Increased Circulating Soluble Junctional Adhesion Molecules in Systemic Sclerosis: Association with Peripheral Microvascular Impairment
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients, Controls, and Serum Samples
2.2. Clinical Assessment
2.3. Assay for Serum sJAM-A
2.4. Assay for Serum sJAM-C
2.5. Statistical Analysis
3. Results
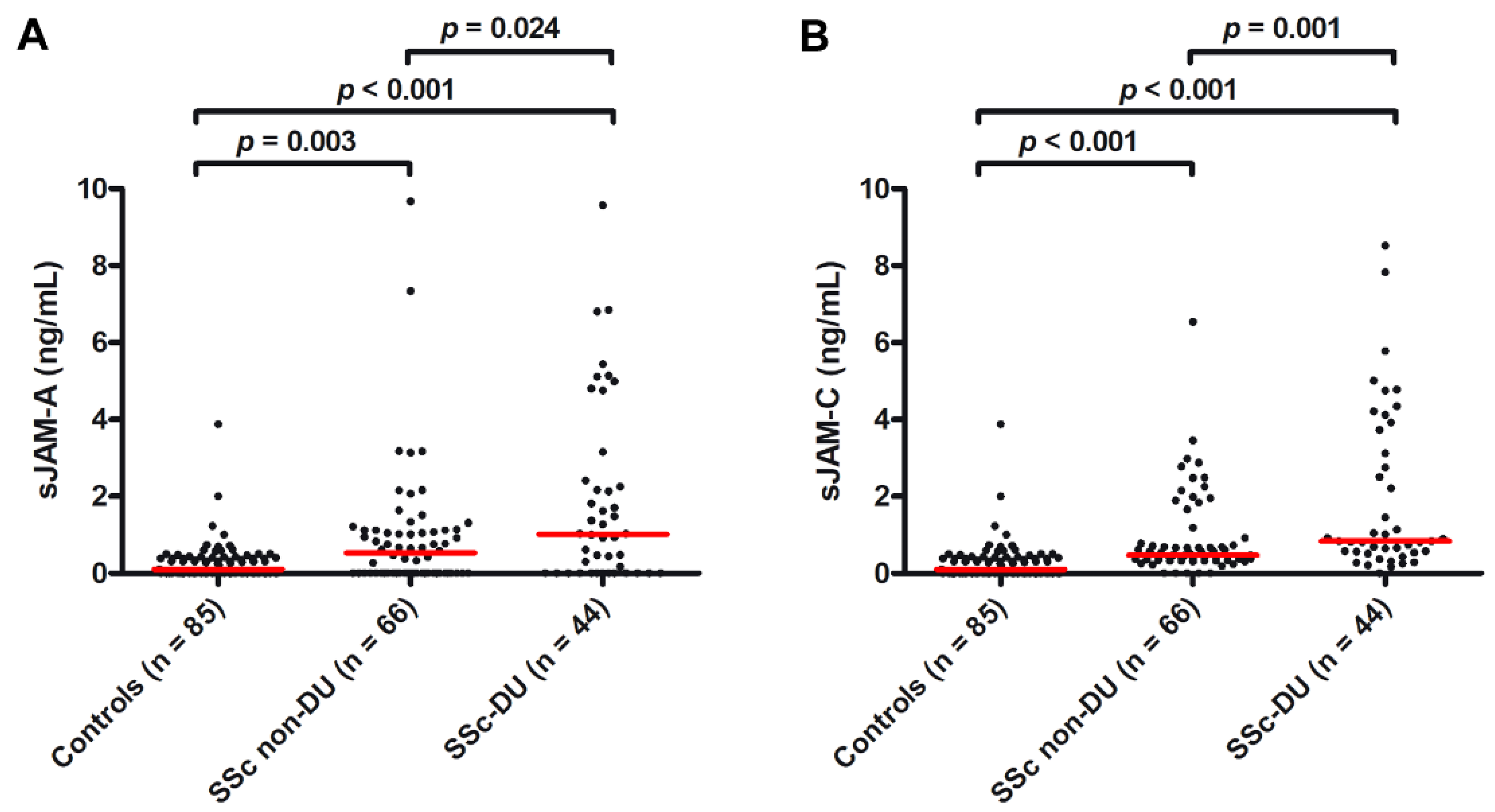
3.1. Serum Levels of Both sJAM-A and sJAM-C Are Increased in SSc Patients
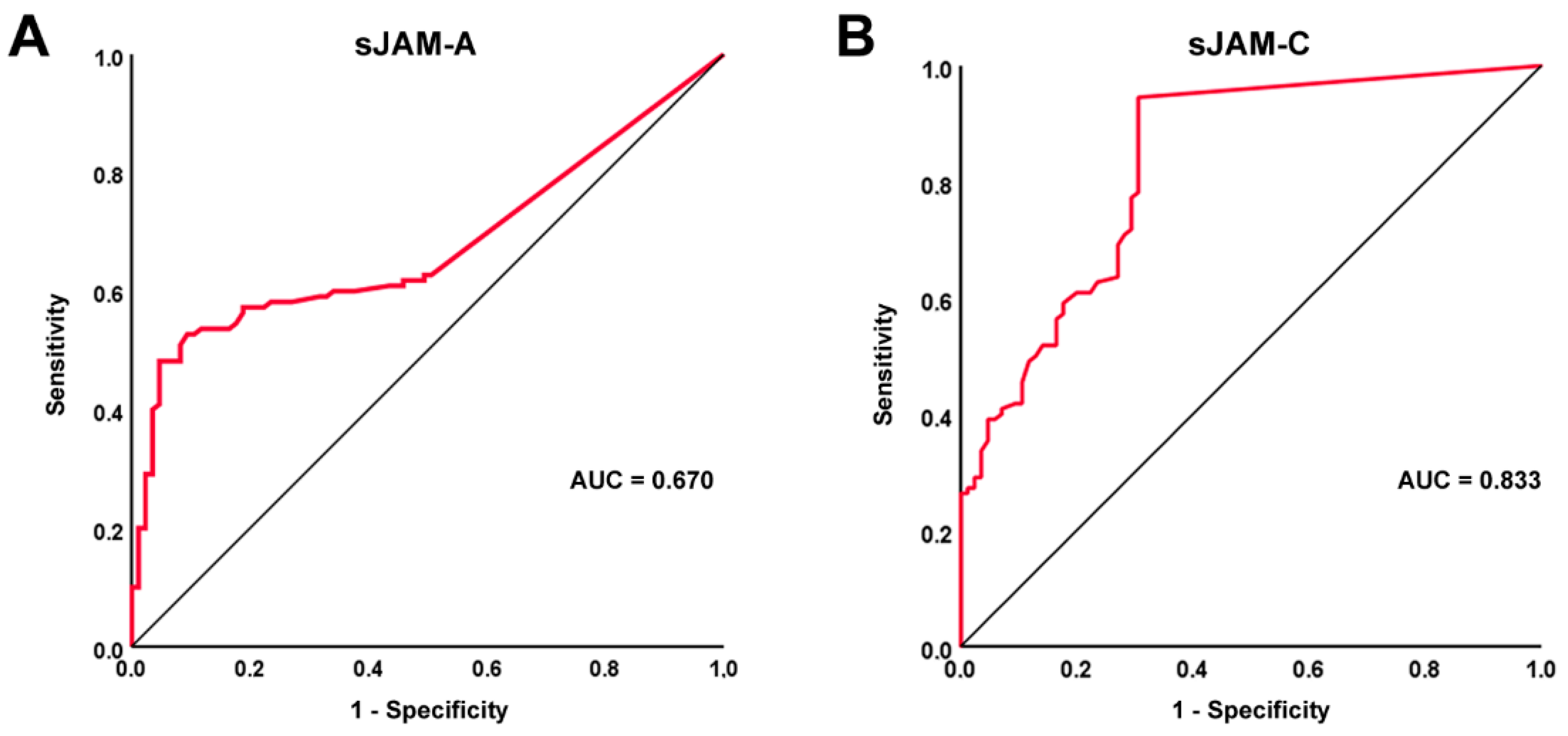
3.2. Diagnostic Accuracy of sJAM-A and sJAM-C for SSc
3.3. Association of sJAM-A and sJAM-C Serum Levels with the Severity of Microvascular Impairment
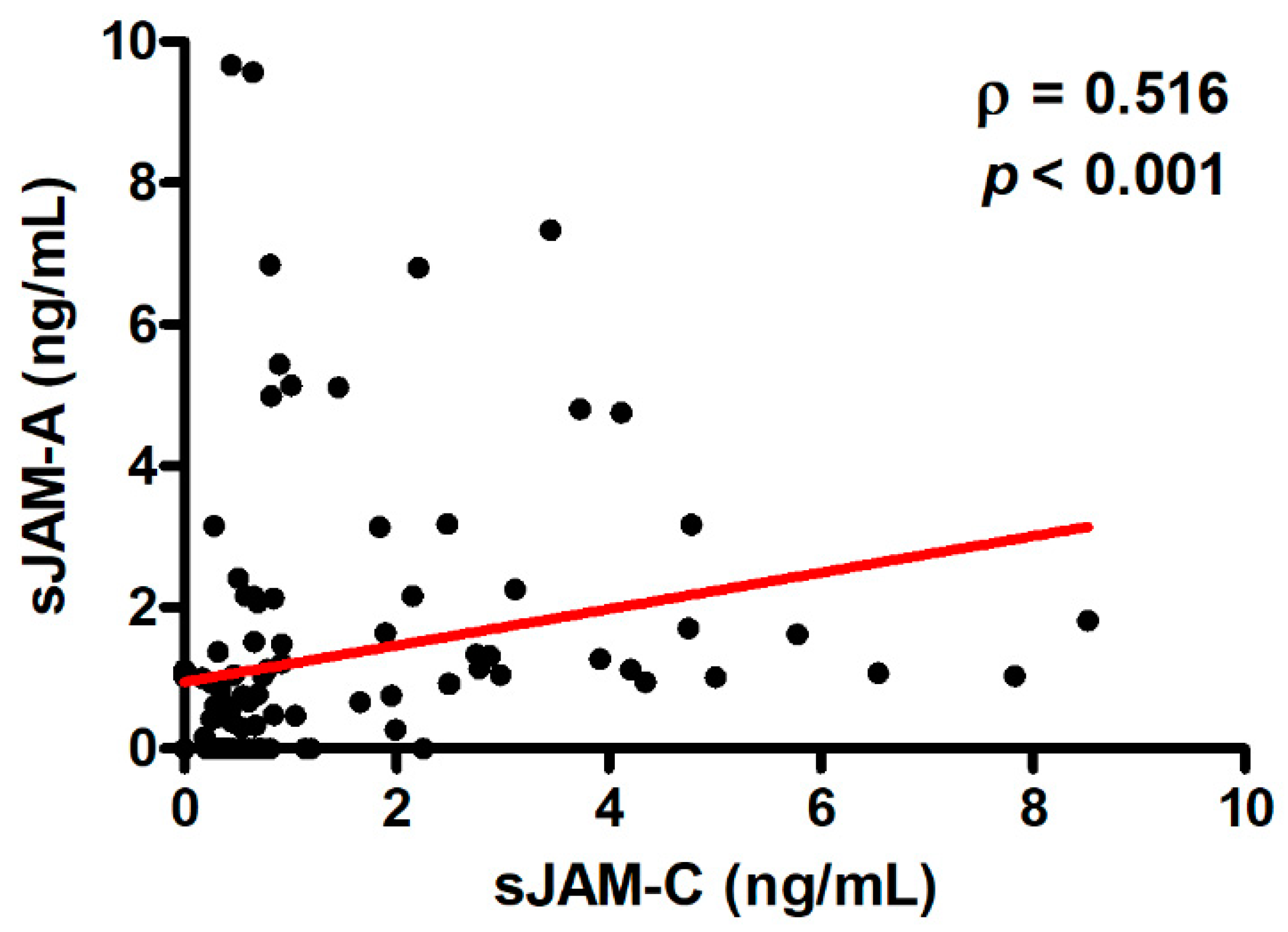
3.4. Correlation between Serum Levels of sJAM-A and sJAM-C and Logistic Regression Model
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef]
- Kobayashi, S.; Nagafuchi, Y.; Shoda, H.; Fujio, K. The pathophysiological roles of regulatory T cells in the early phase of systemic sclerosis. Front. Immunol. 2022, 13, 900638. [Google Scholar] [CrossRef] [PubMed]
- Lescoat, A.; Varga, J.; Matucci-Cerinic, M.; Khanna, D. New promising drugs for the treatment of systemic sclerosis: Pathogenic considerations, enhanced classifications, and personalized medicine. Expert. Opin. Investig. Drugs 2021, 30, 635–652. [Google Scholar] [CrossRef] [PubMed]
- Melissaropoulos, K.; Iliopoulos, G.; Sakkas, L.I.; Daoussis, D. Pathogenetic aspects of systemic sclerosis: A view through the prism of B cells. Front. Immunol. 2022, 13, 925741. [Google Scholar] [CrossRef]
- Zanin-Silva, D.C.; Santana-Gonçalves, M.; Kawashima-Vasconcelos, M.Y.; Oliveira, M.C. Management of endothelial dysfunction in systemic sclerosis: Current and developing strategies. Front. Med. 2021, 8, 788250. [Google Scholar] [CrossRef] [PubMed]
- Hughes, M.; Allanore, Y.; El Aoufy, K.; Denton, C.P.; Khanna, D.; Krieg, T.; Matucci-Cerinic, M. A practical approach to the management of digital ulcers in patients with systemic sclerosis: A narrative review. JAMA Dermatol. 2021, 157, 851–858. [Google Scholar] [CrossRef]
- Manetti, M.; Guiducci, S.; Romano, E.; Rosa, I.; Ceccarelli, C.; Mello, T.; Milia, A.F.; Conforti, M.L.; Ibba-Manneschi, L.; Matucci-Cerinic, M. Differential expression of junctional adhesion molecules in different stages of systemic sclerosis. Arthritis Rheum. 2013, 65, 247–257. [Google Scholar] [CrossRef]
- Bruni, C.; Frech, T.; Manetti, M.; Rossi, F.W.; Furst, D.E.; De Paulis, A.; Rivellese, F.; Guiducci, S.; Matucci-Cerinic, M.; Bellando-Randone, S. Vascular leaking, a pivotal and early pathogenetic event in systemic sclerosis: Should the door be closed? Front. Immunol. 2018, 9, 2045. [Google Scholar] [CrossRef]
- Polverini, P.J. Cellular adhesion molecules: Newly identified mediators of angiogenesis. Am. J. Pathol. 1996, 148, 1023–1029. [Google Scholar]
- Francavilla, C.; Maddaluno, L.; Cavallaro, U. The functional role of cell adhesion molecules in tumor angiogenesis. Semin. Cancer Biol. 2009, 19, 298–309. [Google Scholar] [CrossRef]
- Harjunpää, H.; Llort Asens, M.; Guenther, C.; Fagerholm, S.C. Cell adhesion molecules and their roles and regulation in the immune and tumor microenvironment. Front. Immunol. 2019, 10, 1078. [Google Scholar] [CrossRef] [PubMed]
- Bulska-Będkowska, W.; Czajka-Francuz, P.; Jurek-Cisoń, S.; Owczarek, A.J.; Francuz, T.; Chudek, J. The predictive role of serum levels of soluble cell adhesion molecules (sCAMs) in the therapy of advanced breast cancer-a single-centre study. Medicina 2022, 58, 153. [Google Scholar] [CrossRef] [PubMed]
- Kuryliszyn-Moskal, A.; Klimiuk, P.A.; Sierakowski, S. Soluble adhesion molecules (sVCAM-1, sE-selectin), vascular endothelial growth factor (VEGF) and endothelin-1 in patients with systemic sclerosis: Relationship to organ systemic involvement. Clin. Rheumatol. 2005, 24, 111–116. [Google Scholar] [CrossRef]
- Yanaba, K.; Yoshizaki, A.; Muroi, E.; Ogawa, F.; Shimizu, K.; Sato, S. Increased circulating soluble vascular adhesion protein-1 levels in systemic sclerosis: Association with lower frequency and severity of interstitial lung disease. Int. J. Rheum. Dis. 2013, 16, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, H. The roles of junctional adhesion molecules (JAMs) in cell migration. Front. Cell. Dev. Biol. 2022, 10, 843671. [Google Scholar] [CrossRef]
- Lauko, A.; Mu, Z.; Gutmann, D.H.; Naik, U.P.; Lathia, J.D. Junctional adhesion molecules in cancer: A paradigm for the diverse functions of cell-cell interactions in tumor progression. Cancer Res. 2020, 80, 4878–4885. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, C.; Schwietzer, Y.A.; Otani, T.; Furuse, M.; Ebnet, K. Physiological functions of junctional adhesion molecules (JAMs) in tight junctions. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183299. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, C.S.; Benson, R.A.; Brewer, J.M.; Garside, P. Targeting Opposing immunological roles of the junctional adhesion molecule-A in autoimmunity and cancer. Front. Immunol. 2020, 11, 602094. [Google Scholar] [CrossRef]
- Ong, K.L.; Leung, R.Y.; Babinska, A.; Salifu, M.O.; Ehrlich, Y.H.; Kornecki, E.; Wong, L.Y.; Tso, A.W.; Cherny, S.S.; Sham, P.C.; et al. Elevated plasma level of soluble F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) in hypertension. Am. J. Hypertens. 2009, 22, 500–505. [Google Scholar] [CrossRef]
- Hintermann, E.; Christen, U. The many roles of cell adhesion molecules in hepatic fibrosis. Cells 2019, 8, 1503. [Google Scholar] [CrossRef]
- Rabquer, B.J.; Amin, M.A.; Teegala, N.; Shaheen, M.K.; Tsou, P.S.; Ruth, J.H.; Lesch, C.A.; Imhof, B.A.; Koch, A.E. Junctional adhesion molecule-C is a soluble mediator of angiogenesis. J. Immunol. 2010, 185, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Reglero-Real, N.; Colom, B.; Bodkin, J.V.; Nourshargh, S. Endothelial cell junctional adhesion molecules: Role and regulation of expression in inflammation. Arterioscler Thromb. Vasc. Biol. 2016, 36, 2048–2057. [Google Scholar] [CrossRef] [PubMed]
- Cavusoglu, E.; Kornecki, E.; Sobocka, M.B.; Babinska, A.; Ehrlich, Y.H.; Chopra, V.; Yanamadala, S.; Ruwende, C.; Salifu, M.O.; Clark, L.T.; et al. Association of plasma levels of F11 receptor/junctional adhesion molecule-A (F11R/JAM-A) with human ather-osclerosis. J. Am. Coll. Cardiol. 2007, 50, 1768–1776. [Google Scholar] [CrossRef] [PubMed]
- Rath, D.; Rapp, V.; Schwartz, J.; Winter, S.; Emschermann, F.; Arnold, D.; Rheinlaender, J.; Büttcher, M.; Strebl, M.; Braun, M.B.; et al. Homophilic interaction between transmembrane-JAM-A and soluble JAM-A regulates thrombo-inflammation: Implications for coronary artery disease. JACC Basic Transl. Sci. 2022, 7, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Du, H.J.; Zhou, J.; Hu, D.; Wang, Y.S.; Li, X. Role of junctional adhesion molecule-C in the regulation of inner endothelial blood-retinal barrier function. Front. Cell. Dev. Biol. 2021, 9, 695657. [Google Scholar] [CrossRef]
- Salifu, M.O.; Kolff, Q.; Murty, P.; Haria, D.M.; Zimpa, M.; Shakeel, M.; Lee, H.; Kornecki, E.; Babinska, A. Relationship between the soluble F11 receptor and markers of inflammation in hemodialysis patients. J. Investig. Med. 2007, 55, 115–119. [Google Scholar] [CrossRef]
- Hou, Y.; Rabquer, B.J.; Gerber, M.L.; Del Galdo, F.; Jimenez, S.A.; Haines, G.K., 3rd; Barr, W.G.; Massa, M.C.; Seibold, J.R.; Koch, A.E. Junctional adhesion molecule-A is abnormally expressed in diffuse cutaneous systemic sclerosis skin and mediates myeloid cell adhesion. Ann. Rheum. Dis. 2010, 69, 249–254. [Google Scholar] [CrossRef]
- Rabquer, B.J.; Hou, Y.; Del Galdo, F.; Haines, G.K., 3rd; Gerber, M.L.; Jimenez, S.A.; Seibold, J.R.; Koch, A.E. The proadhesive phenotype of systemic sclerosis skin promotes myeloid cell adhesion via ICAM-1 and VCAM-1. Rheumatology 2009, 48, 734–740. [Google Scholar] [CrossRef]
- van den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A., Jr.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- LeRoy, E.C.; Black, C.; Fleischmajer, R.; Jablonska, S.; Krieg, T.; Medsger, T.A., Jr.; Rowell, N.; Wollheim, F. Scleroderma (systemic sclerosis): Classification, subsets, and pathogenesis. J. Rheumatol. 1988, 15, 202–205. [Google Scholar]
- Valentini, G.; Matucci Cerinic, M. Disease-specific quality indicators, guidelines and outcome measures in scleroderma. Clin. Exp. Rheumatol. 2007, 25, 159–162. [Google Scholar] [PubMed]
- Sulli, A.; Secchi, M.E.; Pizzorni, C.; Cutolo, M. Scoring the nailfold microvascular changes during the capillaroscopic analysis in systemic sclerosis patients. Ann. Rheum. Dis. 2008, 67, 885–887. [Google Scholar] [CrossRef] [PubMed]
- Polo, T.C.F.; Miot, H.A. Use of ROC curves in clinical and experimental studies. J. Vasc. Bras. 2020, 19, e20200186. [Google Scholar] [CrossRef] [PubMed]
- Morris, A.P.; Tawil, A.; Berkova, Z.; Wible, L.; Smith, C.W.; Cunningham, S.A. Junctional Adhesion Molecules (JAMs) are differentially expressed in fibroblasts and co-localize with ZO-1 to adherens-like junctions. Cell. Commun. Adhes. 2006, 13, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Wu, M.; Fan, X.; Chen, Z.; Xia, Z. Lentivirus-mediated subcutaneous JAM-A modification promotes skin wound healing in a mouse model by strengthening the secretory function and proliferation of fibroblasts. Cell. Biol. Int. 2022, 46, 1227–1235. [Google Scholar] [CrossRef] [PubMed]
- Czubak-Prowizor, K.; Babinska, A.; Swiatkowska, M. The F11 receptor (F11R)/junctional adhesion molecule-A (JAM-A) (F11R/JAM-A) in cancer progression. Mol. Cell. Biochem. 2022, 477, 79–98. [Google Scholar] [CrossRef]
- Shi, J.; Barakat, M.; Chen, D.; Chen, L. Bicellular tight junctions and wound healing. Int. J. Mol. Sci. 2018, 19, 3862. [Google Scholar] [CrossRef]
- Kummer, D.; Ebnet, K. Junctional adhesion molecules (JAMs): The JAM-integrin connection. Cells 2018, 7, 25. [Google Scholar] [CrossRef]
- Ebnet, K. Junctional adhesion molecules (JAMs): Cell adhesion receptors with pleiotropic functions in cell physiology and development. Physiol. Rev. 2017, 97, 1529–1554. [Google Scholar] [CrossRef]
- Weber, C.; Fraemohs, L.; Dejana, E. The role of junctional adhesion molecules in vascular inflammation. Nat. Rev. Immunol. 2007, 7, 467–477. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X. Junctional adhesion molecules: Potential proteins in atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 888818. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, E.; Bayer, M.; Ehser, J.; Aurrand-Lions, M.; Pfeilschifter, J.M.; Imhof, B.A.; Christen, U. Murine junctional adhesion molecules JAM-B and JAM-C mediate endothelial and stellate cell interactions during hepatic fibrosis. Cell. Adh. Migr. 2016, 10, 419–433. [Google Scholar] [CrossRef] [PubMed]
- Naik, M.U.; Naik, U.P. Junctional adhesion molecule-A-induced endothelial cell migration on vitronectin is integrin alpha v beta 3 specific. J. Cell. Sci. 2006, 119, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Peddibhotla, S.S.; Brinkmann, B.F.; Kummer, D.; Tuncay, H.; Nakayama, M.; Adams, R.H.; Gerke, V.; Ebnet, K. Tetraspanin CD9 links junctional adhesion molecule-A to αvβ3 integrin to mediate basic fibroblast growth factor-specific angiogenic signaling. Mol. Biol. Cell 2013, 24, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Cooke, V.G.; Naik, M.U.; Naik, U.P. Fibroblast growth factor-2 failed to induce angiogene-sis in junctional adhesion molecule-A-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2005–2011. [Google Scholar] [CrossRef]
- Naik, M.U.; Vuppalanchi, D.; Naik, U.P. Essential role of junctional adhesion molecule-1 in basic fibroblast growth factor-induced endothelial cell migration. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2165–2171. [Google Scholar] [CrossRef]
- Lamagna, C.; Hodivala-Dilke, K.M.; Imhof, B.A.; Aurrand-Lions, M. Antibody against junctional adhesion molecule-C inhibits angiogenesis and tumor growth. Cancer Res. 2005, 65, 5703–5710. [Google Scholar] [CrossRef]
- Solimando, A.G.; Da Vià, M.C.; Leone, P.; Borrelli, P.; Croci, G.A.; Tabares, P.; Brandl, A.; Di Lernia, G.; Bianchi, F.P.; Tafuri, S.; et al. Halting the vicious cycle within the multiple myeloma ecosystem: Blocking JAM-A on bone marrow endothelial cells restores angiogenic homeostasis and suppresses tumor progression. Haematologica 2021, 106, 1943–1956. [Google Scholar] [CrossRef]
- Leinster, D.A.; Colom, B.; Whiteford, J.R.; Ennis, D.P.; Lockley, M.; McNeish, I.A.; Aurrand-Lions, M.; Chavakis, T.; Imhof, B.A.; Balkwill, F.R.; et al. Endothelial cell junctional adhesion molecule C plays a key role in the development of tumors in a murine model of ovarian cancer. FASEB J. 2013, 27, 4244–4253. [Google Scholar]
- Santoso, S.; Orlova, V.V.; Song, K.; Sachs, U.J.; Andrei-Selmer, C.L.; Chavakis, T. The homophilic binding of junctional adhesion molecule-C mediates tumor cell-endothelial cell interactions. J. Biol. Chem. 2005, 280, 36326–36333. [Google Scholar] [CrossRef]
- Fuse, C.; Ishida, Y.; Hikita, T.; Asai, T.; Oku, N. Junctional adhesion molecule-C promotes metastatic potential of HT1080 human fibrosarcoma. J. Biol. Chem. 2007, 282, 8276–8283. [Google Scholar] [CrossRef] [PubMed]
- Hintermann, E.; Bayer, M.; Conti, C.B.; Fuchs, S.; Fausther, M.; Leung, P.S.; Aurrand-Lions, M.; Taubert, R.; Pfeilschifter, J.M.; Friedrich-Rust, M.; et al. Junctional adhesion molecules JAM-B and JAM-C promote autoimmune-mediated liver fibrosis in mice. J. Autoimmun. 2018, 91, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Matucci-Cerinic, M.; Manetti, M.; Bruni, C.; Chora, I.; Bellando-Randone, S.; Lepri, G.; De Paulis, A.; Guiducci, S. The “myth” of loss of angiogenesis in systemic sclerosis: A pivotal early pathogenetic process or just a late unavoidable event? Arthritis Res. Ther. 2017, 19, 162. [Google Scholar] [CrossRef]
- Lazzaroni, M.G.; Piantoni, S.; Angeli, F.; Bertocchi, S.; Franceschini, F.; Airò, P. A narrative review of pathogenetic and histopathologic aspects, epidemiology, classification systems, and disease outcome measures in systemic sclerosis. Clin. Rev. Allergy Immunol. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Moschetti, L.; Piantoni, S.; Vizzardi, E.; Sciatti, E.; Riccardi, M.; Franceschini, F.; Cavazzana, I. Endothelial dysfunction in systemic lupus erythematosus and systemic sclerosis: A common trigger for different microvascular diseases. Front. Med. 2022, 9, 849086. [Google Scholar] [CrossRef] [PubMed]
- Solimando, A.G.; Brandl, A.; Mattenheimer, K.; Graf, C.; Ritz, M.; Ruckdeschel, A.; Stühmer, T.; Mokhtari, Z.; Rudelius, M.; Dotterweich, J.; et al. JAM-A as a prognostic factor and new therapeutic target in multiple myeloma. Leukemia 2018, 32, 736–743. [Google Scholar] [CrossRef] [PubMed]
- Koenen, R.R.; Pruessmeyer, J.; Soehnlein, O.; Fraemohs, L.; Zernecke, A.; Schwarz, N.; Reiss, K.; Sarabi, A.; Lindbom, L.; Hackeng, T.M.; et al. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood 2009, 113, 4799–4809. [Google Scholar] [CrossRef]
- Houri, N.; Huang, K.C.; Nalbantoglu, J. The coxsackievirus and adenovirus receptor (CAR) undergoes ectodomain shedding and regulated intramembrane proteolysis (RIP). PLoS ONE 2013, 8, e73296. [Google Scholar] [CrossRef]
- Colom, B.; Bodkin, J.V.; Beyrau, M.; Woodfin, A.; Ody, C.; Rourke, C.; Chavakis, T.; Brohi, K.; Imhof, B.A.; Nourshargh, S. Leukotriene B4-neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity 2015, 42, 1075–1086. [Google Scholar] [CrossRef]
- Bohgaki, T.; Amasaki, Y.; Nishimura, N.; Bohgaki, M.; Yamashita, Y.; Nishio, M.; Sawada, K.I.; Jodo, S.; Atsumi, T.; Koike, T. Up regulated expression of tumour necrosis factor {alpha} converting enzyme in peripheral monocytes of patients with early systemic sclerosis. Ann. Rheum. Dis. 2005, 64, 1165–1173. [Google Scholar] [CrossRef]
- Cipriani, P.; Di Benedetto, P.; Ruscitti, P.; Liakouli, V.; Berardicurti, O.; Carubbi, F.; Ciccia, F.; Guggino, G.; Zazzeroni, F.; Alesse, E.; et al. Perivascular cells in diffuse cutaneous systemic sclerosis overexpress activated ADAM12 and are involved in myofibroblast transdifferentiation and development of fibrosis. J. Rheumatol. 2016, 43, 1340–1349. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, M.; Sugimoto, K.; Mabuchi, Y.; Yamashita, R.; Ichikawa-Tomikawa, N.; Kaneko, T.; Akazawa, C.; Hasegawa, H.; Imura, T.; Chiba, H. Soluble JAM-C ectodomain serves as the niche for adipose-derived stromal/stem cells. Biomedicines 2021, 9, 278. [Google Scholar] [CrossRef] [PubMed]
- Bellando-Randone, S.; Del Galdo, F.; Matucci-Cerinic, M. Insights into molecular and clinical characteristics of very early systemic sclerosis. Curr. Opin. Rheumatol. 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]


| Characteristics | SSc Patients (n = 110) |
|---|---|
| Age, mean ± SD (years) | 57.1 ± 14.2 |
| Sex | |
| Male | 9 (8.2) |
| Female | 101 (91.8) |
| Disease subset | |
| lcSSc | 72 (65.5) |
| dcSSc | 38 (34.5) |
| Disease duration, mean ± SD (years) | 4.3 ± 2.9 |
| Digital ulcers | 44 (40.0) |
| NVC pattern | |
| Early | 36 (32.7) |
| Active | 44 (40.0) |
| Late | 30 (27.3) |
| Vasoactive/vasodilator drugs | |
| Calcium channel blockers | 66 (60.0) |
| Endothelin receptor antagonists | 16 (14.5) |
| Phosphodiesterase 5 inhibitors | 15 (13.6) |
| Iloprost | 20 (18.2) |
| Early/Active NVC | DUs | ||
|---|---|---|---|
| sJAM-A | OR (95% CI) | 0.65 (0.41–1.04) | 1.28 (1.01–1.61) |
| p | 0.073 | 0.04 | |
| sJAM-C | OR (95% CI) | 0.75 (0.50–1.15) | 1.48 (1.12–1.96) |
| p | 0.189 | 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romano, E.; Rosa, I.; Fioretto, B.S.; Matucci-Cerinic, M.; Manetti, M. Increased Circulating Soluble Junctional Adhesion Molecules in Systemic Sclerosis: Association with Peripheral Microvascular Impairment. Life 2022, 12, 1790. https://doi.org/10.3390/life12111790
Romano E, Rosa I, Fioretto BS, Matucci-Cerinic M, Manetti M. Increased Circulating Soluble Junctional Adhesion Molecules in Systemic Sclerosis: Association with Peripheral Microvascular Impairment. Life. 2022; 12(11):1790. https://doi.org/10.3390/life12111790
Chicago/Turabian StyleRomano, Eloisa, Irene Rosa, Bianca Saveria Fioretto, Marco Matucci-Cerinic, and Mirko Manetti. 2022. "Increased Circulating Soluble Junctional Adhesion Molecules in Systemic Sclerosis: Association with Peripheral Microvascular Impairment" Life 12, no. 11: 1790. https://doi.org/10.3390/life12111790
APA StyleRomano, E., Rosa, I., Fioretto, B. S., Matucci-Cerinic, M., & Manetti, M. (2022). Increased Circulating Soluble Junctional Adhesion Molecules in Systemic Sclerosis: Association with Peripheral Microvascular Impairment. Life, 12(11), 1790. https://doi.org/10.3390/life12111790









