Abstract
An aerobic bacterium, designated as strain KD337-16T, was isolated from the fecal samples of a black pig. It exhibited spherical, non-motile and non–spore-forming, Gram-positive cells. KD337-16T was identified as a member of the genus Micrococcus through 16S rRNA gene sequencing, and its closest relatives were found to be Micrococcus endophyticus YIM 56238T (99.5% similarity), Micrococcus luteus NCTC 2665T (99.1%), Micrococcus yunnanensis YIM 65004T (99.1%), Micrococcus aloeverae AE-6T (99.1%), Micrococcus antarcticus T2T (98.9%), and Micrococcus flavus LW4T (98.7%). Phylogenomic trees were constructed, and strain KD337-16T was found to form its own cluster as an independent lineage of M. flavus LW4T. Between KD337-16T and its close relatives, the average nucleotide identity, average amino acid identity, and digital DNA–DNA hybridization were below the respective species delineation thresholds at 82.1–86.6%, 78.1–86.1%, and 24.4–34.9%. The major cellular fatty acids and polar lipids were anteiso-C15:0 and iso-C15:0, and DPG and PG, respectively. The predominant menaquinone was MK-8(H2). Taken together, the results indicate that strain KD337-16T is a novel species of the genus Micrococcus, for which the name Micrococcus porci sp. nov. is proposed. The type strain is KD337-16T (=BCRC 81318T = NBRC 115578T).
1. Introduction
The genus Micrococcus was first described by Cohn [1], and this description was subsequently amended by Stackebrandt et al. [2] and Wieser et al. [3], with Micrococcus luteus as the type species. The genus Micrococcus belongs to the family Micrococcaceae, order Micrococcales, and phylum Actinomycetota. Nine species have been reported in this genus (https://lpsn.dsmz.de/genus/micrococcus, accessed on 28 October 2022): Micrococcus aloeverae, Micrococcus antarcticus, Micrococcus cohnii, Micrococcus endophyticus, Micrococcus flavus, M. luteus, Micrococcus lylae, Micrococcus terreus, and Micrococcus yunnanensis. Genotypically and phenotypically, the species M. aloeverae, M. endophyticus, M. luteus, M. yunnanensis, M. antarcticus, and M. lylae are closely related and together constitute the M. luteus group [4]. Previous studies have reported the isolation of Micrococcus strains from numerous habitats, including human skin [5], permanently cold samples [6], activated sludge [7], the inner tissues of plants [8,9,10], soil [11], dairy waste [12], air [13], and intestine of several fish species [14,15]. These strains have been described as emerging opportunistic pathogens [16,17].
Pig is not only an important protein source for the human diet, but has also become increasingly important as biomedical animal model of human beings. The pig growth trait has been found to correlate with the gut microbiota [18,19,20], and the Prevotella copri has demonstrated to regulate the fat accumulation through a causal study in pigs [21]. Culturomics is an approach that is based on diverse culture conditions and enables the maximal recovery of culturable microorganisms from biological samples [22,23]. The cultivation projects of swine gut microbiota have been completed in recent years [24,25,26]. However, a huge portion of intestinal bacteria have still not been cultured. For a better understanding of the physiological impact of the gut microbiome of the host, to isolate, identify and characterize of uncultured bacteria is necessary. During a study aimed at isolating novel bacterial species present in the pig intestine using culturomics, one isolated strain, KD337-16T could not be assigned to any recognized species of the genus Micrococcus using matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF MS) analysis. Here, we report the phenotypic, chemotaxonomic and genotypic characterization of strain KD337-16T.
2. Materials and Methods
2.1. Isolation of Strain KD337-16T and Culture Conditions
This study was approved by the Institutional Animal Care and Use Committee of Livestock Research Institute (permit no. LRI IACUC110-35). A fecal sample obtained from a KHAPS black pig (Sus scrofa) was collected from the Kaohsiung Animal Propagation Station in Pingtung County (approximate geographic coordinates: 22.63424° N 20.60237° E), Taiwan, in 2021. Wet-weight feces (1 g) were suspended in 10 mL of phosphate-buffered saline, and the suspension was subsequently homogenized. Serial dilutions were plated on blood agar that contained 5% sheep blood for 48 h of aerobic incubation at 37 °C. All of the isolates were subjected for strain dereplication using a MALDI Microflex LT mass spectrometer (Bruker Daltonics, Bremen, Germany), as previously described [27]. One strain, named KD337-16T, could not be identified confidently. Strain KD337-16T and its phylogenetically closest reference species, including M. endophyticus BCRC 16908T, M. luteus BCRC 80739T, M. yunnanensis BCRC 80243T, M. aloeverae BCRC 80870T, and M. flavus BCRC 80069T, were routinely cultured on trypticase soy agar (TSA) at 37 °C for further taxonomic characterization, and the strains were then preserved in 10% glycerol at −80 °C.
2.2. DNA Extraction and Gene Sequence Comparison
A DNeasy kit (Qiagen, Valencia, CA, USA) was employed to extract and purify bacterial genomic DNA. The 16S rRNA gene and three housekeeping genes (gyrB, recA, and rpoB) were amplified and sequenced using a method reported elsewhere [4,28]. The EzBioCloud database (www.ezbiocloud.net/identify, accessed on 28 October 2022) was employed in subsequent BLAST analyses, and gene sequences were compared with those on NCBI GenBank (www.ncbi.nlm.nih.gov/BLAST/, accessed on 28 October 2022).
2.3. Phylogenetic Analysis
Clustal X (v. 2.1) software was employed for aligning sequences [29]. MEGA (v. 11) software was employed for phylogenetic tree reconstruction [30] based on sequences from the novel strain KD337-16T, its close relative strains, a roughly 1375 bp segment of the 16S rRNA gene, and nearly 1870 bp of the concatenated sequences of the three housekeeping genes (recA, gyrB, and rpoB). The neighbor-joining (NJ) [31], maximum-likelihood (ML) [32] and minimum-evolution (ME) [33] methods and the Kimura two-parameter model were used for tree reconstruction. Bootstrapping analysis with 1000 replicates was employed to determine how statistically reliable the trees were [34].
2.4. Genomic Analysis
A QIAamp PowerFecal Pro DNA Kit (Qiagen, Hilden, Germany) was employed to extract genomic DNA from the KD337-16T strain. Subsequently, the SQK-LSK109 Ligation Sequencing Kit on a PromethION Flow Cell (R9.4.1) and Illumina NovaSeq 6000 in paired-end (2 × 151 bp) mode was used for Oxford Nanopore Technologies (ONT) sequencing. After the sequences had been decoded and refined, Flye version 2.8.3 was employed for assembly of the valid ONT sequences. The primary contigs were polished with Racon v1.4.22 and the Illumina read alignment results constructed using Minimap2 v2.17. The DDBJ Fast Annotation and Submission Tool was used to annotate the genome [35]. Methods described elsewhere [36,37,38] were employed to quantify the digital DNA–DNA hybridization (dDDH), the amino acid identity (AAI), and average nucleotide identity (ANI). The up-to-date bacterial core genes pipeline (http://leb.snu.ac.kr/ubcg2, accessed on 28 October 2022) [39] and EDGAR platform were utilized to construct phylogenomic trees [40], whereas the 3ggNOG 4.5 database and carbohydrate-active enzyme (CAZy) database were employed for functional assignment [41,42]. The OrthoVenn2 webserver was used for pangenome analysis [43]. Finally, AntiSMASH software (v. 6.0) was employed to predict putative biosynthetic gene clusters [44].
2.5. Phenotypic Characterization
For the phenotypic analysis, strain KD337-16T was aerobically cultured on TSA at 37 °C for 48 h, unless otherwise stated. Cell morphology was observed under a phase-contrast microscope (Eclipse E600, Nikon, Tokyo, Japan). Gram staining was performed by using a Gram-staining kit (Difco, St. Louis, MA, USA) according to the manufacturer’s instructions. Growth at different temperatures (4, 15, 20, 28, 30, 37, 42 and 50 °C), NaCl concentrations (0–15% w/v, at 1% intervals) and pH levels (pH 4.0–12.0, at 1.0 pH unit intervals), tested in tryptic soy broth (TSB), were investigated through the standard methods [45]. Catalase activity was determined by the reaction of fresh cells toward 3% hydrogen peroxide (H2O2), and oxidase reaction was tested by using oxidase reagents (bioMerieux, Marcy-l’Étoile, France). The ability of the cells to utilize various sources of carbon and their enzyme activity were evaluated with commercial kits from API ZYM, API 20E, and Biolog GEN III MicroPlate system in accordance with the manufacturer’s instructions.
2.6. Chemotaxonomic Characterization
MALDI-TOF MS was performed for whole-cell protein analysis in accordance with a method described elsewhere [27]. Dendrogram clustering was constructed with the setting of 200 (distance measure: correlation; linkage: average; score oriented) using MALDI BioTyper software (v. 3.1; Bruker Daltonics, Billerica, MA, USA). Biomass for analysis of whole-cell fatty acids, polar lipids and isoprenoid quinone were obtained by culturing strain KD337-16T in TSB for 2 days at 30 °C. A previously reported method and the Sherlock Microbial Identification System (MIDI) were used to analyze whole-cell fatty acids as fatty acid methyl esters [46]. Polar lipids were extracted from 100 mg freeze-dried cells using the method described by Minnikin et al. [47] and analyzed by TLC using chloroform/methanol/water (65:25:4, by vol.) in the first direction and chloroform/acetic acid/methanol/water (80:18:12:5, by vol.) in the second. Lipids were visualized by spraying the TLC plate with 10% molybdophosphoric acid. Anisaldehyde (sugar), Schiff’s reagent (glycol) and Dittmer–Lester reagent (phosphorous) were also used as specific spray reagents for polar lipids. The molecular species and concentrations of isoprenoid quinones were determined as described by Hamada et al. [48].
3. Results and Discussion
MALDI-TOF MS is a phenotype-based method that can be used for the rapid identification and dereplication of numerous isolates on the basis of specific proteomic profiles [49]. The MALDI-TOF MS spectra of type strain KD337-16T, shown in Supplementary Figure S1, could not be reliably identified, yielding a log score of <1.7. On the basis of pairwise sequence analysis of the 16S rRNA gene, strain KD337-16T and its closest relatives, the type strains M. endophyticus YIM 56238T, M. luteus NCTC 2665T, M. yunnanensis YIM 65004T, M. aloeverae AE-6T, M. antarcticus T2T, and M. flavus LW4T, showed high similarity values of 99.5%, 99.1%, 99.1%, 99.1%, 98.9%, and 98.7%, respectively (Table 1).

Table 1.
Sequence similarity of the M. porci sp. nov. KD337-16T and its closely related species.
Through phylogenetic analysis of 16S rRNA gene sequences, the strain was determined to be a member of the M. luteus group (Figure 1). Members of the M. luteus group can be discriminated with good resolution using protein-encoding genes such as gyrB, recA and rpoB [4]. Among these three, gyrB has the highest discriminatory power at the interspecific level. The degrees of similarity between KD337-16T and other members of the M. luteus group were determined to be 90.1–91.6% (Table 1) on the basis of the concatenated sequences (gyrB, recA, and rpoB). When the NJ method was used for reconstruction, the resulting phylogenetic tree showed that strain KD337-16T constituted its own cluster and that this cluster was clearly separate from that of its close relatives (Figure 2). Using the ME or ML method resulted in similar tree topology, which indicated that this strain may be a novel species.
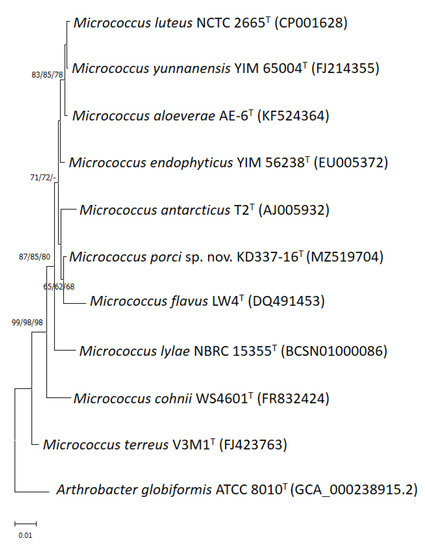
Figure 1.
Phylogenetic tree based on 16S rRNA gene sequences showing the relationship of M. porci sp. nov. KD337-16T with strains of closely related species. The tree was constructed by the neighbor-joining, minimum evolution and maximum-likelihood methods based on a comparison of approximately 1375 bp, and Arthrobacter globiformis ATCC 8010T was used as the outgroup. The type strain of the M. antarcticus CGMCC 1.2372 and JCM 11467 is currently not available from a recognized culture collection. Bootstrap values (>60%) based on 1000 replicates are shown at branch nodes. Bar, 1% sequence divergence.
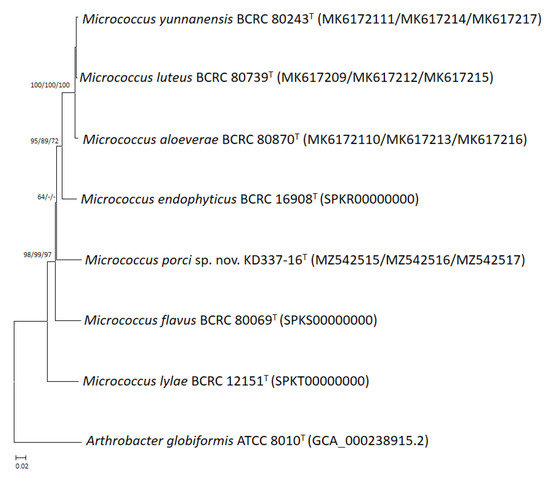
Figure 2.
Phylogenetic tree based on the concatenated housekeeping gene sequences (gyrB, recA, and rpoB) showing the relationship of M. porci sp. nov. KD337-16T with strains of closely related species. The tree was constructed by the neighbor-joining minimum evolution and maximum-likelihood methods based on a comparison of 1873 bp, and A. globiformis ATCC 8010T was used as an outgroup. Bootstrap values based on 1000 replicates are shown at branch nodes. Bar, 2% sequence divergence.
Whole-genome sequencing is currently the most fruitful source of taxonomic information [50,51,52,53]. Comparative genomics, overall genome–related indices (e.g., AAI, ANI, and dDDH), and phylogenomic tree analyses may be critical approaches for estimating evolutionary distances among species and delineating prokaryotic taxa at the species and genus levels. The present study revealed that strain KD337-16T had G+C content of 73.0% and a genome size of 2.64 Mb; the genome contained 2502 coding genes and 59 predicted RNA genes (Table 2). Between KD337-16T and its close relations, the ANI, AAI, and dDDH varied from 82.1% to 86.6%, 78.1% to 86.1%, and 24.4% to 34.9%, respectively (Table 3), lower than 95–96%, 95%, and 70%, the respective generally accepted cutoffs for prokaryotic species. By contrast, the ANI, AAI, and dDDH values among the M. aloeverae, M. luteus, and M. yunnanensis type strains exceeded the species thresholds, indicating that the aforementioned strains are members of the same species [4]. Nevertheless, between M. luteus and M. aloeverae and between M. luteus and M. yunnanensis, the dDDH was 77.8% and 77.9%, respectively, below the threshold for subspecies delineation [54]. The phylogenomic trees were obtained on the basis of 81 and 368 core genes (Figure 3 and Figure 4) shared by the investigated strains and we also discovered an independent cluster formed solely by strain KD337-16T, thus confirming this strain’s status as a novel species. A total of 2313 genes from strain KD337-16T were assigned to 21 functional categories (Supplementary Figure S2). The most common categories among these functional groups belonged to the [L] (recombination and repair; 220 genes) and [E] (amino acid transport and metabolism; 214 genes) clusters. Of identified CAZy families, the strain KD337-16T contained 4 carbohydrate binding modules, 28 glycoside hydrolases, and 12 glycosyl transferases, respectively. In addition, 2012 orthologous protein clusters were discovered in KD337-16T. Of the shared clusters, 502 were common in the five Micrococcus species (Supplementary Figure S3), and 24 protein clusters were unique to the novel Micrococcus porci strain (Supplementary Table S1). KD337-16T appears to produce putative secondary metabolite gene clusters, such as the beta lactone, terpene, and RiPP-like biosynthetic clusters.

Table 2.
Genomic characteristics of M. porci sp. nov. KD337-16T strain.

Table 3.
Average nucleotide identity (ANI), amino acid identity (AAI) and dDDH prediction values (%) between the strain KD337-16T and its closely related species.
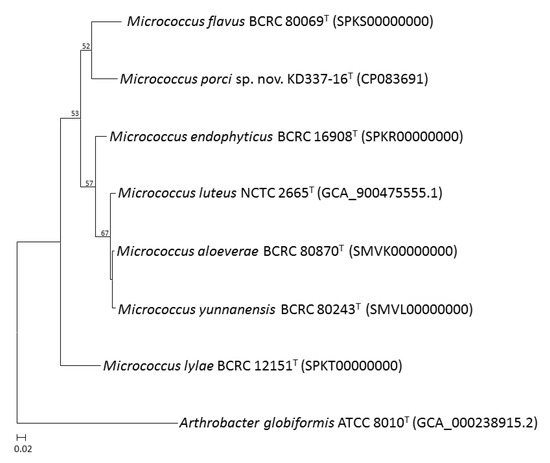
Figure 3.
An UBCG tree based on 81 bacterial core genes of M. porci sp. nov. KD337-16T and closely related species. Bootstrap values greater than 50% are shown at each node and A. globiformis ATCC 8010T was used as an outgroup. Bar, 2% sequence divergence.
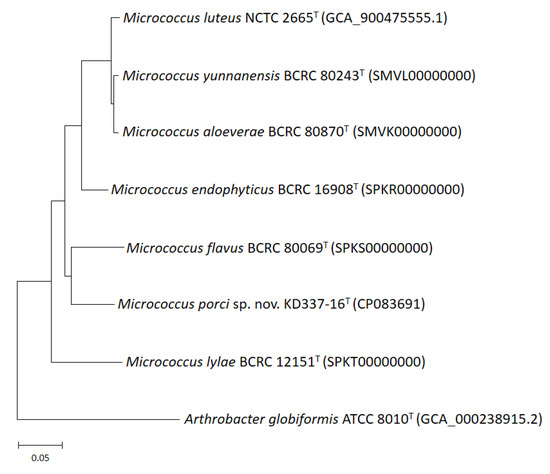
Figure 4.
Phylogenomic tree of M. porci sp. nov. KD337-16T with strains of closely related species. The tree was constructed by the maximum likelihood method the basis of a comparison of 368 core genes, and A. globiformis ATCC 8010T was used as an outgroup. Bar, 5% sequence divergence.
Cells of strain KD337-16T were coccoid shaped (approximately 0.5–1 µm) (Supplementary Figure S4), non-motile and non–spore-forming Gram-, catalase-, and oxidase-positive facultative anaerobic cells. Colonies were cream to yellow, slightly convex, smooth and circular. Table 4 lists the phenotypic characteristics that can be used to distinguish this novel strain from its close relatives. Cluster analysis of MALDI-TOF MS spectra in the 2000–12,000 m/z region of Micrococcus strains revealed the unambiguous grouping of five distinct clusters, each defined by known species and our novel taxon (Supplementary Figure S5). The fatty acid analysis revealed the major fatty acids (>10%) in strain KD337-16T to be anteiso-C15:0 and iso-C15:0; furthermore, this novel strain can be differentiated by analyzing whether C16:0 is present and minor fatty acids such as iso-C16:1 H, anteiso-C17:1ω9c, and summed features 1 and 3 are absent (Table 5). The polar lipids of strain KD337-16T were detected to be diphosphatidylglycerol, phosphatidylglycerol, phosphatidylinositol and unidentified glycolipid (Supplementary Figure S6). The predominant isoprenoid quinone of strain KD337-16T was MK-8(H2); MK-7(H2) and MK-9(H2) were detected as minor components (85:14:1). These features were in agreement with those of the genus Micrococcus [55].

Table 4.
Differential characteristics of M. porci sp. nov. KD337-16T and the phylogenetically closest related species of the genus Micrococcus. Strains: 1, KD337-16T; 2, M. endophyticus BCRC 16908T; 3, M. luteus BCRC 11034T; 4, M. flavus BCRC 80069T. All four strains were tested in parallel in this study for reactions of API ZYM, API 20E, and BIOLOG GENIII. +, Positive; −, Negative. ND, data not available. DPG, diphosphatidylglycerol; PG, phosphatidylglycerol; PI, phosphatidylinositol; PC, phosphatidylcholine; PL, unidentified phospholipids; GL, unidentified glycolipids.

Table 5.
Cellular fatty acid compositions (%) of M. porci sp. nov. KD337-16T and the phylogenetically closest related species of the genus Micrococcus. Scheme 1. KD337-16T; 2, M. endophyticus YIM 56238T; 3, M. luteus DSM 20030T; 4, M. flavus CGMCC 1.5361T [8]. Values are percentages of total fatty acids. Fatty acids present at >10% are indicated in bold. TR, Trace amount (<1.0%). –, not detected.
4. Conclusions
According to the results of polyphasic characterization, strain KD337-16T is genetically and phenotypically discernible from other currently recognized Micrococcus species. Thus, it represents a novel taxon for which the name Micrococcus porci sp. nov. is proposed.
Description of Micrococcus porci sp. nov.
Micrococcus porci (por’ci. L. gen. n. porci of a pig).
Cells are Gram-positive, aerobic, non-spore-forming, non-motile cocci with approximately 0.5–1 µm in diameter. Colonies are cream–yellow, slightly convex and smooth on TSA after 2 days at 37 °C under aerobic culture conditions. Growth occurs at 15–40 °C (not obviously at 15 °C) and pH 6–11, and tolerate 0–10% (w/v) NaCl. Cells are positive for catalase and oxidase. In API ZYM and API 20E system, positive reactions are obtained from the tests of alkaline phosphatase, esterase, esterase lipase, leucine arylamidase, valine arylamidase, cystine arylamidase, acid phosphatase, naphthol-AS-BI-phosphohydrolase, α-glucosidase, acetoin production and gelatinase, but not from lipase, trypsin, α-chymotrypsin, α-galactosidase, β-galactosidase, β-glucuronidase, β-glucosidase, N-acetyl-β-glucosaminidase, α-mannosidase, α-fucosidase, arginine dihydrolase, lysine decarboxylase, ornithine decar, boxylase, citrate utilization, urease, tryptophane deaminse and indole production. Biolog GEN III Microplate assays showed as positive for the utilization of dextrin, d-maltose, d-turanose, α-d-glucose, pectin, sodium butyrate, p-hydroxy-phenylacetic acid, l-lactic acid, α-hydroxy butyric acid, α-keto-butyric acid, potassium tellurite, d-fucose, l-fucose, acetoacetic acid and acetic acid; and negative for the utilization of d-trehalose, d-cellobiose, gentiobiose, sucrose, stachyose, d-raffinose, α-d-lactose, d-melibiose, β-methyl-d-glucoside, d-salicin, N-acetyl-β-d-mannosamine, N-acetyl galactosamine, N-acetyl neuraminic acid, d-mannose, d-fructose, 3-methyl glucose, myo-inositol, d-glucose-6-PO4, d-fructose-6-PO4, gelatin, l-alanine, l-arginine, l-aspartic acid, l-glutamic acid, l-histidine, l-pyroglutamic acid, d-gluconic acid, d-glucuronic acid, glucuronamide, mucic acid, quinic acid, d-saccharic acid, tetrazolium blue, methyl pyruvate, α-keto-glutaric acid, l-malic acid, bromo-succinic acid, Tween 40, propionic acid, sodium bromate, d-serine, troleandomycin, rifamycin SV, linomycin, tetrazolium violet, aztreonam, N-acetyl-d-glucosamine, d-aspartic acid, glycyl-l-proline, l-serine, d-lactic acid methyl ester, d-malic acid, γ-amino-butyric acid, β-hydroxy-d,l-butyric acid, d-galactose, l-rhamnose, d-galacturonic acid, l-galactonic acid lactone, fusidic acid, minocycline, guanidine HCl, niaproof 4, vancomycin, inosine, d-sorbitol, d-mannitol, d-arabitol, glycerol, citric acid and formic acid. The major cellular fatty acids and polar lipids are anteiso-C15:0 and iso-C15:0, and DPG and PG, respectively. The predominant menaquinone is MK-8(H2). The type strain KD337-16T (=BCRC 81318T = NBRC 115578T) was isolated from fecal samples of a black pig. The genome size of the type strain is 2.64 Mb and has a DNA G + C content of 73.0 mol%. The GenBank/EMBL/DDBJ accession numbers are MZ519704 (16S rRNA gene), MZ542515 (gyrB gene), MZ542516 (recA gene), MZ542517 (rpoB gene) and CP083691 (chromosome).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/life12111749/s1, Figure S1: Representative spectra from M. porci sp. nov. KD337-16T type strain. Figure S2: Results of an eggNOG functional category analysis of 2313 genes. Figure S3: Venn diagram showing the number of core, shared and unique genes (orthologous clusters) for each genome. Figure S4: Electron microscopic photograph of strain KD337-16T after aerobic cultivation on TSA plate at 37 °C for 1 day. Figure S5: Dendrogram showing the clustering of the Micrococcus strains based on MALDI-TOF MS analysis. Figure S6: Two-dimensional thin-layer chromatograms of polar lipids from strain KD337-16T. Table S1: Genomic characteristics of M. porci sp. nov. KD337-16T strain.
Author Contributions
Conceptualization, C.-H.H.; formal analysis, A.-Y.L., C.-H.C., C.-H.H., J.-S.L., Y.-C.L., M.H., Y.-T.W., C.-C.C., L.-L.P., S.-C.C. and C.-F.L.; funding acquisition, C.-H.H.; methodology, C.-H.H., A.-Y.L. and M.H.; project administration, C.-H.H.; visualization, C.-H.H.; writing—original draft, A.-Y.L. and C.-H.H.; writing—review and editing, C.-H.H. and L.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Council of Agriculture, Taiwan, ROC (project no. COA 111AS-2.1.1-LI-L6) and Ministry of Economic Affairs, Taiwan, ROC (project no. 111-EC-17-A-22-0525).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequencing data has been uploaded to GenBank (MZ519704, MZ542515~MZ542517, and CP083691).
Acknowledgments
We would like to thank Chii-Cherng Liao and Sung-Yuan Hsieh (Food Industry Research and Development Institute, Hsinchu, Taiwan) for their encouragement during the course of this research activity.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cohn, F. Untersuchungen über Bakterien. Beitr. Biol. Pflanz. 1872, 1, 127–244. [Google Scholar]
- Stackebrandt, E.; Koch, C.; Gvozdiak, O.; Schumann, P. Taxonomic dissection of the genus Micrococcus: Kocuria gen. nov., Nesterenkonia gen. nov., Kytococcus gen. nov., Dermacoccus gen. nov., and Micrococcus Cohn 1872 gen. emend. Int. J. Syst. Bacteriol. 1995, 45, 682–692. [Google Scholar] [CrossRef] [PubMed]
- Wieser, M.; Denner, E.B.; Kämpfer, P.; Schumann, P.; Tindallm, B.; Steiner, U.; Vybiral, D.; Lubitz, W.; Maszenan, A.M.; Patel, B.K.; et al. Emended descriptions of the genus Micrococcus, Micrococcus luteus (Cohn 1872) and Micrococcus lylae (Kloos et al., 1974). Int. J. Syst. Evol. Microbiol. 2002, 52, 629–637. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Wang, C.L.; Liou, J.S.; Lee, A.Y.; Blom, J.; Huang, L.; Watanabe, K. Reclassification of Micrococcus aloeverae and Micrococcus yunnanensis as later heterotypic synonyms of Micrococcus luteus. Int. J. Syst. Evol. Microbiol. 2019, 69, 3512–3518. [Google Scholar] [CrossRef] [PubMed]
- Kloos, W.E.; Tornabene, T.G.; Schleifer, K.H. Isolation and characterization of micrococci from human skin, including two new species: Micrococcus lylae and Micrococcus kristinae. Int. J. Syst. Bacteriol. 1974, 24, 79–101. [Google Scholar] [CrossRef]
- Liu, H.; Xu, Y.; Ma, Y.; Zhou, P. Characterization of Micrococcus antarcticus sp. nov., a psychrophilic bacterium from Antarctica. Int. J. Syst. Evol. Microbiol. 2000, 50, 715–719. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wang, B.J.; Jiang, C.Y.; Liu, S.J. Micrococcus flavus sp. nov., isolated from activated sludge in a bioreactor. Int. J. Syst. Evol. Microbiol. 2007, 57, 66–69. [Google Scholar] [CrossRef]
- Chen, H.H.; Zhao, G.Z.; Park, D.J.; Zhang, Y.Q.; Xu, L.H.; Lee, J.C.; Kim, C.J.; Li, W.J. Micrococcus endophyticus sp. nov., isolated from surface-sterilized Aquilaria sinensis roots. Int. J. Syst. Evol. Microbiol. 2009, 59, 1070–1075. [Google Scholar] [CrossRef]
- Prakash, O.; Nimonkar, Y.; Munot, H.; Sharma, A.; Vemuluri, V.R.; Chavadarm, M.S.; Schouche, Y.S. Description of Micrococcus aloeverae sp. nov., an endophytic actinobacterium isolated from Aloe vera. Int. J. Syst. Evol. Microbiol. 2014, 64, 3427–3433. [Google Scholar] [CrossRef]
- Zhao, G.Z.; Li, J.; Qin, S.; Zhang, Y.Q.; Zhu, W.Y.; Jiang, C.L.; Xu, L.H.; Li, W.J. Micrococcus yunnanensis sp. nov., a novel actinobacterium isolated from surfacesterilized Polyspora axillaris roots. Int. J. Syst. Evol. Microbiol. 2009, 59, 2383–2387. [Google Scholar] [CrossRef]
- Zhang, J.Y.; Liu, X.Y.; Liu, S.J. Agrococcus terreus sp. nov. and Micrococcus terreus sp. nov., isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2010, 60, 1897–1903. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chittpurna; Singh, P.K.; Verma, D.; Pinnaka, A.K.; Mayilraj, S.; Korpole, S. Micrococcus lactis sp. nov., isolated from dairy industry waste. Int. J. Syst. Evol. Microbiol. 2011, 61, 2832–2836. [Google Scholar] [CrossRef]
- Rieser, G.; Scherer, S.; Wenning, M. Micrococcus cohnii sp. nov., isolated from the air in a medical practice. Int. J. Syst. Evol. Microbiol. 2013, 63, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Akayli, T.; Albayrak, G.; Ürkü, Ç.; Çanak, Ö.; Yörük, E. Characterization of Micrococcus luteus and Bacillus marisflavi recovered from common dentex (Dentex dentex) larviculture system. Mediterr. Mar. Sci. 2016, 17, 163–169. [Google Scholar] [CrossRef]
- Saleh, O.; Mohamed, M.; El-Galil, A.; Abd El-Aziz, M.; El-Kamed, A.; Sayed, H. Isolation and Characterization of Micrococcus luteus from Oreochromis niloticus in Egypt. J. Curr. Vet. Res. 2021, 3, 16–23. [Google Scholar] [CrossRef]
- Zhu, M.; Zhu, Q.; Yang, Z.; Liang, Z. Clinical Characteristics of Patients with Micrococcus luteus Bloodstream Infection in a Chinese Tertiary-Care Hospital. Pol. J. Microbiol. 2021, 70, 321–326. [Google Scholar] [CrossRef]
- Pękala, A.; Paździor, E.; Antychowicz, J.; Bernad, A.; Głowacka, H.; Więcek, B.; Niemczuk, W. Kocuria rhizophila and Micrococcus luteus as emerging opportunist pathogens in brown trout (Salmo trutta Linnaeus, 1758) and rainbow trout (Oncorhynchus mykiss Walbaum, 1792). Aquaculture 2018, 486, 285–289. [Google Scholar] [CrossRef]
- Ding, X.; Lan, W.; Liu, G.; Ni, H.; Gu, J.D. Exploring possible associations of the intestine bacterial microbiome with the pre-weaned weight gaining performance of piglets in intensive pig production. Sci. Rep. 2019, 9, 15534. [Google Scholar] [CrossRef]
- Ramayo-Caldas, Y.; Mach, N.; Lepage, P.; Levenez, F.; Denis, C.; Lemonnier, G.; Leplat, J.J.; Billon, Y.; Berri, M.; Doré, J.; et al. Phylogenetic network analysis applied to pig gut microbiota identifies an ecosystem structure linked with growth traits. ISME 2016, 10, 2973–2977. [Google Scholar] [CrossRef]
- Quan, J.; Cai, G.; Ye, J.; Yang, M.; Ding, R.; Wang, X.; Zheng, E.; Fu, D.; Li, S.; Zhou, S.; et al. A global comparison of the microbiome compositions of three gut locations in commercial pigs with extreme feed conversion ratios. Sci. Rep. 2018, 8, 4536. [Google Scholar] [CrossRef]
- Chen, C.; Fang, S.; Wei, H.; He, M.; Fu, H.; Xiong, X.; Zhou, Y.; Wu, J.; Gao, J.; Yang, H.; et al. Prevotella copri increases fat accumulation in pigs fed with formula diets. Microbiome 2021, 9, 175. [Google Scholar] [CrossRef]
- Bilen, M.; Dufour, J.C.; Lagier, J.C.; Cadoret, F.; Daoud, Z.; Dubourg, G.; Raoult, D. The contribution of culturomics to the repertoire of isolated human bacterial and archaeal species. Microbiome 2018, 6, 94. [Google Scholar] [CrossRef]
- Lagier, J.C.; Armougom, F.; Million, M.; Hugon, P.; Pagnier, I.; Robert, C.; Bittar, F.; Fournous, G.; Gimenez, G.; Maraninchi, M.; et al. Microbial culturomics: Paradigm shift in the human gut microbiome study. Clin. Microbiol. Infect. 2012, 18, 1185–1193. [Google Scholar] [CrossRef]
- Fenske, G.; Ghimire, S.; Antony, L.; Christopher-Hennings, J.; Scaria, J. Comprehensive Cultivation of the Swine Gut Microbiome Reveals High Bacterial Diversity and Guides Bacterial Isolation in Pigs. FEMS Microbiol. Ecol. 2020, 96, fiaa022. [Google Scholar] [CrossRef]
- Wylensek, D.; Hitch, T.C.A.; Riedel, T.; Afrizal, A.; Kumar, N.; Wortmann, E.; Liu, T.; Devendran, S.; Lesker, T.R.; Hernández, S.B.; et al. A collection of bacterial isolates from the pig intestine reveals functional and taxonomic diversity. Nat. Commun. 2020, 11, 6389. [Google Scholar] [CrossRef]
- Wang, X.; Howe, S.; Wei, X.; Deng, F.; Tsai, T.; Chai, J.; Xiao, Y.; Yang, H.; Maxwell, C.V.; Li, Y.; et al. Comprehensive Cultivation of the swine gut microbiome reveals high bacterial diversity and guides bacterial isolation in pigs. mSystems 2021, 6, e0047721. [Google Scholar] [CrossRef]
- Huang, C.H.; Huang, L. Rapid species- and subspecies-specific level classification and identification of Lactobacillus casei group members using MALDI Biotyper combined with ClinProTools. J. Dairy Sci. 2018, 101, 979–991. [Google Scholar] [CrossRef]
- Huang, C.H.; Liou, J.S.; Lee, A.Y.; Tseng, M.; Miyashita, M.; Huang, L.; Watanabe, K. Polyphasic characterization of a novel species in the Lactobacillus casei group from cow manure of Taiwan: Description of L. chiayiensis sp. nov. Syst. Appl. Microbiol. 2018, 41, 270–278. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Plewniak, F.; Jeanmougin, F.; Higgins, D.G. The CLUSTAL X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997, 25, 4876–4882. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular Evolutionary Genetics Analysis Version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [PubMed]
- Chor, B.; Hendy, M.D.; Snir, S. Maximum likelihood Jukes-Cantor triplets: Analytic solutions. Mol. Biol. Evol. 2006, 23, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Rzhetsky, A.; Nei, M. A simple method for estimating and testing minimum-evolution trees. Mol. Biol. Evol. 1992, 9, 945–967. [Google Scholar]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Tanizawa, Y.; Fujisawa, T.; Kaminuma, E.; Nakamura, Y.; Arita, M. DFAST and DAGA: Web-based integrated genome annotation tools and resources. Biosci. Microbiota Food Health 2016, 35, 173–184. [Google Scholar] [CrossRef]
- Lee, I.; Kim, Y.O.; Park, S.C.; Chun, J. OrthoANI: An improved algorithm and software for calculating average nucleotide identity. Int. J. Syst. Evol. Microbiol. 2016, 66, 1100–1103. [Google Scholar] [CrossRef]
- Konstantinidis, K.T.; Tiedje, J.M. Towards a genome-based taxonomy for prokaryotes. J. Bacteriol. 2005, 187, 6258–6264. [Google Scholar] [CrossRef]
- Meier-Kolthoff, J.P.; Auch, A.F.; Klenk, H.P.; Göker, M. Genome sequence-based species delimitation with confidence intervals and improved distance functions. BMC Bioinform. 2013, 14, 60. [Google Scholar] [CrossRef]
- Kim, J.; Na, S.I.; Kim, D.; Chun, J. UBCG2: Up-to-date bacterial core genes and pipeline for phylogenomic analysis. J. Microbiol. 2021, 59, 609–615. [Google Scholar] [CrossRef]
- Blom, J.; Albaum, S.P.; Doppmeier, D.; Pühler, A.; Vorhölter, F.J.; Zakrzewski, M.; Goesmann, A. EDGAR: A software framework for the comparative analysis of prokaryotic genomes. BMC Bioinform. 2009, 10, 154. [Google Scholar] [CrossRef]
- Huerta-Cepas, J.; Szklarczyk, D.; Forslund, K.; Cook, H.; Heller, D.; Walter, M.D.; Rattei, T.; Mende, D.R.; Sunagawa, S.; Kuhn, M.; et al. eggNOG 4.5: A hierarchical orthology framework with improved functional annotations for eukaryotic, prokaryotic and viral sequences. Nucleic Acids Res. 2016, 44, D286–D293. [Google Scholar] [CrossRef]
- Lombard, V.; Golacondaamulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, D490–D495. [Google Scholar] [CrossRef]
- Xu, L.; Dong, Z.; Fang, L.; Luo, Y.; Wei, Z.; Guo, H.; Zhang, G.; Gu, Y.Q.; Coleman-Derr, D.; Xia, Q.; et al. OrthoVenn2: A web server for whole-genome comparison and annotation of orthologous clusters across multiple species. Nucleic Acids Res. 2019, 47, W52–W58. [Google Scholar] [CrossRef]
- Blin, K.; Shaw, S.; Kloosterman, A.M.; Charlop-Powers, Z.; van Wezel, G.P.; Medema, M.H.; Weber, T. antiSMASH 6.0: Improving cluster detection and comparison capabilities. Nucleic Acids Res. 2021, 49, W29–W35. [Google Scholar] [CrossRef]
- Barrow, G.I.; Feltham, R.K. Cowan and Steel’s Manual for the Identification of Medical Bacteria, 3rd ed.; Cambridge University Press: London, UK, 1993. [Google Scholar]
- Chern, L.L.; Stackebrandt, E.; Lee, S.F.; Lee, F.L.; Chen, J.K.; Fu, H.M. Chitinibacter tainanensis gen. nov., sp. nov., a chitin-degrading aerobe from soil in Taiwan. Int. J. Syst. Evol. Microbiol. 2004, 54, 1387–1391. [Google Scholar] [CrossRef]
- Minnikin, D.E.M.; Collins, D.; Goodfellow, M. Fatty acid and polar lipid composition in the classification of Cellulomonas, Oerskovia and related taxa. J. Appl. Bacteriol. 1975, 47, 87–95. [Google Scholar] [CrossRef]
- Hamada, M.; Yamamura, H.; Komukai, C.; Tamura, T.; Suzuki, K.I.; Hayakawa, M. Luteimicrobium album sp. nov., a novel actinobacterium isolated from a lichen collected in Japan, and emended description of the genus Luteimicrobium. J. Antibiot. 2012, 65, 427–431. [Google Scholar] [CrossRef]
- Ghyselinck, J.; Van Hoorde, K.; Hoste, B.; Heylen, K.; De Vos, P. Evaluation of MALDI-TOF MS as a tool for high-throughput dereplication. J. Microbiol. Methods 2011, 86, 327–336. [Google Scholar] [CrossRef]
- Chun, J.; Oren, A.; Ventosa, A.; Christensen, H.; Arahal, D.R.; da Costa, M.S.; Rooney, A.P.; Yi, H.; Xu, X.W.; De Meyer, S.; et al. Proposed minimal standards for the use of genome data for the taxonomy of prokaryotes. Int. J. Syst. Evol. Microbiol. 2018, 68, 461–466. [Google Scholar] [CrossRef]
- Sant’ Anna, F.H.; Bach, E.; Porto, R.Z.; Guella, F.; Sant’Anna, E.H.; Passaglia, L.M. Genomic metrics made easy: What to do and where to go in the new era of bacterial taxonomy. Crit. Rev. Microbiol. 2019, 45, 182–200. [Google Scholar] [CrossRef]
- Zong, Z. Genome-based Taxonomy for Bacteria: A Recent Advance. Trends Microbiol. 2020, 28, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wittouck, S.; Salvetti, E.; Franz, C.M.A.P.; Harri, H.M.B.; Mattarelli, P.; O’Toole, P.W.; Pot, B.; Vandamme, P.; Walter, J.; et al. A taxonomic note on the genus Lactobacillus: Description of 23 novel genera, emended description of the genus Lactobacillus Beijerinck 1901, and union of Lactobacillaceae and Leuconostocaceae. Int. J. Syst. Evol. Microbiol. 2020, 70, 2782–2858. [Google Scholar] [CrossRef] [PubMed]
- Meier-Kolthoff, J.P.; Hahnke, R.L.; Petersen, J.; Scheuner, C.; Michael, V.; Fiebig, A.; Rohde, C.; Rohde, M.; Fartmann, B.; Goodwin, L.A.; et al. Complete genome sequence of DSM 30083(T), the type strain (U5/41(T)) of Escherichia coli, and a proposal for delineating subspecies in microbial taxonomy. Stand. Genom. Sci. 2014, 9, 2. [Google Scholar] [CrossRef] [PubMed]
- Dastager, S.G.; Krishnamurthi, S.; Rameshkumar, N.; Dharne, M. The Family Micrococcaceae. In The Prokaryotes–Actinobacteria; Rosenberg, E., DeLong, E.F., Lory, S., Stackebrandt, E., Thompson, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2014; pp. 455–498. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).