealed by compositional and gene expression profiling. Phytochemistry 2015, 115, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Zupanska, A.K.; Denison, F.C.; Ferl, R.J.; Paul, A.L. Spaceflight engages heat shock protein and other molecular chaperone genes in tissue culture cells of Arabidopsis thaliana. Am. J. Bot. 2013, 100, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Li, M.Y.; Phillips, A.; Li, L.; Ali, U.; Li, Q.; Lu, S.P.; Hong, Y.Y.; Wang, X.M.; Guo, L. Nonspecific phospholipase C4 hydrolyzes phosphosphingolipids and sustains plant root growth during phosphate deficiency. Plant Cell 2021, 33, 766–780. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kordyum, E.L.; Artemenko, O.A.; Hasenstein, K.H. Lipid Rafts and Plant Gravisensitivity. Life 2022, 12, 1809. https://doi.org/10.3390/life12111809
Kordyum EL, Artemenko OA, Hasenstein KH. Lipid Rafts and Plant Gravisensitivity. Life. 2022; 12(11):1809. https://doi.org/10.3390/life12111809
Chicago/Turabian StyleKordyum, Elizabeth L., Olga A. Artemenko, and Karl H. Hasenstein. 2022. "Lipid Rafts and Plant Gravisensitivity" Life 12, no. 11: 1809. https://doi.org/10.3390/life12111809
APA StyleKordyum, E. L., Artemenko, O. A., & Hasenstein, K. H. (2022). Lipid Rafts and Plant Gravisensitivity. Life, 12(11), 1809. https://doi.org/10.3390/life12111809







