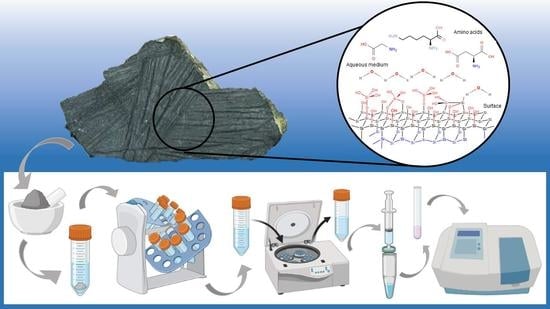
Komatiites as Complex Adsorption Surfaces for Amino Acids in Prebiotic Environments, a Prebiotic Chemistry Essay
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Cleaning of Geological Material
2.2.2. Characterization of Komatiite Sample by X-ray Diffraction Analysis
2.2.3. Estimation of the Point of Zero Charge of Geological Materials
2.2.4. Quantification of Amino Acids
2.2.5. Effect of pH Variation
2.2.6. Experimental Systems
2.2.7. Adsorption Experiments
3. Results
3.1. Characterization of Geological Materials
3.1.1. Mineral Phases in Komatiite Sample
3.1.2. Point of Zero Charge
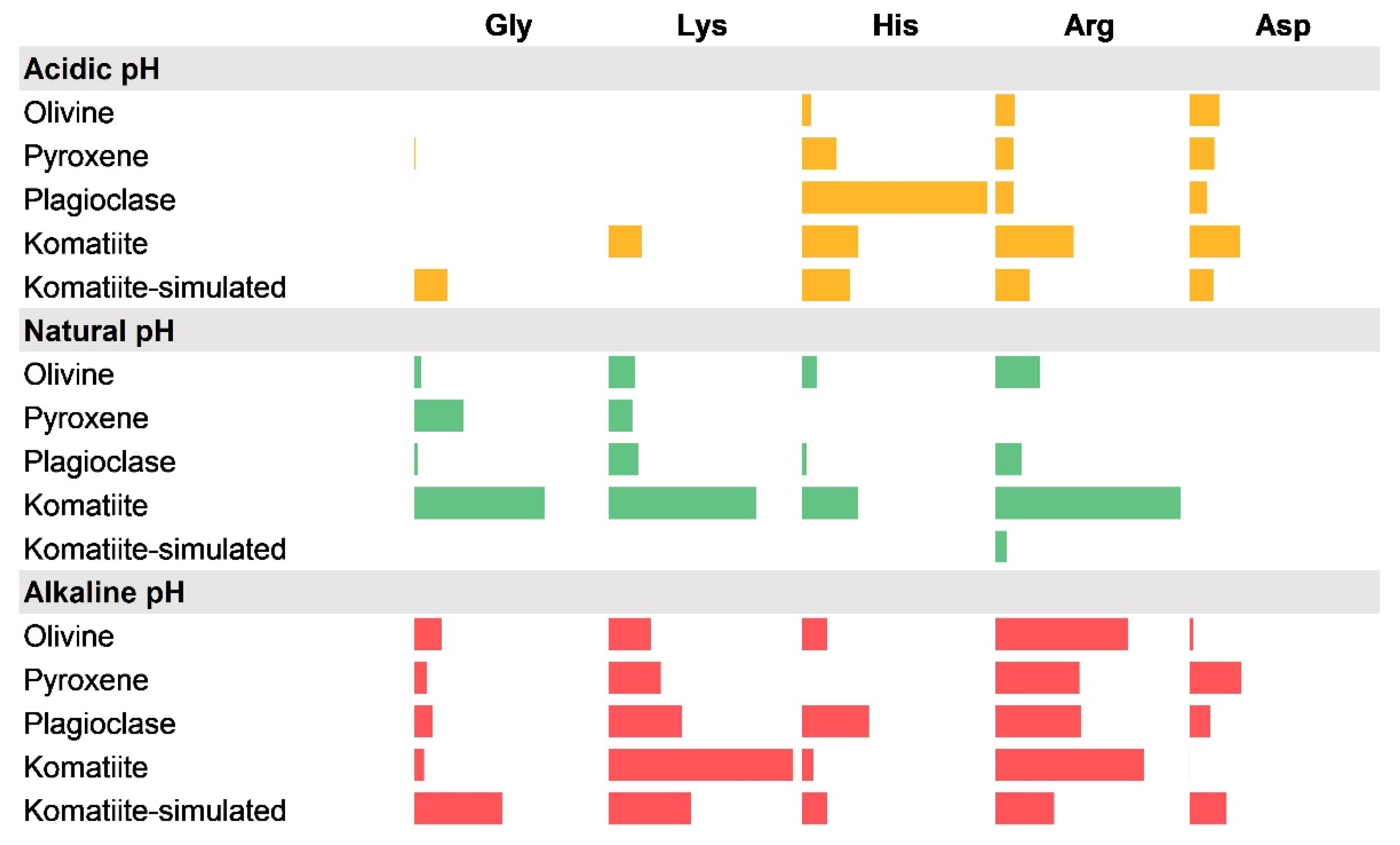
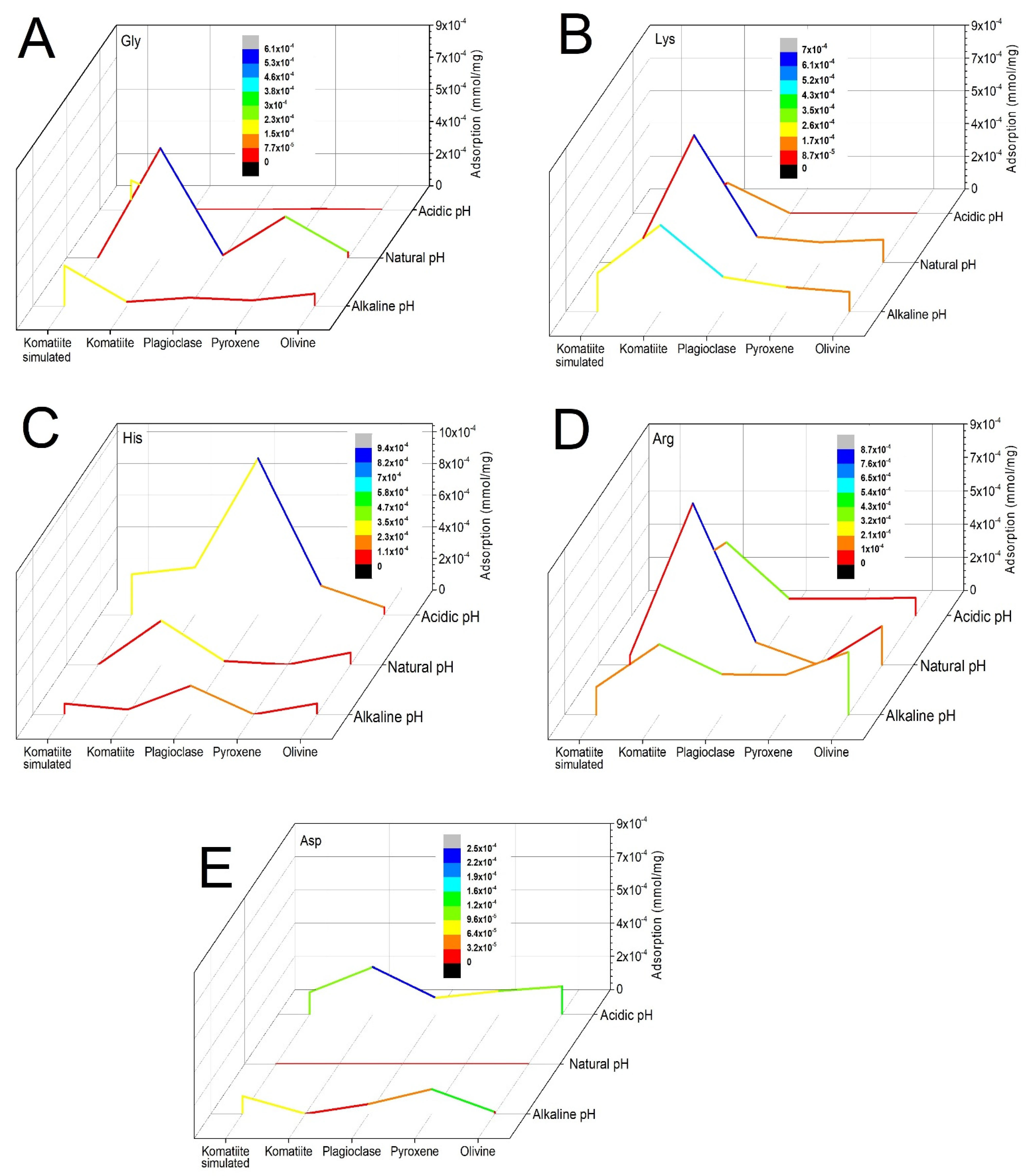
3.2. Adsorption Experiments
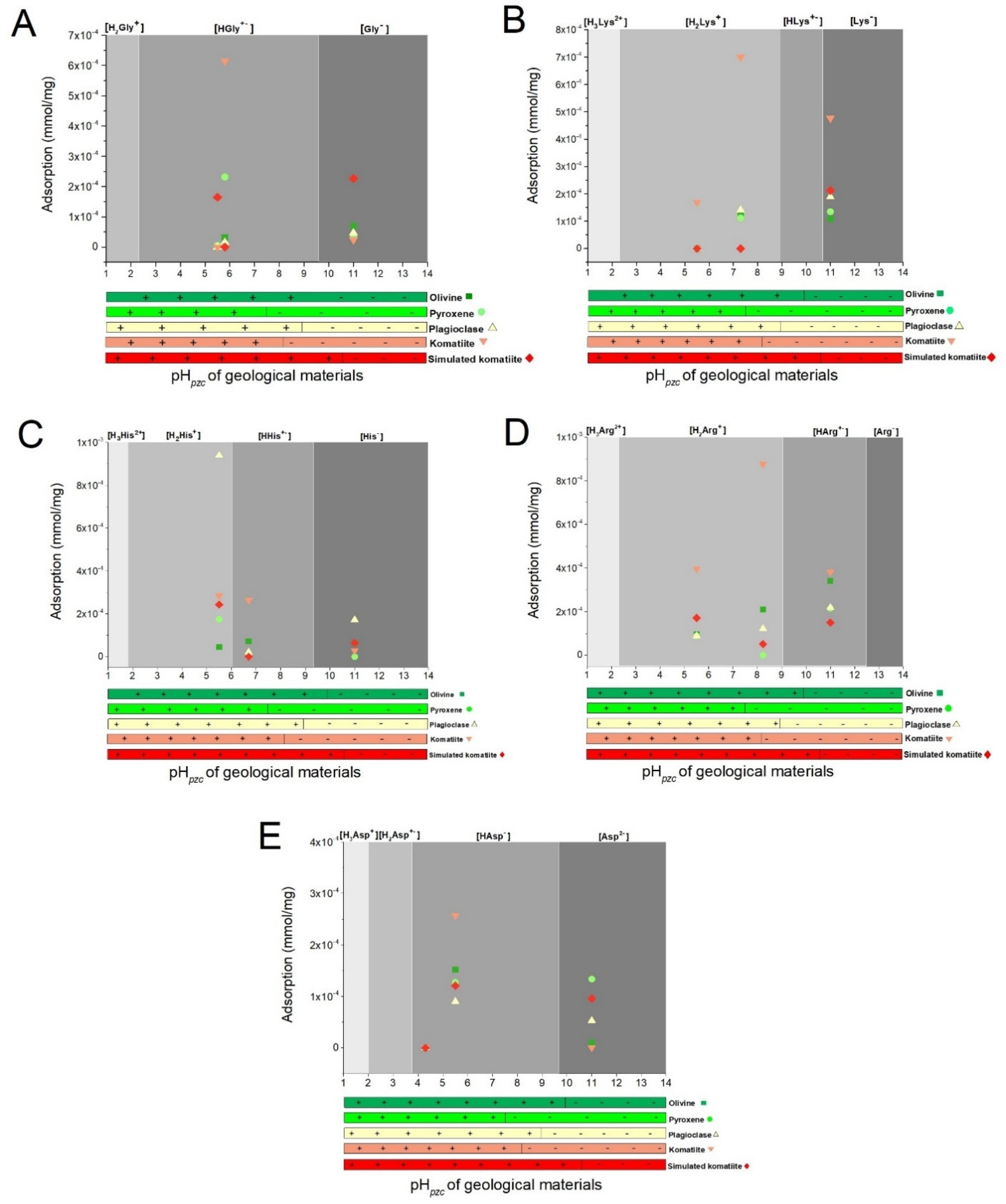
3.2.1. Adsorption Experiments at Natural pH Values
3.2.2. Adsorption Experiments at Acidic pH
3.2.3. Adsorption Experiments at Alkaline pH
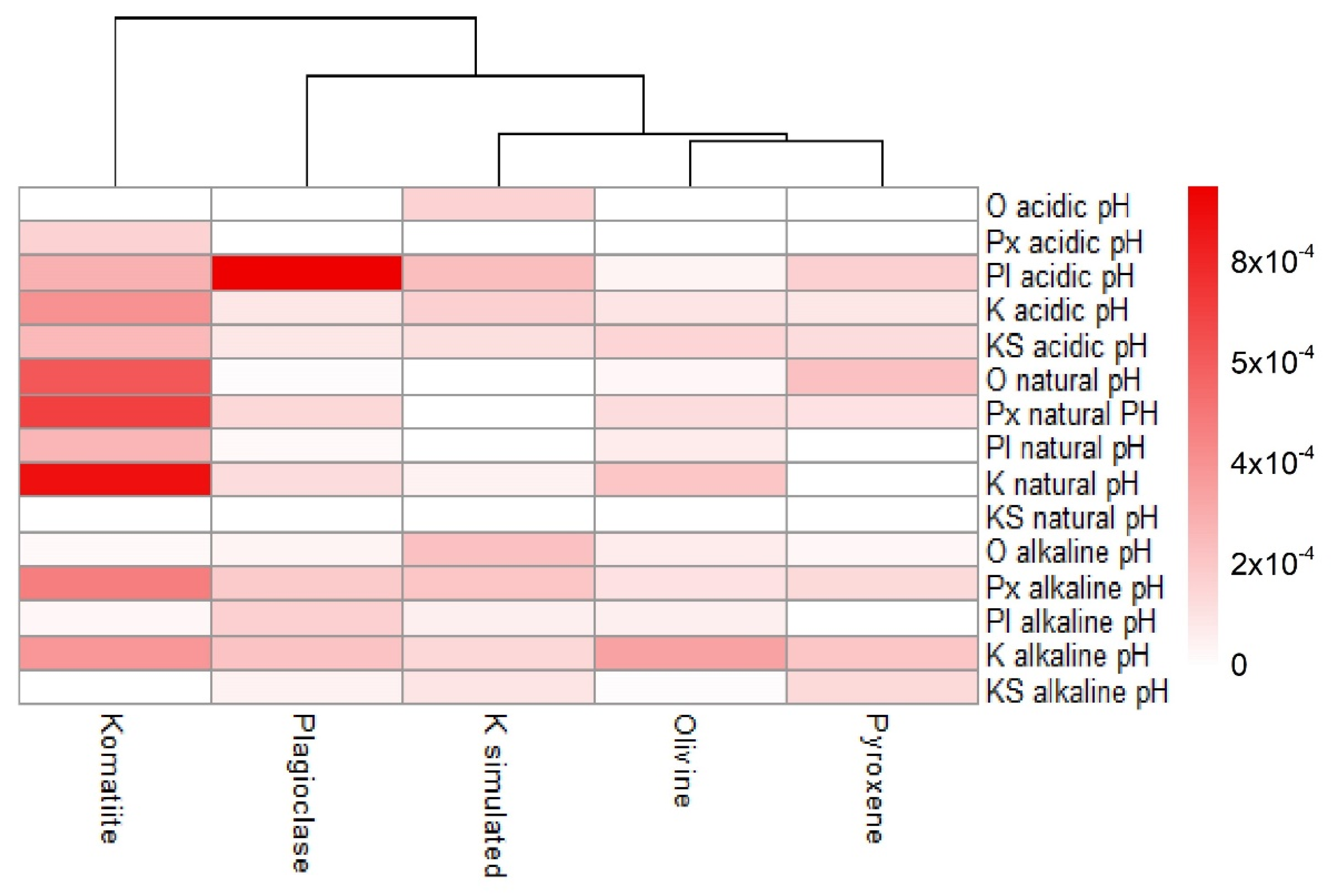
3.2.4. Adsorption Trends under Different pH Values
4. Discussion
4.1. Mineral Phases in Komatiite Sample
4.2. pzc Estimations
4.3. Adsorption of Amino Acids in Geological Materials
4.3.1. Characteristics of the Amino Acids
4.3.2. Properties of the Geological Materials
4.4. Adsorption of Amino Acids in Geological Materials under Different pH Values
4.5. Implications for Prebiotic Chemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hazen, R.M.; Papineau, D.; Bleeker, W.; Downs, R.T.; Ferry, J.M.; McCoy, T.J.; Sverjensky, D.A.; Yang, H. Mineral Evolution. Am. Mineral. 2008, 93, 1693–1720. [Google Scholar] [CrossRef]
- Ichikawa, H.; Gréaux, S.; Azuma, S. Subduction of the Primordial Crust into the Deep Mantle. Geosci. Front. 2017, 8, 347–354. [Google Scholar] [CrossRef]
- Elkins-Tanton, L.T. Linked Magma Ocean Solidification and Atmospheric Growth for Earth and Mars. Earth Planet. Sci. Lett. 2008, 271, 181–191. [Google Scholar] [CrossRef]
- Hazen, R.M.; Ferry, J.M. Mineral Evolution: Mineralogy in the Fourth Dimension. Elements 2010, 6, 9–12. [Google Scholar] [CrossRef]
- Santosh, M.; Arai, T.; Maruyama, S. Hadean Earth and Primordial Continents: The Cradle of Prebiotic Life. Geosci. Front. 2017, 8, 309–327. [Google Scholar] [CrossRef]
- Nna-Mvondo, D.; Martinez-Frias, J. Review Komatiites: From Earth’s Geological Settings to Planetary and Astrobiological Contexts. Earth Moon Planets 2007, 100, 157–179. [Google Scholar] [CrossRef]
- Martin, R. Earth’s Evolving Systems: The History of Planet Earth; Jones & Bartlett Publishers: Burlington, MA, USA, 2013; ISBN 0-7637-8001-4. [Google Scholar]
- Srivastava, R.K.; Ellam, R.M.; Gautam, G.C. Sr–Nd Isotope Geochemistry of the Early Precambrian Sub-Alkaline Mafic Igneous Rocks from the Southern Bastar Craton, Central India. Mineral. Petrol. 2009, 96, 71. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Lazcano, A. The Origin of Biomolecules. In Chemical Evolution II: From the Origins of Life to Modern Society; ACS Symposium Series; American Chemical Society: New York, NY, USA, 2009; Volume 1025, pp. 17–43. ISBN 978-0-8412-6980-4. [Google Scholar]
- Kitadai, N.; Maruyama, S. Origins of Building Blocks of Life: A Review. Geosci. Front. 2018, 9, 1117–1153. [Google Scholar] [CrossRef]
- Cockell, C.S. The Origin and Emergence of Life under Impact Bombardment. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1845–1856. [Google Scholar] [CrossRef]
- Schwartz, A.W. Did Minerals Perform Prebiotic Combinatorial Chemistry? Chem. Biol. 1996, 3, 515–518. [Google Scholar] [CrossRef]
- Ferris, J.P. Prebiotic Synthesis on Minerals: Bridging the Prebiotic and RNA Worlds. Biol. Bull. 1999, 196, 311–314. [Google Scholar] [CrossRef] [PubMed]
- Goldschmidt, V. Geochemical Aspects of the Origin of Complex Organic Molecules on the Earth, as Precursors to Organic Life. New Biol. 1952, 12, 97–105. [Google Scholar]
- Hartman, H.; Cairns-Smith, A.G. Clay Minerals and the Origin of Life; CUP Archive; Cambridge University Press: Cambridge, UK, 1986; ISBN 0-521-32408-4. [Google Scholar]
- Schoonen, M.; Smirnov, A.; Cohn, C. A Perspective on the Role of Minerals in Prebiotic Synthesis. Ambio 2004, 33, 539–551. [Google Scholar] [CrossRef]
- Zaia, D.A.M. A Review of Adsorption of Amino Acids on Minerals: Was It Important for Origin of Life? Amino Acids 2004, 27, 113–118. [Google Scholar] [CrossRef]
- Lambert, J.-F. Adsorption and Polymerization of Amino Acids on Mineral Surfaces: A Review. Orig. Life Evol. Biosph. 2008, 38, 211–242. [Google Scholar] [CrossRef] [PubMed]
- Zaia, D.A.M.; Zaia, C.T.B.V. A Few Experimental Suggestions Using Minerals to Obtain Peptides with a High Concentration of L-Amino Acids and Protein Amino Acids. Symmetry 2020, 12, 2046. [Google Scholar] [CrossRef]
- Cleaves, H.J.; Aubrey, A.D.; Bada, J.L. An Evaluation of the Critical Parameters for Abiotic Peptide Synthesis in Submarine Hydrothermal Systems. Orig. Life Evol. Biosph. 2009, 39, 109–126. [Google Scholar] [CrossRef]
- Kitadai, N. Energetics of Amino Acid Synthesis in Alkaline Hydrothermal Environments. Orig. Life Evol. Biosph. 2015, 45, 377–409. [Google Scholar] [CrossRef]
- Pizzarello, S. Molecular Asymmetry in Prebiotic Chemistry: An Account from Meteorites. Life 2016, 6, 18. [Google Scholar] [CrossRef]
- Sugahara, H.; Mimura, K. Peptide Synthesis Triggered by Comet Impacts: A Possible Method for Peptide Delivery to the Early Earth and Icy Satellites. Icarus 2015, 257, 103–112. [Google Scholar] [CrossRef]
- Goldman, N.; Reed, E.J.; Fried, L.E.; William Kuo, I.-F.; Maiti, A. Synthesis of Glycine-Containing Complexes in Impacts of Comets on Early Earth. Nat. Chem. 2010, 2, 949–954. [Google Scholar] [CrossRef]
- Dose, K. Peptides and Amino Acids in the Primordial Hydrosphere. In The Origin of Life and Evolutionary Biochemistry; Dose, K., Fox, S.W., Deborin, G.A., Pavlovskaya, T.E., Eds.; Springer: Boston, MA, USA, 1974; pp. 69–77. ISBN 978-1-4684-2115-6. [Google Scholar]
- Stribling, R.; Miller, S.L. Energy Yields for Hydrogen Cyanide and Formaldehyde Syntheses: The Hcn and Amino Acid Concentrations in the Primitive Ocean. Orig. Life Evol. Biosph. 1987, 17, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Rode, B.M. Peptides and the Origin of Life1. Peptides 1999, 20, 773–786. [Google Scholar] [CrossRef]
- Zaia, D.A.M.; Zaia, C.T.B.V.; De Santana, H. Which Amino Acids Should Be Used in Prebiotic Chemistry Studies? Orig. Life Evol. Biosph. 2008, 38, 469–488. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Samanta, M.; Ashkenasy, G.; Leman, L.J. Prebiotic Peptides: Molecular Hubs in the Origin of Life. Chem. Rev. 2020, 120, 4707–4765. [Google Scholar] [CrossRef]
- Colín-García, M.; Heredia, A.; Cordero, G.; Camprubí, A.; Negrón-Mendoza, A.; Ortega-Gutiérrez, F.; Beraldi, H.; Ramos-Bernal, S.; Colín-García, M.; Heredia, A.; et al. Hydrothermal Vents and Prebiotic Chemistry: A Review. Boletín La Soc. Geológica Mex. 2016, 68, 599–620. [Google Scholar] [CrossRef]
- Raggi, L.; Bada, J.L.; Lazcano, A. On the Lack of Evolutionary Continuity between Prebiotic Peptides and Extant Enzymes. Phys. Chem. Chem. Phys. 2016, 18, 20028–20032. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Haynes, J.W.; Petrov, A.S.; Burcar, B.T.; Krishnamurthy, R.; Hud, N.V.; Leman, L.J.; Williams, L.D. Selective Incorporation of Proteinaceous over Nonproteinaceous Cationic Amino Acids in Model Prebiotic Oligomerization Reactions. Proc. Natl. Acad. Sci. USA 2019, 116, 16338–16346. [Google Scholar] [CrossRef]
- de Souza, C.M.D.; Carneiro, C.E.A.; Baú, J.P.T.; da Costa, A.C.S.; Ivashita, F.F.; Paesano, A.; di Mauro, E.; de Santana, H.; Holm, N.G.; Neubeck, A.; et al. Interaction of Forsterite-91 with Distilled Water and Artificial Seawater: A Prebiotic Chemistry Experiment. Int. J. Astrobiol. 2013, 12, 135–143. [Google Scholar] [CrossRef]
- Starcher, B. A Ninhydrin-Based Assay to Quantitate the Total Protein Content of Tissue Samples. Anal. Biochem. 2001, 292, 125–129. [Google Scholar] [CrossRef]
- Sacchi, M.; Jenkins, S.J. Co-Adsorption of Water and Glycine on Cu{110}. Phys. Chem. Chem. Phys. 2014, 16, 6101–6107. [Google Scholar] [CrossRef] [PubMed]
- Samulewski, R.B.; Guimarães, R.T.D.; Zaia, D.A.M. Histidine Adsorption onto Modified Montmorillonite under Prebiotic Chemistry Conditions: A Thermodynamic and Kinetic Study. Int. J. Astrobiol. 2021, 20, 81–92. [Google Scholar] [CrossRef]
- Arndt, N.; Lesher, M.C.; Barnes, S.J. Komatiite; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Aitken, B.G.; Echeverría, L.M. Petrology and Geochemistry of Komatiites and Tholeiites from Gorgona Island, Colombia. Contrib. Mineral. Petrol. 1984, 86, 94–105. [Google Scholar] [CrossRef]
- Serrano, L.; Ferrari, L.; Martínez, M.L.; Petrone, C.M.; Jaramillo, C. An Integrative Geologic, Geochronologic and Geochemical Study of Gorgona Island, Colombia: Implications for the Formation of the Caribbean Large Igneous Province. Earth Planet. Sci. Lett. 2011, 309, 324–336. [Google Scholar] [CrossRef]
- Gansser, A.; Dietrich, V.J.; Cameron, W.E. Palaeogene Komatiites from Gorgona Island. Nature 1979, 278, 545–546. [Google Scholar] [CrossRef]
- Dietrich, V.J.; Gansser, A.; Sommerauer, J.; Cameron, W.E. Palaeogene Komatiites from Gorgona Island, East Pacific—A Primary Magma for Ocean Floor Basalts? Geochem. J. 1981, 15, 141–161. [Google Scholar] [CrossRef]
- Kosmulski, M. IEP as a Parameter Characterizing the PH-Dependent Surface Charging of Materials Other than Metal Oxides. Adv. Colloid Interface Sci. 2012, 171–172, 77–86. [Google Scholar] [CrossRef]
- Luce, R.W.; Parks, G.A. Point of Zero Charge of Weathered Forsterite. Chem. Geol. 1973, 12, 147–153. [Google Scholar] [CrossRef]
- Cristiano, E.; Hu, Y.-J.; Siegfried, M.; Kaplan, D.; Nitsche, H. A Comparison of Point of Zero Charge Measurement Methodology. Clays Clay Miner. 2011, 59, 107–115. [Google Scholar] [CrossRef]
- Bertus, L.M.; Carcel, R.A. Prediction of TiO2 and WO3 Nanopowders Surface Charge by Evaluation of Point of Zero Charge (PZC). Environ. Eng. Manag. J. (EEMJ) 2011, 10, 1021–1026. [Google Scholar] [CrossRef]
- Kosmulski, M. The PH-Dependent Surface Charging and the Points of Zero Charge. J. Colloid Interface Sci. 2002, 253, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Pokrovsky, O.S.; Schott, J. Forsterite Surface Composition in Aqueous Solutions: A Combined Potentiometric, Electrokinetic, and Spectroscopic Approach. Geochim. Cosmochim. Acta 2000, 64, 3299–3312. [Google Scholar] [CrossRef]
- Lasaga, A.C. Chemical Kinetics of Water-Rock Interactions. J. Geophys. Res. Solid Earth 1984, 89, 4009–4025. [Google Scholar] [CrossRef]
- Lambert, J.-F.; Stievano, L.; Lopes, I.; Gharsallah, M.; Piao, L. The Fate of Amino Acids Adsorbed on Mineral Matter. Planet. Space Sci. 2009, 57, 460–467. [Google Scholar] [CrossRef]
- Lomenech, C.; Bery, G.; Costa, D.; Stievano, L.; Lambert, J.F. Theoretical and Experimental Study of the Adsorption of Neutral Glycine on Silica from the Gas Phase. ChemPhysChem 2005, 6, 1061–1070. [Google Scholar] [CrossRef] [PubMed]
- Escamilla-Roa, E.; Moreno, F. Adsorption of Glycine by Cometary Dust: Astrobiological Implications. Planet. Space Sci. 2012, 70, 1–9. [Google Scholar] [CrossRef]
- dos Santos, R.; Patel, M.; Cuadros, J.; Martins, Z. Influence of Mineralogy on the Preservation of Amino Acids under Simulated Mars Conditions. Icarus 2016, 277, 342–353. [Google Scholar] [CrossRef]
- Niehues, G.; Heyden, M.; Schmidt, D.A.; Havenith, M. Exploring Hydrophobicity by THz Absorption Spectroscopy of Solvated Amino Acids. Faraday Discuss. 2011, 150, 193–207. [Google Scholar] [CrossRef]
- Costa, D.; Lomenech, C.; Meng, M.; Stievano, L.; Lambert, J.-F. Microsolvation of Glycine by Silanol Ligands: A DFT Study. J. Mol. Struct. Theochem 2007, 806, 253–259. [Google Scholar] [CrossRef]
- Bilgiç, C. Investigation of the Factors Affecting Organic Cation Adsorption on Some Silicate Minerals. J. Colloid Interface Sci. 2005, 281, 33–38. [Google Scholar] [CrossRef]
- Hossain, A.; Roy, S.; Dolui, B.K. Effects of Thermodynamics on the Solvation of Amino Acids in the Pure and Binary Mixtures of Solutions: A Review. J. Mol. Liq. 2017, 232, 332–350. [Google Scholar] [CrossRef]
- Liao, S.-M.; Du, Q.-S.; Meng, J.-Z.; Pang, Z.-W.; Huang, R.-B. The Multiple Roles of Histidine in Protein Interactions. Chem. Cent. J. 2013, 7, 44. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, Y.; Takani, M.; Yamauchi, O. Metal Complexes of Amino Acids and Amino Acid Side Chain Groups. Structures and Properties. Dalton Trans. 2009, 38, 7854–7869. [Google Scholar] [CrossRef] [PubMed]
- Skinner, B.J. Earth Resources. Proc. Natl. Acad. Sci. USA 1979, 76, 4212–4217. [Google Scholar] [CrossRef] [PubMed]
- Ueda, H.; Shibuya, T. Composition of the Primordial Ocean Just after Its Formation: Constraints from the Reactions between the Primitive Crust and a Strongly Acidic, CO2-Rich Fluid at Elevated Temperatures and Pressures. Minerals 2021, 11, 389. [Google Scholar] [CrossRef]
- Gao, Q.; Xu, W.; Xu, Y.; Wu, D.; Sun, Y.; Deng, F.; Shen, W. Amino Acid Adsorption on Mesoporous Materials: Influence of Types of Amino Acids, Modification of Mesoporous Materials, and Solution Conditions. J. Phys. Chem. B 2008, 112, 2261–2267. [Google Scholar] [CrossRef]
- Galvez-Martinez, S.; Escamilla-Roa, E.; Zorzano, M.-P.; Mateo-Marti, E. Defects on a Pyrite(100) Surface Produce Chemical Evolution of Glycine under Inert Conditions: Experimental and Theoretical Approaches. Phys. Chem. Chem. Phys. 2019, 21, 24535–24542. [Google Scholar] [CrossRef]
- Bada, J.L.; Miller, S.L. Ammonium Ion Concentration in the Primitive Ocean. Science 1968, 159, 423–425. [Google Scholar] [CrossRef]
- Zaia, D.A.M. Adsorption of Amino Acids and Nucleic Acid Bases onto Minerals: A Few Suggestions for Prebiotic Chemistry Experiments. Int. J. Astrobiol. 2012, 11, 229–234. [Google Scholar] [CrossRef]
- Villafañe-Barajas, S.A.; Colín-García, M.; Negrón-Mendoza, A.; Ruiz-Bermejo, M. An Experimental Study of the Thermolysis of Hydrogen Cyanide: The Role of Hydrothermal Systems in Chemical Evolution. Int. J. Astrobiol. 2020, 19, 369–378. [Google Scholar] [CrossRef]
- Damodaran, S. Amino Acids, Peptides and Proteins. Fennemas Food Chem. 2008, 4, 217–329. [Google Scholar]
- Churchill, H.; Teng, H.; Hazen, R.M. Correlation of PH-Dependent Surface Interaction Forces to Amino Acid Adsorption: Implications for the Origin of Life. Am. Mineral. 2004, 89, 1048–1055. [Google Scholar] [CrossRef]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Role of Mineral Surfaces in Prebiotic Chemical Evolution. In Silico Quantum Mechanical Studies. Life 2019, 9, 10. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-López, A.; Colín-García, M.; Ortega-Gutiérrez, F.; Cruz-Castañeda, J. Role of the Interchangeable Cations on the Sorption of Fumaric and Succinic Acids on Montmorillonite and Its Relevance in Prebiotic Chemistry. Orig. Life Evol. Biosph. 2021, 51, 87–116. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.M.; Methivier, C.; Dubot, P.; Pradier, C.M. Adsorption of (S)-Histidine on Cu(110) and Oxygen-Covered Cu(110), a Combined Fourier Transform Reflection Absorption Infrared Spectroscopy and Force Field Calculation Study. J. Phys. Chem. B 2003, 107, 10785–10792. [Google Scholar] [CrossRef]
- Dąbrowski, A. Adsorption—From Theory to Practice. Adv. Colloid Interface Sci. 2001, 93, 135–224. [Google Scholar] [CrossRef]
- Hazen, R.M.; Eldredge, N. Themes and Variations in Complex Systems. Elements 2010, 6, 43–46. [Google Scholar] [CrossRef]
- Bost, N.; Loiselle, L.; Foucher, F.; Ramboz, C.; Westall, F.; Gaillard, F.; Auguste, J.-L. Synthesis of Gusev Crater Analogue Basalts, Mars: Interest for Astrobiology. In Proceedings of the 44th Lunar and Planetary Science Conference, The Woodlands, TX, USA, 18–22 March 2013; Lunar and Planetary Institute (LPI)/Universities Space Research Association (USRA). Abstract Number (1457l). Available online: https://www.lpi.usra.edu/meetings/lpsc2013/pdf/1457.pdf (accessed on 1 November 2022).
- Dass, A.V.; Hickman-Lewis, K.; Brack, A.; Kee, T.P.; Westall, F. Stochastic Prebiotic Chemistry within Realistic Geological Systems. ChemistrySelect 2016, 1, 4906–4926. [Google Scholar] [CrossRef]
- Galvez-Martinez, S.; Escamilla-Roa, E.; Zorzano, M.-P.; Mateo-Marti, E. Ar+ Ion Bombardment Dictates Glycine Adsorption on Pyrite (100) Surface: X-Ray Photoemission Spectroscopy and DFT Approach. Appl. Surf. Sci. 2020, 530, 147182. [Google Scholar] [CrossRef]
- Villafañe-Barajas, S.A.; Ruiz-Bermejo, M.; Rayo-Pizarroso, P.; Gálvez-Martínez, S.; Mateo-Martí, E.; Colín-García, M. A Lizardite-HCN Interaction Leading the Increasing of Molecular Complexity in an Alkaline Hydrothermal Scenario: Implications for Origin of Life Studies. Life 2021, 11, 661. [Google Scholar] [CrossRef]
- Potenti, S.; Manini, P.; Fornaro, T.; Poggiali, G.; Crescenzi, O.; Napolitano, A.; Brucato, J.R.; Barone, V.; d’Ischia, M. Solid State Photochemistry of Hydroxylated Naphthalenes on Minerals: Probing Polycyclic Aromatic Hydrocarbon Transformation Pathways under Astrochemically-Relevant Conditions. ACS Earth Space Chem. 2018, 2, 977–1000. [Google Scholar] [CrossRef]

| Amino Acid | Classification | Formula | pKa | pI | ||
|---|---|---|---|---|---|---|
| pK1 | pK2 | pK3 | ||||
| Gly | Neutral | 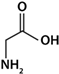 | 2.34 | 9.60 | - | 5.98 |
| Lys | Basic | 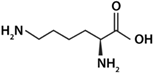 | 2.18 | 8.95 | 10.53 | 9.74 |
| His | Basic | 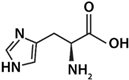 | 1.82 | 9.17 | 6.00 | 7.59 |
| Arg | Basic | 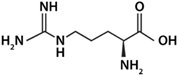 | 2.17 | 9.04 | 12.48 | 10.76 |
| Asp | Acid | 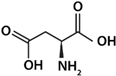 | 1.88 | 9.60 | 3.65 | 2.77 |
| Amino Acid | pH Value |
|---|---|
| Gly | 5.8 ± 0.51 |
| Lys | 7.3 ± 0.26 |
| His | 6.7 ± 0.23 |
| Arg | 8.23 ± 0.6 |
| Asp | 4.3 ± 0.28 |
| Mineral Phases | SemiQuant RIR (%) |
|---|---|
| Olivine (forsterite: Mg2SiO4) | 10 |
| Pyroxene (diopside: CaMgSi2O8) | 14 |
| Plagioclase (bytownite: (NaCa)AlSi3O8) | 26 |
| Serpentine (lizardite:Mg3Si2O5(OH)4) | 29 |
| Other phyllosilicates ~14 Å ppb type of chlorite | 21 |
| Total | 100 |
| Geological Materials | pzc |
|---|---|
| Olivine | 9.9 |
| Pyroxene | 7.5 |
| Plagioclase | 8.9 |
| Komatiite | 8.2 |
| Komatiite-simulated Olivine 49.29% Plagioclase 29.57% Pyroxene 21.12% | 10.05 |
| Geological Materials | Adsorption (mmol/mg) | ||||
|---|---|---|---|---|---|
| Gly ± SD | Lys ± SD | His ± SD | Arg ± SD | Asp | |
| Olivine | 3.18 × 10−5 ± 2.2 × 10−6 | 1.27 × 10−4 ± 4.9 × 10−6 | 7.17 × 10−5 ± 1.9 × 10−6 | 2.11 × 10−4 ± 5.1 × 10−6 | - |
| Pyroxene | 2.31 × 10−4 ± 7 × 10−6 | 1.12 × 10−4 ± 2 × 10−6 | - | - | - |
| Plagioclase | 1.55 × 10−5 ± 1.4 × 10−7 | 1.41 × 10−4 ± 9.8 × 10−6 | 2.15 × 10−5 ± 2.1 × 10−8 | 1.22 × 10−4 ± 1.8 × 10−7 | - |
| Komatiite | 6.14 × 10−4 ± 3.3 × 10−6 | 6.99 × 10−4 ± 4.3 × 10−5 | 2.64 × 10−4 ± 1.0 × 10−6 | 8.76 × 10−4 ± 1.4 × 10−4 | - |
| Komatiite-simulated | - | - | - | 5.14 × 10−5 ± 4.1 × 10−7 | - |
| Geological Materials | Adsorption (mmol/mg) | ||||
|---|---|---|---|---|---|
| Gly ± SD | Lys ± SD | His ± SD | Arg ± SD | Asp ± SD | |
| Olivine | - | - | 4.52 × 10−5 ± 3.8 × 10−6 | 9.55 × 10−5 ± 1 × 10−6 | 1.52 × 10−4 ± 1.4 × 10−6 |
| Pyroxene | 4.74 × 10−6 ± 1.9 × 10−8 | - | 1.75 × 10−4 ± 4.9 × 10−6 | 8.94 × 10−5 ± 1.8 × 10−7 | 1.27 × 10−4 ± 2.1 × 10−6 |
| Plagioclase | - | - | 9.40 × 10−4 ± 1.2 × 10−5 | 8.93 × 10−5 ± 2.7 × 10−7 | 9.00 × 10−5 ± 5.4 × 10−7 |
| Komatiite | - | 1.69 × 10−4 ± 4.6 × 10−6 | 2.85 × 10−4 ± 4.2 × 10−5 | 3.96 × 10−4 ± 2.4 × 10−6 | 2.57 × 10−4 ± 1 × 10−5 |
| Komatiite-simulated | 1.65 × 10−4 ± 3.3 × 10−6 | - | 2.44 × 10−4 ± 2.5 × 10−6 | 1.72 × 10−4 ± 4.1 × 10−6 | 1.21 × 10−4 ± 4.5 × 10−6 |
| Geological Materials | Adsorption (mmol/mg) | ||||
|---|---|---|---|---|---|
| Gly ± SD | Lys ± SD | His ± SD | Arg ± SD | Asp ± SD | |
| Olivine | 6.90 × 10−5 ± 2.6 × 10−6 | 1.08 × 10−4 ± 2.1 × 10−6 | 6.52 × 10−5 ± 2.7 × 10−6 | 3.40 × 10−4 ± 1.3 × 10−5 | 1.04 × 10−5 ± 3.5 × 10−7 |
| Pyroxene | 3.18 × 10−5 ± 3.4 × 10−7 | 1.35 × 10−4 ± 1.5 × 10−6 | - | 2.15 × 10−4 ± 1.6 × 10−6 | 1.34 × 10−4 ± 2.7 × 10−6 |
| Plagioclase | 4.68 × 10−5 ± 1.4 × 10−6 | 1.90 × 10−4 ± 1.2 × 10−5 | 1.72 × 10−4 ± 1.1 × 10−5 | 2.19 × 10−4 ± 9.3 × 10−6 | 5.29 × 10−5 ± 1.7 × 10−6 |
| Komatiite | 2.39 × 10−5 ± 2.9 × 10−7 | 4.76 × 10−4 ± 1.5 × 10−5 | 2.91 × 10−5 ± 3 × 10−7 | 3.82 × 10−4 ± 8.8 × 10−5 | 5.70 × 10−7 ± 2.7 × 10−8 |
| Komatiite-simulated | 2.27 × 10−4 ± 4.5 × 10−6 | 2.12 × 10−4 ± 3.6 × 10−6 | 6.45 × 10−5 ± 2.3 × 10−6 | 1.50 × 10−4 ± 3.3 × 10−5 | 5.57 × 10−5 ± 6.8 × 10−7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz-Hernández, A.E.; Colín-García, M.; Ortega-Gutiérrez, F.; Mateo-Martí, E. Komatiites as Complex Adsorption Surfaces for Amino Acids in Prebiotic Environments, a Prebiotic Chemistry Essay. Life 2022, 12, 1788. https://doi.org/10.3390/life12111788
Cruz-Hernández AE, Colín-García M, Ortega-Gutiérrez F, Mateo-Martí E. Komatiites as Complex Adsorption Surfaces for Amino Acids in Prebiotic Environments, a Prebiotic Chemistry Essay. Life. 2022; 12(11):1788. https://doi.org/10.3390/life12111788
Chicago/Turabian StyleCruz-Hernández, Abigail E., María Colín-García, Fernando Ortega-Gutiérrez, and Eva Mateo-Martí. 2022. "Komatiites as Complex Adsorption Surfaces for Amino Acids in Prebiotic Environments, a Prebiotic Chemistry Essay" Life 12, no. 11: 1788. https://doi.org/10.3390/life12111788
APA StyleCruz-Hernández, A. E., Colín-García, M., Ortega-Gutiérrez, F., & Mateo-Martí, E. (2022). Komatiites as Complex Adsorption Surfaces for Amino Acids in Prebiotic Environments, a Prebiotic Chemistry Essay. Life, 12(11), 1788. https://doi.org/10.3390/life12111788