Abstract
What kinds of CHOS compounds might be formed in a prebiotic milieu by reducing CO2 in the presence of H2 and H2S? How might the presence of sulfur influence the chemical composition of the mixture? We explore these questions by using first-principles quantum chemistry to calculate the free energies of CHOS compounds in aqueous solution, by first generating a thermodynamic map of one- and two-carbon species. We find that while thiols are thermodynamically favored, thioesters, thioacids, and thiones are less favorable than their non-sulfur counterparts. We then focus on the key role played by mercaptoacetaldehyde in sulfur analogs of the autocatalytic formose reaction, whereby the thiol group introduces asymmetry and potential thermodynamic selectivity of some compounds over others.
1. Introduction
Sulfur has been implicated in origin-of-life scenarios, from the discovery of chemotrophic organisms at hydrothermal vents and the proposal of pyrite surfaces [1] as a driving force for prebiotic metabolism, to De Duve’s thioester world [2] and more recent work invoking a cyanosulfidic world [3] as prebiotic milieus. The core of extant metabolism [4] consists of a small subset of molecules containing the elements carbon, hydrogen, and oxygen. Sulfur participates in the form of Coenzyme A at specific junctures, embedded in a system that is overall highly regulated by enzymes and co-factors. Sulfur also participates through amino acids in extant life; see Youssef-Saliba and Vallée for a recent review in their role and significance in prebiotic chemistry [5].
Energy is at the heart of metabolism in extant life, and we expect it to be the driving force in the dynamic creation of proto-metabolic chemical systems. Our most recent study presented first-principles quantum chemical calculations of the relative free energies in aqueous solution of a wide range of potential CHO-containing metabolites [6]. In that scenario, sulfur was not included, and the free energies of CHO compounds were calculated with reference to carbon dioxide (specifically H2CO3 as the aqueous form) and molecular hydrogen as the reducing agent. In that work, we zeroed in on the smallest potential autocatalytic cycle that utilized a C1 molecule as the “food” source, and a linchpin C2 molecule that is regenerated in a three-step reaction cycle: C2 + C1 → C3, C3 + C1 → C4, and C4 → C2 + C2. This smallest cycle is present in the autocatalytic formose reaction [7], amidst a mess of larger cycles and side reactions. The result is a complex and messy mixture with a large array of compounds [8]. A recent excellent review on the formose reaction in prebiotic chemistry including issues of chirality has been written by Martinez et al. [9].
Our present study treads similar ground, but includes the addition of H2S as both reactant and reductant; H2S is the source of incorporating sulfur in the formation of CHOS compounds. To get a lay of the land, we first calculate the relative free energies of the C1 and C2 CHOS compounds that are potentially formed, and compare these to the CHO compounds from our previous work. We find that thiols are thermodynamically favored over their alcohol counterparts, whereas thiones (C=S) are disfavored relative to carbonyl (C=O) groups. (We use the nomenclature for sulfur compounds provided by Toohey and Cooper [10]). Thiocarboxylic acids (both the carbothioic O-acid and S-acid) are also disfavored relative to carboxylic acids; we will refer to these as the thione-acid and the thioacid, respectively, for short. In examining the thermodynamics of coupling reactions that form C-S bonds, we find that formation of dialkylsulfides is exergonic, while condensation reactions to form thioesters are endergonic.
This paper then focuses on investigating both the thermodynamics and kinetics of the simplest formose-like autocatalytic cycle for CHOS compounds, comparing our free energy map to our previous calculations on the CHO system [11]. The inclusion of sulfur in the system increases the number of possible chemical species in the mixture. Furthermore, the asymmetry provided by having thiol groups leads to energetic differences favoring some compounds over others. In a sense, the thiol acts as a “directing” group that influences both the kinetics and thermodynamics of the aldol-like reactions in this system. We also analyze how sulfur influences the disproportionation (Cannizzaro) reactions that give rise to a range of compounds with different oxidation states. These “side” reactions (which are arguably critical towards generating a diverse mixture) are a significant part of the messy formose reaction [8].
While thioesters are of interest in the construction of proto-metabolic cycles, they are not a focus of this paper. The present work on the CHOS analog to autocatalytic cycles in the formose reaction sets the stage for exploring the role of thioesters in such cycles, and we are actively generating data that will be presented in a follow-up paper. The present paper is structured as follows. After describing our computational methods, the combined results and discussion section (Section 3) will cover (1) formation of C1 CHOS compounds, (2) a survey of the energy landscape of C2 CHOS compounds, (3) a discussion of C-S bond forming reactions, (4) our detailed examination of the CHOS analog of the formose reaction, (5) investigating if thiol groups can influence the selection of some sugars over others, and (6) products formed in the CHOS analog to the Cannizzaro reaction.
2. Materials and Methods
Since we will be comparing our calculations on the CHOS system to our previous work on the CHO system, we use the same computational protocol found in those papers [6,11]. We provide herein a brief description of that protocol for the convenience of our readers. Some of the text in this section is reproduced from our most recent work [6] since we think our previous description is both clear and succinct. Essentially, we calculate the free energies using quantum chemical methods, and our protocol shows good agreement with available experimental results for CHO systems [11,12,13]. Here, are the computational details:
The structure of each molecule is optimized and its electronic energy calculated at the B3LYP [14,15,16,17] flavor of density functional theory with the 6-311G** basis set. To maximize the probability of finding global minima, multiple conformers are generated using molecular mechanics (MMFFs force field [18]). The optimized structures are embedded in a Poisson-Boltzmann continuum to calculate the aqueous solvation contribution to the free energy. While this does not provide a specific concentration, it assumes a dilute solution such that the electrostatic field generated by a neighboring solute molecule is effectively screened by the water solvent. One can consider all solutes to have the same relative concentrations in our calculations. Zero-point energy corrections are included, and we apply the standard temperature-dependent enthalpy correction term (for 298.15 K) from statistical mechanics by assuming translational and rotational corrections are a constant times kT, and that low frequency vibrational modes generally cancel out when calculating enthalpy differences. So far, this is standard fare.
However, entropic corrections in aqueous solution are more problematic [19,20,21]. Changes in free energy terms for translation and rotation are poorly defined in solution due to restricted complex motion, particularly as the size of the molecule increases (thus increasing its conformational entropy). Free energy corrections come from two different sources: thermal corrections and implicit solvent. Neither of these parameters is easily separable, nor do they constitute all the required parts of the free energy. We follow the approach of Deubel & Lau [22], assigning the solvation entropy of each species as half its gas-phase entropy (calculated using standard statistical mechanics approximations similar to the enthalpy calculations described above), based on proposals by Wertz [23] and Abraham [24] that upon dissolving in water, molecules lose a constant fraction (~0.5) of their entropy.
To estimate activation energies, transition states were optimized by including several explicit water molecules (two is usually optimal, but occasionally one or three give lower barriers) to aid proton transfer. All calculated transition states have one significant negative eigenvalue corresponding to the reaction coordinate (eigenvector) involving bond breaking/forming. Several conformers are tested in each case and we only report the lowest calculated barriers.
When put to the test by first calculating the equilibrium concentrations in a self-oligomerizing solution of 1 M glycolaldehyde at 298 K, our protocol fared very well compared to subsequent NMR measurements [13]. Our relative Gibbs free energies in aqueous solution are typically within 0.5 kcal/mol compared to experiment. That being said, our protocol did show systematic errors of 2–3 kcal/mol when calculating barriers and comparing to experimental results. Going to a higher level of theory does not reduce this error [25]. This may seem surprising but quantum chemistry is about error cancellation, and our protocol (with its foibles, including the simplistic entropy correction) has worked well even with this systematic error for activation barriers. Thus, we do well on thermodynamics and just okay on kinetics (but at least we’re in the ballpark).
The relative aqueous Gibbs free energies, designated Gr0, are calculated with respect to the reference molecules: CO2, H2, H2S and H2O. These molecules are assigned Gr0 = 0.0 kcal/mol. (Note that in our most recent work, we used H2CO3 instead of CO2 as the reference to directly compare with experimentally derived thermodynamic data from Alberty [26], but since this same experimental data is not available for the sulfur-compounds, it is “cleaner” to use CO2 as the reference.) Our reported Gr0 values are for the lowest energy conformer of each structure. Assigning reference molecules allows us to quickly compare energies among various compounds. For a chemical reaction, the difference in free energies will be designated ΔG, calculated as Gr0(products) − Gr0(reactants). Sample calculations will be shown in the first part of the Results and Discussion section (Section 3).
3. Results and Discussion
3.1. Formation of C1 Compounds and Choosing Reference States
With the reference molecules (CO2, H2, H2S and H2O) assigned Gr0 = 0.0 kcal/mol, we can determine Gr0 values for CHOS species by calculating ΔG for the formation reaction of each compound where the carbon “food” source is CO2, the sulfur source is H2S, additional source of hydrogen as a reductant comes from H2, and H2O is a byproduct of the reduction reaction. We group compounds by oxidation number of carbon, formally calculated by assigning the oxidation numbers of H (+1), O(−2) and S(−2). Thus, in CO2, carbon has oxidation number +4. If CO2 is fully reduced to methane (carbon in −4 oxidation state),
and therefore, Gr0 of CH4 is −39.5 kcal/mol. If CO2 is not fully reduced, there are several possibilities.
CO2 + 4 H2 → CH4 + 2 H2O ΔG = –39.5 kcal
- (a)
- Carbon in −2 oxidation state:
CO2 + 3 H2 → CH3OH + H2O ΔG = –11.2 kcal
CO2 + H2S + 2 H2 → CH3SH + 2 H2O ΔG = –19.2 kcal
Thus, Gr0 of CH3OH and CH3SH are −11.2 and −19.2 kcal/mol respectively. Both reactions are exergonic. The thiol is more stable than the alcohol by 8 kcal/mol. In a prebiotic environment where H2S is present to reduce CO2, we expect to observe methanethiol. The relative amount of H2S versus H2 would lead to different relative ratios of CH3SH and CH3OH in the product mixture (There would of course be many other compounds!).
- (b)
- Carbon in zero oxidation state:
CO2 + 2 H2 → CH2O + H2O ΔG = +7.9 kcal
CO2 + H2S + H2 → CH2S + 2 H2O ΔG = +21.2 kcal
Thus, Gr0 of CH2O and CH2S are +7.9 and +21.2 kcal/mol respectively. Both reactions are endergonic, i.e., it is thermodynamically unfavorable to form formaldehyde and its thione counterpart by reducing CO2. Forming CH2S with its weaker C=S double bond is significantly unfavorable. However, in aqueous solution, hydration can take place across the double bond.
CO2 + 2 H2 → CH2(OH)2 ΔG = +3.3 kcal
CO2 + H2S + H2 → CH2(SH)(OH) + H2O ΔG = +4.0 kcal
For the hydrates, the sulfur-containing compound is marginally less stable (by 0.7 kcal/mol) than its counterpart. Both hydrates are still slightly higher in free energy compared to the reactants, but are now likely to be accessible.
- (c)
- Carbon in +2 oxidation state:
CO2 + H2 → H(C=O)OH ΔG = +2.7 kcal
CO2 + H2S + H2 → H(C=O)SH + H2O ΔG = +12.8 kcal
CO2 + H2S + H2 → H(C=S)OH + H2O ΔG = +15.4 kcal
CO2 + 2 H2S → H(C=S)SH + H2O ΔG = +18.6 kcal
While formation of formic acid is only marginally endergonic from CO2 reduction, all three sulfur analogs are significantly higher in free energy. The thioacid, the best of the three, is 10 kcal/mol less stable than its carboxylic acid counterpart. (The two thione acids are even less stable.) This suggests that if a thioacid can be formed in some way, its hydrolysis to the carboxylic acid would be 10 kcal downhill and can be utilized to drive an uphill C–C bond-forming carboxylation reaction, typically 4–8 kcal endergonic based on our previous work. (We will see in a later section that thioesters are typically 6–7 kcal uphill from their hydrolyzed product.)
In prebiotic experiments for carbon fixation, COS has been used as an activating reagent (and the carbon source). The reaction CO2 + H2S → COS + H2O is endergonic by 10.5 kcal. Thus, we can assign Gr0 of COS as +10.5 kcal/mol. Carbon monoxide has also been used as an activated reactant in prebiotic chemistry. The reaction CO2 + H2 → CO + H2O is endergonic by 11.3 kcal. Thus, we assign Gr0 of CO as +11.3 kcal/mol. If either COS or CO are used as the carbon source rather than CO2, the formation of formaldehyde, its hydrate, or CH2(SH)(OH) are now exergonic reactions. Formic acid is also downhill ~8 kcal/mol, and the thioacid is now only marginally higher in energy (than COS or CO) and likely to be accessible.
The relative free energies of the possible C1 compounds are shown in Figure 1, grouped by oxidation state of carbon. On the left are the more reduced compounds, CH3SH and CH3OH with oxidation state of −2. In the center are CH2O, CH2S, and their hydrates at zero oxidation state. Furthermore, on the right are the acids with oxidation state +2. Carbon monoxide (the dehydrate of formic acid) is in this group, and because of the similar prebiotic chemistry of COS and CO, we have grouped them together. While we have formally assigned sulfur an oxidation number of −2 (so it can be grouped alongside oxygen for ease of analyzing the results), the electronegativity of sulfur is not too different from carbon. Our formal assignments are a bookkeeping method for ease of presentation, allowing us to group together compounds that only differ by swapping an S with an O or vice versa.
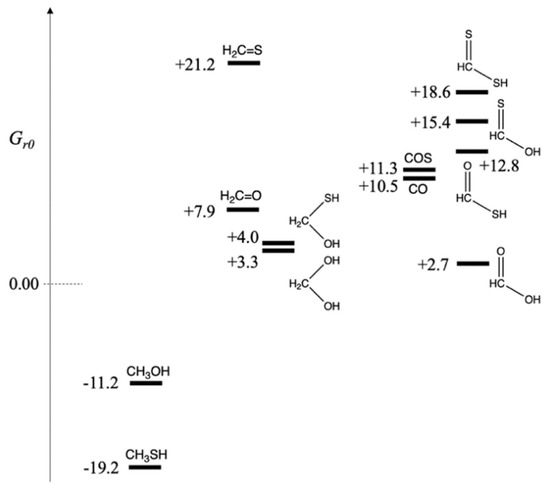
Figure 1.
Free Energy Map of C1 CHOS compounds (Gr0 values in kcal/mol).
Figure 1 makes it clear which compounds are accessible downstream using CO or COS as the carbon source rather than CO2. The fact that both CO and COS are ~11 kcal/mol less stable in free energy than CO2 allows them to function as activated reactants and drive subsequent reactions along a downhill thermodynamic gradient. However, if only CO2 was available as the carbon ‘food’ source, it is less likely that thioacids or thiones would be accessible; and the main C1 sulfur-containing compound would be CH3SH.
3.2. The Free Energy Landscape of C2 Compounds
We now turn our attention to the C2 compounds of CHOS and compare them to their CHO counterparts. Do the same trends we’ve seen for the C1 compounds hold in the C2 cases? In Figure 2, we have grouped the compounds according to the total formal oxidation state of the carbons, e.g., ethanethiol (CH3CH2SH) has six hydrogens (+1 each) and one sulfur (−2), and thus the carbons must add up to −4 for an overall neutral molecule.
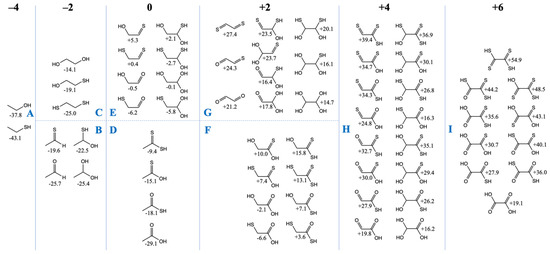
Figure 2.
Free Energies of C2 CHOS compounds by oxidation groups (Gr0 values in kcal/mol).
Similar to our more extensive study of CHO compounds [6], Gr0 values are lowest for the most reduced compounds and Gr0 values increase with oxidation. All compounds in the −4 and −2 oxidation groups have negative Gr0 values, i.e., they are more stable relative to the reference reactants CO2, H2 and H2S. Similar to the C1 case, thiol groups are favored over alcohols. In Figure 2A, ethanethiol is more stable than ethanol by ~5 kcal/mol, and in Figure 2C, replacing an OH by an SH is favorable by 5–6 kcal/mol. In Figure 2B, ethanal is 6 kcal/mol more stable than its counterpart with a C=S thione group (unlike the large gap of 13 kcal/mol in the C1 case). For the C2 case, hydrating the aldehyde hardly changes its Gr0 value, while hydrating the thione stabilizes it by ~3 kcal/mol.
In the CHO compounds from our previous study [6], having a carbonyl group was always more stable than having two separate alcohol groups (on different carbons) by a significant amount (over 10 kcal). However, this trend is reversed with sulfur; having two separate thiol groups (on different carbons) is more stable than the thione (with its weaker C=S pi-bond). For the −2 oxidation group, this leads to the most stable compounds in Figure 2B (the aldehyde and its hydrate) having a similar Gr0 value to the most stable compound (the dithiol) in Figure 2C.
For the zero oxidation group, the acids (Figure 2D) are significantly more stable than their isomers (Figure 2E) which have separate C=X and C–XH groups. In Figure 2D, the trend is similar to the C1 compounds: the carboxylic acid is more stable than the thioacid by 10 kcal/mol, and the thioacid is more stable than its thione isomer by 3 kcal/mol. (The CSSH compound is further destabilized by ~6 kcal/mol.) In Figure 2E, the most stable compound is mercaptoacetaldehyde (Gr0 = −6.2 kcal/mol), as expected, because thiols have lower Gr0 values than alcohols. Glycolaldehyde (Gr0 = −0.5 kcal/mol) is close in stability to its sulfur counterpart (Gr0 = +0.4 kcal/mol) because thiol stabilization over the alcohol is almost equally balanced by carbonyl stabilization over the thione. Hydration trends are similar to what we saw in Figure 2B.
The two sets of compounds in the +2 oxidation group are glycolic acid with its sulfur analogs in Figure 2F, and glyoxal with its sulfur analogs in Figure 2G. The mercaptoacid (Gr0 = −6.6 kcal/mol) is the most stable, followed by glycolic acid (Gr0 = −2.1 kcal). These are the only two compounds with negative Gr0 values in this group. Overall trends comparing the substitution of oxygen with sulfur are similar to previous cases, although we note that the gap between the thiol versus alcohol is now only 3–4 kcal/mol (instead of 5–6 kcal/mol). In Figure 2G, the gap between a thione and aldehyde has also reduced further to ~3 kcal/mol.
In Figure 2G (the +4 oxidation group), energy trends are similar to previous cases both for hydration reactions and for O to S substitutions in functional groups. There are two exceptions: (OH)2CHCSSH (Gr0 = +35.1 kcal/mol) is ~3 kcal/mol higher than expected from the general trend; and S=CC(=O)SH (Gr0 = +34.3 kcal/mol) is ~4 kcal/mol higher than expected from the general trend. It is unclear why this is so, but we do not expect these sulfur analogs to play an important role given their very positive Gr0 values. The most stable compounds in this group are the glyoxylic acid hydrate (Gr0 = +16.2 kcal/mol) and its thione hydrate counterpart Gr0 = +16.3 kcal/mol). Glyoxylic acid is an activated species in proto-metabolism, as discussed in our previous work [6], and not surprisingly is used (as glyoxylate) experimentally to drive proto-metabolic reactions in prebiotic chemistry.
Oxalic acid (Gr0 = +19.1 kcal/mol) is the most stable compound in the +6 oxidation group (Figure 2I). All its sulfur counterparts have very positive Gr0 values and they are not expected to be accessible or utilized in a sulfur-containing proto-metabolism.
3.3. Thermodynamics of C–S Coupling Reactions
Now that we have a lay of the land with our preliminary map of Gr0 values for C1 and C2 CHOS compounds, we can begin to assess the thermodynamics of forming C–S bonds if these are to play a role in proto-metabolic reactions.
In a prebiotic setting where CO2 is reduced by a mixture of H2 and H2S, two CHOS C1 compounds that we might expect to see are methanethiol (CH3SH) and the thione-hydrate CH2(OH)(SH). We also expect the CHO compounds methanol, formaldehyde (and its hydrate), and formic acid to be present. (Methane, the most thermodynamically favorable product, will also be present but is unlikely to react any further in a reducing environment and can be considered a “waste” molecule.) In our previous work on formaldehyde oligomerization [11], polyols and oxanes are produced in condensation reactions forming new C–O bonds. These polyols and oxanes are marginally unfavorable thermodynamically compared to the monomer (hydrate) but the free energy difference is very small. How does forming new C–S bonds fare?
As shown in the first two reactions of Figure 3, the formation of dimethylsulfide from methanethiol is exergonic. We calculate ΔG of the reaction by subtracting Gr0 of the reactants from Gr0 of the products. (Recall that reference molecules have zero Gr0 values.) Thus, ΔG = (−41.7 + 0.0) − 2(−19.2) = −3.4 kcal. The condensation of methanethiol and methanol to form dimethylsulfide is more exergonic: ΔG = (−41.7 + 0.0) − (−11.2 + (−19.2)) = −11.3 kcal. It is certainly more favorable than forming dimethylether (ΔG = +5.5 kcal). Thus, we expect dialkylsulfides to be formed if methylsulfide is present.

Figure 3.
Selected C–S bond forming reactions, Gr0 values next to structures are in kcal/mol.
If hydrated formaldehyde and its counterpart C1 thione are present, their condensation reactions are mildly exergonic, and so we might expect to see HO–CH2–X–CH2–XH compounds (X = O or S) as shown in the middle set of reactions in Figure 3. Forming the C–O–C compound is marginally more favorable than the C–S–C in this case. Thus, one might expect to see mixed polyol/polythiols depending on the concentrations of monomers. In an aqueous solution, the equilibrium will shift towards hydrolysis back into the monomers. For a 1 M solution, where water molecules outnumber solutes by 55:1, the correction factor is 2.4 kcal/mol in favor of hydrolysis [13]. We expect that for dilute solutions, monomers will be favored over condensation reactions that form C–X–C bonds (while releasing water), and hence we have not pursued calculating the free energies of polythiols or thiolanes. For the energetics of oxane/polyol formation from formaldehyde, the reader can refer to our previous work [11].
The final pair of reactions in Figure 3 illustrate thioester formation from the reaction of CH3SH with formic or acetic acid. These reactions are endergonic by 5.3 and 6.2 kcal respectively. In contrast, as we saw in the previous two sections, thioacid formation is endergonic by ~10 kcal. Since compounds with thiol groups are thermodynamically favored over their alcohol counterparts, and carboxylic acid groups (if they can be formed) are the most stable compounds in an oxidation group, this hints towards the role of thioesters in a prebiotic milieu as an important intermediate in chemical processes that couple endergonic and exergonic reactions.
In extant biochemical reactions involving the coenzyme CoA, forming the thioester is typically ~7 kcal uphill. Using the small molecule analog shown in Figure 4, we calculate that its condensation with acetic acid and succinic acid are +6.9 kcal and +7.5 kcal respectively. Thus, exergonic hydrolysis of such thioesters can potentially be coupled to proto-metabolite C–C bond formation where the carboxylation reactions are endergonic by 4–7 kcal, as shown in our previous work on CHO systems [6]. Our preliminary results, while promising, would not do justice to the complexity of the system, and we expect to provide a detailed examination of the connection between thioesters and potential CHO proto-metabolic systems in a future publication.

Figure 4.
Small molecule analog of CoA.
3.4. Sulfur Analogs of the Formose Reaction
In extant biochemical cycles, the reduction of CO2 to build biomass can proceed through cycles analogous to the reverse TCA cycle. We explored the thermodynamics of four such cycles in CHO systems in our previous work [6], the most interesting being the 3-hydroxypropionate/4-hydroxybutyrate (3HP/4HB) cycle because it does not involve CHO compounds with more than four carbons and avoids forming the less stable oxaloacetate. In that work, we proposed alternative pathways that could be thermodynamically more feasible than the 3HP/4HB cycle thereby avoiding some of the more challenging kinetic barriers, but we also noted that in the absence of (specialized) enzyme catalysts there would still be kinetically unfeasible steps in a prebiotic milieu.
There is a known autocatalytic reaction that builds up progressively larger CHO compounds from a C1 species—the formose reaction [7]. It takes advantage of aldol reactions to form new C–C bonds, and autocatalysis is aided by a retro-aldol transformation of a C4 species into two C2 compounds. It is thus analogous to the 3HP/4HB cycle, but much simpler because it avoids redox reactions: formaldehyde is the C1 ‘food’ species, glycolaldehyde is the linchpin C2 species, and all compounds involved remain in the zero oxidation group. In contrast for the 3HP/4HB cycle, while acetate (the C2 linchpin) is in the zero oxidation group, CO2 (+4 oxidation group) is the C1 food species and therefore reducing equivalents of H2 are required for the cycle to be realized.
The problem with the formose reaction is that it is a mess [8], and a slew of compounds are formed in an essentially uncontrolled reaction. Could the presence of sulfur introduce some form of thermodynamic control to the reaction? How might the kinetics change? Is there a path towards taming the formose reaction as a stepping stone towards proto-metabolic cycles that more closely resemble what extant life uses? Building on what we have learned from our survey of CHOS C1 and C2 compounds described earlier, this subsection presents our free energy map of sulfur analogs to the formose reaction. A brief summary of the key compounds in the (non-sulfur-containing) formose reaction are shown in Figure 5.
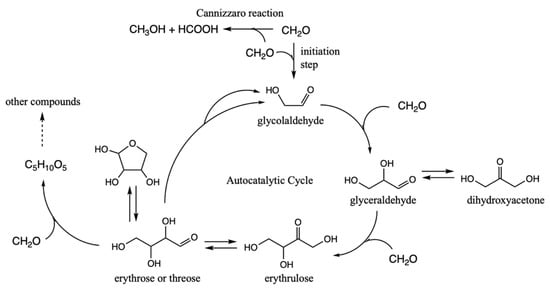
Figure 5.
Key features of the formose reaction autocatalytic cycle.
In discussing the results, we will repeatedly make reference to our earlier free energy map of the thermodynamics and kinetics of the formose reaction (up to C4); this paragraph provides the highlights from that work [11]. Forming glycoaldehyde directly from CH2O is very challenging kinetically. We previously calculated the barrier for direct dimerization to be 45.3 kcal. Experimentally, in a solution only containing CH2O, there is a long induction period. However once even a small amount of glycolaldehyde is formed (or added to the solution as an initiator), the reaction proceeds rapidly producing a wide variety of sugars, mostly in the C4 to C7 range. With C2 present, the difficult C1 + C1 → C2 reaction is bypassed by the much lower barrier C2 + C1 → C3 and C3 + C1 → C4 reactions. The retro-aldol C4 → C2 + C2 reaction regenerates (more) C2 and accelerates the consumption of C1 making the cycle autocatalytic. CH2O can also form polyols and oxanes but hydrolysis in an aqueous solution favors re-forming the monomer. On the other hand, the Cannizzaro disproportionation reaction parasitizes the cycle (to be discussed in a later subsection of this paper). Extensive documentation of experimental results on the formose reaction can be found in a long article by Mizuno and Weiss [27].
If H2S was present as a source of sulfur, one might expect a starting mixture of the hydrates CH2(OH)2 and CH2(SH)(OH) in aqueous solution, as they are relatively close in energy with Gr0 values of +3.3 and +4.0 kcal respectively. Our calculated barrier for the direct C–C coupling reaction of CH2O and CH2S is 26.0 kcal, which is much lower than 45.3 kcal for the direct dimerization of CH2O, but recall from Figure 1 that CH2S is 13.3 kcal/mol less stable than CH2O, which accounts for two-thirds of the difference. Mercaptoacetaldehyde (Gr0 = −6.2 kcal) is the C2 species formed, and the reaction is thermodynamically favorable (ΔG = −19.7 kcal from the hydrates). Since a range of C1 and larger species (C2, C3, etc.) are observed experimentally in prebiotic reactions [28,29,30,31,32,33] by reducing CO2 (or bicarbonate or CO or COS) simulating hydrothermal vent prebiotic chemistry, and since the C1 + C1 → C2 initiation step is not important for the cycle, we need not worry about the initiation step. Our starting point will be the C2 linchpin species, mercaptoacetaldehyde, the thiol analog of glycolaldehyde. Mercaptoacetaldehyde has also been proposed as central in prebiotic scenarios involving the amino acid cysteine [34].
Since Gr0 = −6.2 kcal/mol for mercaptoacetaldehyde, it is favorable thermodynamically to be (one among many possible compounds) produced prebiotically from a source containing CO2, H2 and H2S. (It may not be as easily observed experimentally because it participates in further reactions.) Mercaptoacetaldehyde can also potentially be formed from glycolaldehyde in the presence of H2S as shown in the top row of Figure 6. The reaction is overall thermodynamically favorable, ΔG = −6.2 − (−0.5) = −5.7 kcal. Note that the cis-enol of mercaptoacetaldehyde as shown in Figure 6 is more stable than the trans-enol (not shown) by ~2 kcal/mol in our calculation of Gr0.
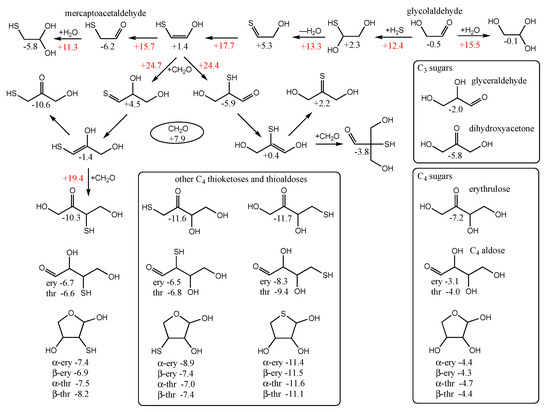
Figure 6.
Selected C2 to C4 thermodynamics and kinetics of thioaldoses and thioketoses, Gr0 values next to structures and arrows are in kcal/mol.
By calculating the energies of the transition states (Gr0 values in red next to arrows), we can estimate the reaction kinetics. For example, the first step of adding H2S to glycolaldehyde has a barrier of +12.4 − (−0.5) = 12.9 kcal. (The corresponding dehydration barrier in the reverse reaction is +12.4 − (+2.3) = 10.1 kcal.) The calculated stepwise barriers for the overall transformation of glycolaldehyde to mercaptoacetaldehyde (involving formation of the thione intermediate and its enol) are in the 11–14 kcal range. (At both ends on the top row, we also show the hydration reactions of mercaptoacetaldehyde and glycolaldehyde for completeness; see Supplementary Materials for transition state structures.)
In our previous work on CH2O oligomerization [11], aldol additions of CH2O proceed via the enol. We see the same for mercaptoacetaldehyde, except that its asymmetry allows for two possible products: the less favorable thione (Gr0 = +4.5 kcal) and the more favorable aldehyde (Gr0 = −5.9 kcal) that has a thiol on the central carbon. Kinetically, we might also expect the aldehyde to be favored because the thiol carbon of the enol is a better nucleophile than the alcohol carbon. However, our calculated barriers are essentially identical; this is after optimizing multiple transition states and the lowest energy structures are shown in Figure 7.
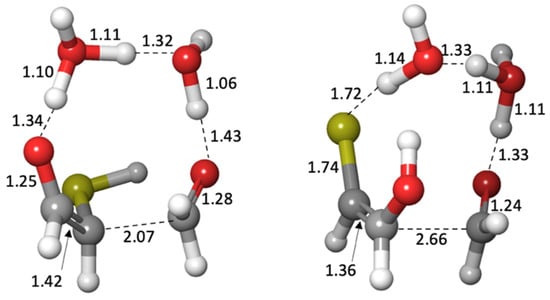
Figure 7.
Transition states for the aldol addition of CH2O to mercaptoacetaldehyde. Bond distances shown are in Å.
Considering the enol (Gr0 = +1.4 kcal) and CH2O (Gr0 = +7.9 kcal) as the reactants, the barrier to forming the C3 aldehyde is 24.4 − (1.4 + 7.9) = 13.3 kcal, and the barrier to the C3 thione is 24.7 − (1.4 + 7.9) = 13.6 kcal. If mercaptoacetaldehyde and CH2O were the reactants, the barriers would, respectively, be 24.4 − (−6.2 + 7.9) = 22.7 kcal and 24.7 − (−6.2 + 7.9) = 23.0 kcal. These calculated barriers are very similar to our previous work for the C1 + C2 → C3 aldol addition of glycolaldehyde and CH2O of 22.3 kcal (or 13.0 kcal from the enol). Thus, in a mixture that contained glycolaldehyde, mercaptoacetaldehyde, and CH2O, the kinetics for this first aldol addition (C1 + C2 → C3) are similar and both C2 “reactants” will consume the C1 food source (CH2O) at similar rates.
Let us now consider the thermodynamics of the C1 + C2 aldol addition. In the CHO system, forming glyceraldehyde (Gr0 = −2.0 kcal) is favorable with ΔG = −2.0 −(−0.5 + 7.9) = −9.4 kcal. The analogous reaction in the CHOS system with mercaptoacetaldehyde, forming the C3 aldehyde-thiol, is similarly favorable: ΔG = −5.9 − (−6.2 + 7.9) = −7.6 kcal. On the other hand, forming the thione-diol is slightly unfavorable: ΔG = +4.5 − (−6.2 + 7.9) = +2.8 kcal. Since the C3 aldehyde-thiol has the lower Gr0 value, it could be thermodynamically favored over glyceraldehyde in an equilibrating mixture with multiple reactants.
However, the situation is more complicated because “globally” among the C3 structures, the thioketose (Gr0 = −10.6 kcal, leftmost structure in the second row of Figure 6) is the most stable, and access to it via enolization comes from the less thermodynamically favorable aldol addition. The intermediate enol with a terminal thiol (Gr0 = −1.4 kcal, leftmost structure in the third row of Figure 6) is also the starting point for further aldol addition of CH2O to form the linear C4 thiosugars. On the other hand, the enol of the C3 aldehyde-thiol would lead to a branched C4 thiosugar (Gr0 = −3.8 kcal), assuming our earlier argument that the thiol carbon of the enol is the better nucleophile. However, as we saw for C1 + C2 → C3, addition to the alcohol side of the enol is just as viable kinetically, and likely more so in this case to avoid steric hindrance. Thus, access to the linear C4 thio-sugars is possible through both branches. What role might the C3 thioketose play? Analogous to dihydroxyacetone, as discussed in our previous work [6], it may be an “off-cycle” compound that forms an equilibrating pool of inter-connected compounds [35] that could stabilize the cycle and provide a form (albeit simple) of regulatory control. (Dehydrations of C3 sugars may also be a part of this pool; see Supporting Materials).
The C1 + C3 addition to form the C4 thioketose (Gr0 = −10.3 kcal/mol, left side of Figure 6) is thermodynamically favorable with ΔG = −10.3 − (−10.6 + 7.9) = −7.4 kcal, very similar to the aforementioned C1 + C2 → C3 addition of ΔG = −7.6 kcal. The barrier for the C1 + C3 → C4 aldol addition is 19.4 − (1.4 + 7.9) = 8.1 kcal from the enol, noticeably lower than 13.3 kcal in the analogous C1 + C2 → C3. In our previous work on the CHO system [11] (leading to erythrulose), the barrier is 8.5 kcal for C1 + C3 → C4, which is similarly lower than the 13.0 kcal barrier for C1 + C2 → C3. Thus, kinetically, we expect the CHOS analog of the formose reaction to show similar behavior as the CHO system under appropriate experimental conditions that facilitate the reaction. Thermodynamically (left side of Figure 6), the thioketose is ~4 kcal more stable than its open-chain thioaldoses, while the ring structures are ~1 kcal more stable than the open thioaldoses. Once again, this is similar to the non-sulfur analogs (bottom right box in Figure 6) of erythrulose, erythrose, threose, and the ring structures. We can think of the ketose, the open chain aldose, and the furanose as an equilibrating pool of compounds.
For the C4 sugars, the 3-thioketose turns out to be marginally less stable than both the 1-thioketose and 4-thioketose that have terminal thiols (Figure 6, central lower box). As for the aldoses, the 3-thioaldose and 2-thioaldose have similar energies, while the 4-thioaldose with its terminal thiol is the most stable. This is also true for the ring structures, and interestingly the 4-thioaldose rings (Gr0 values of −11.1 to −11.6 kcal/mol) are similar in stability to the 4-thioketose (Gr0 = −11.7 kcal/mol). This suggests that a possible role played by (terminal) thiol groups in a prebiotic setting is to stabilize the corresponding aldose rings.
A key autocatalytic step in the formose reaction is the retroaldol reaction of the C4 aldose back into two C2 linchpin molecules. In the CHO system, this reaction starting from threose is marginally uphill with ΔG = 2(−0.5) − (−4.0) = +3.0 kcal. In the sulfur analog, the thioaldose splits into mercaptoacetaldehyde and glycolaldehyde. For 4-thiothreose, we see a similar result: ΔG = (−0.5) + (−6.2) − (−9.4) = +2.7 kcal. However, for 3-thiothreose, the reaction is now energetically neutral with ΔG = (−0.5) + (−6.2) − (−6.6) = −0.1 kcal. (2-thiothreose shows a similar result with ΔG = +0.1 kcal). Thus, considering only the C4 species for the moment, we might expect over time a depletion of the 2- and 3-thio-sugars, and possible accumulation of the 4-thiosugars, favoring the aldose rings that are more resistant to hydrolysis. The reality would be a lot messier with other aldol and retro-aldol reactions occurring, alongside Cannizzaro side-reactions.
Stepping back to look at the overall thermodynamic map, we see that the C3 and C4 species show similar trends as the C1 and C2 species discussed earlier. Compared to the reference compounds, thiol groups are favored over their alcohol counterparts and are most stable in the terminal position. Thiones with their weaker C=S bonds are less stable than their carbonyl counterparts. We also have preliminary data (for a future publication) showing that the trends for sulfur analogs for the larger molecular acids mirror those for we previously discussed for the smaller molecules. Overall, we see many similarities for both the thermodynamics and kinetics when comparing individual steps in the formose reaction of the CHO system to its sulfur analogs.
3.5. Can Dithiol Groups Influence Sugar Formation?
Could having a thiol group in a sugar make a relevant and interesting difference? One possibility we explore in this subsection is based on the experimental work of Eschenmoser and colleagues [36], where they found that starting with glycolaldehyde-2-phosphate and formaldehyde led to a higher yield of ribose among the pentose-2,4-diphosphates formed. If phosphate can “direct” the reaction to favor certain products over others (in a messy formose-like reaction), can sulfur do the same? If sulfur was primordial to phosphate in prebiotic systems, could it have played an analogous role?
Considering mercaptoacetaldehyde as the sulfur analog of glycolaldehyde-2-phosphate, in the presence of formaldehyde we expect aldol addition to favorably form the C3 aldehyde-thiol (as discussed in the previous section), i.e., the analog of glyceraldehyde-2-phosphate. Aldol addition of mercaptoacetaldehyde (via its enol) with the C3 aldehyde-thiol leads to 2,4-dithioaldoses (the sulfur analogs of the C5 aldose-2,4-diphosphates) as shown in Figure 8. The rings are more stable than the open chain structures. Unlike the CHO sugars, the pyranoses are not more stable than the furanoses but have comparable free energies. This is because having sulfur in the ring provides a 2–3 kcal/mol stabilization (as seen for the thiotetroses in Figure 6).
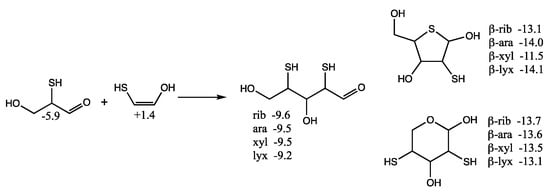
Figure 8.
Formation of C5 2,4-dithioaldoses from aldol C2 + C3 reaction; Gr0 values next to structures are in kcal/mol.
For the open chain pentoses, our calculated Gr0 values have ribose being the most stable followed by arabinose, xylose, lyxose. However, the difference in free energy is tiny and certainly within the computational error; we cannot claim that incorporation of sulfur favors ribose over the other aldopentoses. For the β-pyranoses, we see the same order of stability as the open chain structures, and again the differences are tiny and within the computational error. For the β-furanoses, arabinose and lyxose have lower Gr0 values than ribose with xylose being the least stable. We have no explanation why this is or if this is some artifact of the calculation (possibly not finding the best conformers in some cases).
Although we expect the C2 + C3 addition to form the C5 2,4-dithioaldoses to be kinetically favored (because the thiol carbon of the enol is more nucleophilic than the alcohol carbon), we also consider the possibility of forming 1,4-dithio-2-ketopentose since it leads to more thermodynamically favored ketoses as shown in Figure 9. (We have switched the position of the OH and SH in the enol in Figure 9 compared to Figure 8 to make the aldol addition clearer.) The thione intermediate formed is likely to isomerize to the more favored keto form. Our calculated Gr0 values have the open chain sulfur analog of xylulose more stable than the ribulose by 1 kcal/mol. The furanoses are not as stable as the open chain structures.
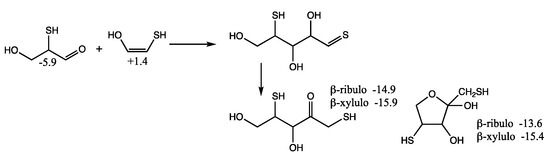
Figure 9.
Formation of C5 1,4-dithio-2-ketoses from aldol C2 + C3 reaction; Gr0 values next to structures are in kcal/mol.
However, there is another possibility. If C3 acts as the enol (rather than the C2), a C5 thione intermediate can be formed that could subsequently isomerize into a 1,4-dithio-3-ketose or a 2,5-dithioaldose, as shown in Figure 10. The most sTable 3-ketose has Gr0 = −15.0 kcal/mol (its diastereomer is only 0.3 kcal less stable). For the open chain 2,5-dithioaldoses, the ribose analog is the most stable followed by arabinose, xylose, and lyxose. The furanoses, with a pendant thiol in the 5-position are slightly more stable than the open chain structures. The pyranoses with sulfur in the ring are the most stable group. For the ring structures, we have no explanation for the relative ordering of the most stable different stereoisomers according to our calculated Gr0 values.
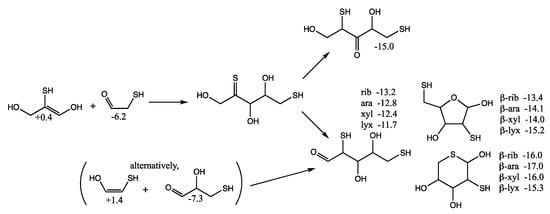
Figure 10.
Formation of C5 1,4-dithio-3-ketoses and 2,5-dithioaldoses from aldol C2 + C3 reaction; Gr0 values next to structures are in kcal/mol.
An alternative route (see parenthesis in Figure 10) to the C5 2,5-dithioaldoses is the aldol addition of the C2 enol with 3-thioglyceraldehyde (Gr0 = −7.3 kcal/mol), the isomer of the C3 ketone (the most stable C3 sulfur analog in Figure 6 with Gr0 = −10.6 kcal/mol). If thiols could be precursors to phosphates in a prebiotic world, the 2,5-dithioribose analog could be a stand-in for ribose-2,5-diphosphate.
There are other possible products from aldol additions of these sulfur analogs that we have not discussed. (See Supplementary Materials). For example, in the C2 + C3 → C5 addition, one of the species may not contain sulfur, and this would lead to a range of sugars with just one thiol group rather than two. As a second example, we have not discussed the C4 + C1 → C5 addition, which would lead to other isomers such as 3,5-dithio-2-ketoses, 1,3-dithio-2-ketoses and 3,5-dithioaldoses. Furthermore, we have mainly focused on non-branched sugars, and we have only shown one example, the branched tetrose in Figure 6. We expect these thermodynamically less stable branched sugars to be less prevalent than their straight-chain counterparts.
Our limited foray into sulfur analogs of the formose reaction is clearly not exhaustive. Our goal here is to provide a flavor of the myriad possible reactions, intermediates, and products, in this system. Based on our limited analyses, we can draw some general conclusions. Substituting an alcohol with a thiol group is thermodynamically favorable. Thiol groups in the terminal position are particularly favored. Sulfur in the sugar ring is thermodynamically favored. Furthermore, the presence of sulfur provides some asymmetry to the aldol addition reactions, and the lower electronegativity of sulfur means that in an enol, the thiol carbon is a better nucleophile which may provide some “directing” ability that favors some subsets of products over others.
While we have speculated about the possibility that thiols might be precursors to phosphates in aldol reactions of sugars, our results thus far are inconclusive on this topic. However, there are tantalizing analogies. In the pentose phosphate pathway, the sugars involved have terminal phosphates, and our limited study finds that terminal thiols are thermodynamically favored. By including thiols in the mix, we find that aldoses can be as thermodynamically stable as ketoses for C4 and C5, while this is not so in CHO sugars where the ketose is typically 2–3 kcal more stable than the aldose.
3.6. Sulfur Analogs of the Cannizzaro Reaction
As this article has focused on the sulfur analogs of the sugars in the formose reaction, we would be remiss by not (briefly) discussing the Cannizzaro side-reactions. Let us first consider the simplest case involving monomeric formaldehyde. The reaction CH2O + CH2(OH)2 → CH3OH + HCOOH is thermodynamically favorable (ΔG = −20.7 kcal) and kinetically favorable (barrier of 20.3 kcal) [11], and certainly outcompetes the dimerization of CH2O to glycolaldehyde. It is also a disproportionation reaction, converting the zero-oxidation formaldehyde into reduced and oxidized products (−2 for methanol and +2 for formic acid respectively). These reactions are why the formose reaction is messy and the products include a range of alcohols and carboxylic acids (or carboxylates in the alkaline solution used experimentally) [8]. Does the presence of sulfur analogs make a difference?
In the presence of H2S, we expect some amount of CH2(SH)(OH) to be present in solution as a starting point. Using the Gr0 values from Figure 1, we can consider the following possibilities.
CH2O + CH2(SH)(OH) → CH3SH + HC(=O)OH ΔG = −28.6 kcal
CH2O + CH2(SH)(OH) → CH3OH + HC(=O)SH ΔG = −10.3 kcal
Based on the trends discussed earlier—thiols are more stable than alcohols, and carboxylic acids are more stable than thioacids—it is no surprise that thermodynamically, methanethiol and formic acid are the preferred products. Our calculated barrier is 21.5 kcal, not too different from the non-sulfur Cannizzaro reaction.
C2 compounds in the mixture could also undergo disproportionation. The four main possibilities involving a C1 and a C2 species are shown in Figure 11; we have not included higher-energy starting reactants that are unlikely to be present in the mixture such as H2C=S (which would undoubtedly lead to significantly exergonic reactions as a highly activated species). The disproportionation of glycolaldehyde with (hydrated) formaldehyde leading to ethylene glycol and formic acid is exergonic by 14.9 kcal. Not too different is the disproportionation of mercaptoacetaldehyde with formaldehyde leading to mercaptoethanol (Gr0 = −19.1 kcal) and formic acid (ΔG = −13.5 kcal). Reactions with CH2(SH)(OH) leading to the thioacid are still exergonic but less favorable.
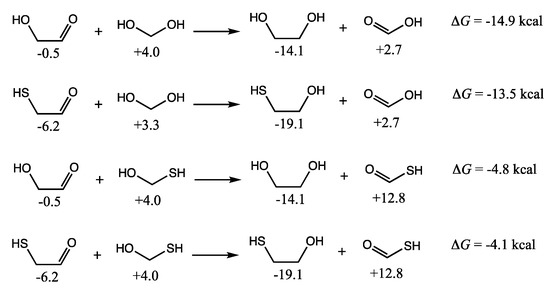
Figure 11.
C2 + C1 disproportionation reactions (Gr0 values next to structures in kcal/mol).
Mercaptoethanol could play an important role as a precursor to CoA. For example, the condensation of mercaptoethanol with acetamide leads to the CoA analog shown in Figure 4. Cannizzaro disproportionation reactions such as those shown in Figure 11 could be a source for mercaptoethanol. One could imagine a range of other sulfur-containing molecules produced by disproportionation, which we have not catalogued in the present work, but we do expect the trends in free energy to be similar to what we have analyzed thus far.
4. Conclusions and Future Work
In this article, we present a thermodynamic free energy map for the C1 and C2 compounds that may be present in a (possibly hydrothermal) prebiotic milieu where CO2, H2, H2S are present as reactants. We find that thiols are significantly more stable than alcohols, while thiones are significantly less stable than carbonyls. However, hydrated thiones are only marginally less stable than their aldehyde counterparts leaving the door open to the possibility that thiones could still play a role as reaction intermediates. Based on our calculated Gr0 values, we expect (thermodynamically favored) CH3SH to be present and participate in reactions to form dialkylsulfides.
Our main focus was tracing the key reactions of CHOS compounds in the smallest autocatalytic cycle of the formose reaction. Mercaptoacetaldehyde is the linchpin C2 species that plays the role analogous to glycolaldehyde. The asymmetry of the mercaptoacetaldehyde enol (and by extension other related enols) potentially favors some aldol additions over others, opening the possibility to different kinetic and/or thermodynamic controls, which could lead to reaction channels that favor some reaction intermediates and products over others. Thiols are favored in the terminal positions of the sugar, possibly analogous to what we see in the sugar phosphates of extant biochemistry. Sulfur in ring structures of sugars also shift the aldehyde-ketone equilibrium away from exclusively favoring the ketoses over the aldoses.
Cannizzaro reactions may produce both carboxylic acids and thioacids, along with thiols and thio-alcohols such as mercaptoethanol. The presence of these substances could lead to a rich world of thioester chemistry, and these compounds could further participate in autocatalytic cycles that provide the beginnings of a proto-metabolic chemistry. Although thioacids are ~10 kcal less stable than their carboxylic acid counterparts, thioesters are 5–7 kcal less stable than their hydrolysis products of carboxylic acid and thiol. Hydrolysis of thioesters could help drive the carboxylation reactions needed to build CHO compounds in autocatalytic cycles. In this article, we did not further discuss these reactions because as we embarked on exploring this chemical space, it has turned out to be far larger than we anticipated. We are actively working on filling the large gaps of a more extensive free energy map involving thioesters, and we look forward to providing those results to our readers in the near future. For an example of how thioesters and dithio compounds can be experimentally coupled in prebiotic chemistry, see [37].
Our present work provides a baseline free energy map for potential CHOS metabolites in the core, and future work includes extending this map to include nitrogen-containing compounds such as amino acids, pterins, pyrroles, and pyrimidines, that may play a role in wider intermediary metabolism. Such compounds are also expected to play a role as primitive catalysts and co-factors in the establishing of proto-metabolic cycles.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/life12111763/s1, Supplementary Materials document. (1) Reaction schemes to form dithiopentoses; (2) Gr0 values for zero-oxidation C3 CHOS compounds; (3) Transition state structures for conversion of glycolaldehyde to mercaptoacetaldehyde.
Author Contributions
Conceptualization, J.K.; methodology, J.K.; formal analysis, J.K. and N.A.M.; validation, J.K. and N.A.M.; investigation, J.K. and N.A.M.; writing—original draft preparation, J.K.; writing—review and editing, J.K.; visualization, J.K.; supervision, J.K.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Materials.
Acknowledgments
This research was supported by the University of San Diego. Shared computing facilities were provided by the saber3 high-performance computing clusters at the University of San Diego.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Wachtershauser, G. Before enzymes and templates: Theory of surface metabolism. Microbiol. Rev. 1988, 52, 452–484. [Google Scholar] [CrossRef] [PubMed]
- De Duve, C. The Beginnings of Life on Earth. Am. Sci. 1995, 83, 428–437. [Google Scholar]
- Patel, B.H.; Percivalle, C.; Ritson, D.J.; Duffy, C.D.; Sutherland, J.D. Common origins of RNA, protein and lipid precursors in a cyanosulfidic protometabolism. Nat. Chem. 2015, 7, 301–307. [Google Scholar] [CrossRef]
- Braakman, R.; Smith, E. The compositional and evolutionary logic of metabolism. Phys. Biol. 2013, 10, 011001. [Google Scholar] [CrossRef] [PubMed]
- Youssef-Saliba, S.; Vallee, Y. Sulfur Amino Acids: From Prebiotic Chemistry to Biology and Vice Versa. Synthesis-Stuttgart 2021, 53, 2798–2808. [Google Scholar] [CrossRef]
- Kua, J.; Hernandez, A.L.; Velasquez, D.N. Thermodynamics of Potential CHO Metabolites in a Reducing Environment. Life 2021, 11, 1025. [Google Scholar] [CrossRef]
- Breslow, R. On the Mechanism of the Formose Reaction. Tetrahedron Lett. 1959, 22–26. [Google Scholar] [CrossRef]
- Omran, A.; Menor-Salvan, C.; Springsteen, G.; Pasek, M. The Messy Alkaline Formose Reaction and Its Link to Metabolism. Life 2020, 10, 125. [Google Scholar] [CrossRef]
- Martinez, R.F.; Cuccia, L.A.; Viedma, C.; Cintas, P. On the Origin of Sugar Handedness: Facts, Hypotheses and Missing Links-A Review. Orig. Life Evol. Biosph. 2022, 52, 21–56. [Google Scholar] [CrossRef]
- Toohey, J.I.; Cooper, A.J. Thiosulfoxide (sulfane) sulfur: New chemistry and new regulatory roles in biology. Molecules 2014, 19, 12789–12813. [Google Scholar] [CrossRef]
- Kua, J.; Avila, J.E.; Lee, C.G.; Smith, W.D. Mapping the Kinetic and Thermodynamic Landscape of Formaldehyde Oligomerization under Neutral Conditions. J. Phys. Chem. A 2013, 117, 12658–12667. [Google Scholar] [CrossRef] [PubMed]
- Kua, J.; Hanley, S.W.; De Haan, D.O. Thermodynamics and Kinetics of Glyoxal Dimer Formation: A Computational Study. J. Phys. Chem. A 2008, 112, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Kua, J.; Galloway, M.M.; Millage, K.D.; Avila, J.E.; De Haan, D.O. Glycolaldehyde Monomer and Oligomer Equilibria in Aqueous Solution: Comparing Computational Chemistry and NMR Data. J. Phys. Chem. A 2013, 117, 2997–3008. [Google Scholar] [CrossRef] [PubMed]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Halgren, T.A. MMFF VII. Characterization of MMFF94, MMFF94s, and other widely available force fields for conformational energies and for intermolecular-interaction energies and geometries. J. Comput. Chem. 1999, 20, 730–748. [Google Scholar] [CrossRef]
- Warshel, A.; Florian, J. Computer simulations of enzyme catalysis: Finding out what has been optimized by evolution. Proc. Natl. Acad. Sci. USA 1998, 95, 5950–5955. [Google Scholar] [CrossRef]
- Wiberg, K.B.; Bailey, W.F. Chiral diamines 4: A computational study of the enantioselective deprotonation of Boc-pyrrolidine with an alkyllithium in the presence of a chiral diamine. J. Am. Chem. Soc. 2001, 123, 8231–8238. [Google Scholar] [CrossRef]
- Nielsen, R.J.; Keith, J.M.; Stoltz, B.M.; Goddard, W.A., 3rd. A computational model relating structure and reactivity in enantioselective oxidations of secondary alcohols by (-)-sparteine-Pd(II) complexes. J. Am. Chem. Soc. 2004, 126, 7967–7974. [Google Scholar] [CrossRef] [PubMed]
- Deubel, D.V.; Lau, J.K. In silico evolution of substrate selectivity: Comparison of organometallic ruthenium complexes with the anticancer drug cisplatin. Chem. Comm. 2006, 23, 2451–2453. [Google Scholar] [CrossRef] [PubMed]
- Wertz, D.H. Relationship between the gas-phase entropies of molecules and their entropies of solvation in water and 1-octanol. J. Am. Chem. Soc. 1980, 102, 5316–5322. [Google Scholar] [CrossRef]
- Abraham, M.H. Relationship between solution entropies and gas phase entropies of nonelectrolytes. J. Am. Chem. Soc. 1981, 103, 6742–6744. [Google Scholar] [CrossRef]
- Krizner, H.E.; De Haan, D.O.; Kua, J. Thermodynamics and Kinetics of Methylglyoxal Dimer Formation: A Computational Study. J. Phys. Chem. A 2009, 113, 6994–7001. [Google Scholar] [CrossRef]
- Alberty, R.A. Thermodynamics of Biochemical Reactions; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2003. [Google Scholar]
- Mizuno, T.W.; Alvin, H. Synthesis and Utilization of Formose Sugars. Adv. Carbohydr. Chem. Biochem. 1974, 29, 173–227. [Google Scholar] [CrossRef]
- Huber, C.; Wachtershauser, G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science 1997, 276, 245–247. [Google Scholar] [CrossRef]
- Heinen, W.; Lauwers, A.M. Organic sulfur compounds resulting from the interaction of iron sulfide, hydrogen sulfide and carbon dioxide in an anaerobic aqueous environment. Orig. Life Evol. Biosph. 1996, 26, 131–150. [Google Scholar] [CrossRef]
- Cody, G.D.; Boctor, N.Z.; Filley, T.R.; Hazen, R.M.; Scott, J.H.; Sharma, A.; Yoder, H.S., Jr. Primordial carbonylated iron-sulfur compounds and the synthesis of pyruvate. Science 2000, 289, 1337–1340. [Google Scholar] [CrossRef]
- Leman, L.; Orgel, L.; Ghadiri, M.R. Carbonyl sulfide-mediated prebiotic formation of peptides. Science 2004, 306, 283–286. [Google Scholar] [CrossRef]
- Hudson, R.; de Graaf, R.; Strandoo Rodin, M.; Ohno, A.; Lane, N.; McGlynn, S.E.; Yamada, Y.M.A.; Nakamura, R.; Barge, L.M.; Braun, D.; et al. CO2 reduction driven by a pH gradient. Proc. Natl. Acad. Sci. USA 2020, 117, 22873–22879. [Google Scholar] [CrossRef] [PubMed]
- He, D.; Wang, X.; Yang, Y.; He, R.; Zhong, H.; Wang, Y.; Han, B.; Jin, F. Hydrothermal synthesis of long-chain hydrocarbons up to C24 with NaHCO3-assisted stabilizing cobalt. Proc. Natl. Acad. Sci. USA 2021, 118, e2115059118. [Google Scholar] [CrossRef] [PubMed]
- Shalayel, I.; Youssef-Saliba, S.; Vazart, F.; Ceccarelli, C.; Bridoux, M.; Vallee, Y. Cysteine Chemistry in Connection with Abiogenesis. Eur. J. Org. Chem. 2020, 2020, 3019–3023. [Google Scholar] [CrossRef]
- Igamberdiev, A.U.; Eprintsev, A.T. Organic Acids: The Pools of Fixed Carbon Involved in Redox Regulation and Energy Balance in Higher Plants. Front Plant Sci. 2016, 7, 1042. [Google Scholar] [CrossRef]
- Mueller, D.; Pitsch, S.; Kittaka, A.; Wagner, E.; Wintner, C.E.; Eschenmoser, A. Chemistry of a-aminonitriles. Aldomerization of glycolaldehyde phosphate to rac-hexose 2,4,6-triphosphates and (in presence of formaldehyde) rac-pentose 2,4-diphosphates: Rac-allose 2,4,6-triphosphate and rac-ribose 2,4-diphosphate are the main reaction products. Helv. Chim. Acta 1990, 73, 1410–1468. [Google Scholar] [CrossRef]
- Leqraa, N.; Nicolet, Y.; Milet, A.; Vallee, Y. A way to thioacetate esters compatible with non-oxidative prebiotic conditions. Sci Rep. 2020, 10, 14488. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).