Sex-Related Differences in Short-Term Outcomes after Mobile VA-ECMO Implantation: Five-Year Experience of an ECMO Retrieval Program
Abstract
1. Introduction
2. Materials and Methods
2.1. ECMO-Center Protocol
2.2. Data Collection
2.3. Outcome Analysis
2.4. Ethics
2.5. Statistical Methods
3. Results
3.1. Demographic, Clinical Characteristics and Postimplantation Data
3.2. Laboratory Parameter 24 and 48 h after VA-ECMO Implantation
3.3. Primary and Secondary Outcome Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ostadal, P.; Rokyta, R.; Kruger, A.; Vondrakova, D.; Janotka, M.; Šmíd, O.; Šmalcová, J.; Hromadka, M.; Linhart, A.; Bělohlávek, J. Extra corporeal membrane oxygenation in the therapy of cardiogenic shock (ECMO-CS): Rationale and design of the multicenter randomized trial. Eur. J. Heart Fail. 2017, 19, 124–127. (In English) [Google Scholar] [CrossRef]
- Gaisendrees, C.; Djordjevic, I.; Sabashnikov, A.; Adler, C.; Eghbalzadeh, K.; Ivanov, B.; Walter, S.G.; Braumann, S.; Wörmann, J.; Suhr, L.; et al. Gender-related differences in treatment and outcome of extracorporeal cardiopulmonary resuscitation-patients. Artif. Organs 2020, 45, 488–494. (In English) [Google Scholar] [CrossRef] [PubMed]
- Gerfer, S.; Gaisendrees, C.; Djordjevic, I.; Ivanov, B.; Merkle, J.; Eghbalzadeh, K.; Schlachtenberger, G.; Rustenbach, C.; Sabashnikov, A.; Kuhn-Régnier, F.; et al. Gender-related propensity score match analysis of ECMO therapy in postcardiotomy cardiogenic shock in patients after myocardial revascularization. Perfusion 2021, 37, 470–476. (In English) [Google Scholar] [CrossRef]
- Wernly, B.; Büter, S.; Masyuk, M.; Saeed, D.; Albert, A.; Fuernau, G.; Aubin, H.; Kelm, M.; Westenfeld, R.; Jung, C. Sex-specific Outcomes of Patients Treated With Extracorporeal Cardiopulmonary Resuscitation. J. Invasive Cardiol. 2020, 32, 422–426. (In English) [Google Scholar] [PubMed]
- Casey, S.D.; Mumma, B.E. Sex, race, and insurance status differences in hospital treatment and outcomes following out-of-hospital cardiac arrest. Resuscitation 2018, 126, 125–129. (In English) [Google Scholar] [CrossRef] [PubMed]
- Liaudat, C.C.; Vaucher, P.; De Francesco, T.; Jaunin-Stalder, N.; Herzig, L.; Verdon, F.; Favrat, B.; Locatelli, I.; Clair, C. Sex/gender bias in the management of chest pain in ambulatory care. Women’s Health 2018, 14, 5641. (In English) [Google Scholar] [CrossRef]
- Hsu, K.-H.; Chi, N.-H.; Yu, H.-Y.; Wang, C.-H.; Huang, S.-C.; Wang, S.-S.; Ko, W.-J.; Chen, Y.-S. Extracorporeal membranous oxygenation support for acute fulminant myocarditis: Analysis of a single center’s experience. Eur. J. Cardio Thorac. Surg. 2011, 40, 682–688. (In English) [Google Scholar] [CrossRef]
- Chung, S.-Y.; Sheu, J.-J.; Lin, Y.-J.; Sun, C.-K.; Chang, L.-T.; Chen, Y.-L.; Tsai, T.-H.; Chen, C.-J.; Yang, C.-H.; Leu, S.; et al. Outcome of patients with profound cardiogenic shock after cardiopulmonary resuscitation and prompt extracorporeal membrane oxygenation support. Circ. J. 2012, 76, 1385–1392. (In English) [Google Scholar] [CrossRef]
- Rajagopal, S.K.; Almond, C.S.; Laussen, P.C.; Rycus, P.T.; Wypij, D.; Thiagarajan, R.R. Extracorporeal membrane oxygenation for the support of infants, children, and young adults with acute myocarditis: A review of the extracorporeal life support organization registry. Crit. Care Med. 2010, 38, 382–387. (In English) [Google Scholar] [CrossRef]
- Hodgson, C.L.; Hayes, K.; Everard, T.; Nichol, A.D.; Davies, A.R.; Bailey, M.J.; Tuxen, D.V.; Cooper, D.J.; Pellegrino, V. Long-term quality of life in patients with acute respiratory distress syndrome requiring extracorporeal membrane oxygenation for refractory hypoxaemia. Crit. Care 2012, 16, R202. (In English) [Google Scholar] [CrossRef]
- Hemmila, M.R.; Rowe, S.A.; Boules, T.N.; Miskulin, J.; McGillicuddy, J.W.; Schuerer, D.J.; Haft, J.W.; Swaniker, F.; Arbabi, S.; Hirschl, R.B.; et al. Extracorporeal life support for severe acute respiratory distress syndrome in adults. Ann. Surg. 2004, 240, 595–605; discussion 605–607. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ting, P.-S.; Chen, L.; Yang, W.-C.; Huang, T.-S.; Wu, C.-C.; Chen, Y.-Y. Gender and age disparity in the initiation of life-supporting treatments: A population-based cohort study. BMC Med. Ethic 2017, 18, 62. (In English) [Google Scholar] [CrossRef] [PubMed]
- Akahane, M.; Ogawa, T.; Koike, S.; Tanabe, S.; Horiguchi, H.; Mizoguchi, T.; Yasunaga, H.; Imamura, T. The effects of sex on out-of-hospital cardiac arrest outcomes. Am. J. Med. 2011, 124, 325–333. (In English) [Google Scholar] [CrossRef] [PubMed]
- Sabashnikov, A.; Djordjevic, I.; Deppe, A.-C.; Kuhn, E.W.; Merkle, J.; Weber, C.; Sindhu, D.; Eghbalzadeh, K.; Zeriouh, M.; Liakopoulos, O.J.; et al. Managing traps and pitfalls during initial steps of an ECMO retrieval program using a miniaturized portable system: What have we learned from the first two years? Artif. Organs 2017, 42, 484–492. (In English) [Google Scholar] [CrossRef]
- Djordjevic, I.; Gaisendrees, C.; Adler, C.; Eghbalzadeh, K.; Braumann, S.; Ivanov, B.; Merkle, J.; Deppe, A.-C.; Kuhn, E.; Stangl, R.; et al. Extracorporeal cardiopulmonary resuscitation for out-of-hospital cardiac arrest: First results and outcomes of a newly established ECPR program in a large population area. Perfusion 2021, 37, 249–256. (In English) [Google Scholar] [CrossRef]
- Lorusso, R.; Shekar, K.; MacLaren, G.; Schmidt, M.; Pellegrino, V.; Meyns, B.; Haft, J.; Vercaemst, L.; Pappalardo, F.; Bermudez, C.; et al. ELSO interim guidelines for venoarterial extracorporeal membrane oxygenation in adult cardiac patients. ASAIO J. 2021, 67, 827–844. (In English) [Google Scholar] [CrossRef]
- Navarro-Gastón, D.; Munuera-Martínez, P.V. Prevalence of preoperative anxiety and its relationship with postoperative pain in foot nail surgery: A cross-sectional study. Int. J. Environ. Res. Public Health 2020, 17, 4481. (In English) [Google Scholar] [CrossRef]
- Tanaka, D.; Hirose, H.; Cavarocchi, N.; Entwistle, J.W. The impact of vascular complications on survival of patients on venoarterial extracorporeal membrane oxygenation. Ann. Thorac. Surg. 2016, 101, 1729–1734. (In English) [Google Scholar] [CrossRef]
- Lamb, K.M.; DiMuzio, P.J.; Johnson, A.; Batista, P.; Moudgill, N.; McCullough, M.; Eisenberg, J.A.; Hirose, H.; Cavarocchi, N.C. Arterial protocol including prophylactic distal perfusion catheter decreases limb ischemia complications in patients undergoing extracorporeal membrane oxygenation. J. Vasc. Surg. 2017, 65, 1074–1079. (In English) [Google Scholar] [CrossRef]
- Kasirajan, V.; Smedira, N.G.; McCarthy, J.F.; Casselman, F.; Boparai, N.; McCarthy, P.M. Risk factors for intracranial hemorrhage in adults on extracorporeal membrane oxygenation 1. Eur. J. Cardio-Thoracic Surg. 1999, 15, 508–514. (In English) [Google Scholar] [CrossRef]
- Berdowski, J.; Berg, R.A.; Tijssen, J.G.; Koster, R.W. Global incidences of out-of-hospital cardiac arrest and survival rates: Systematic review of 67 prospective studies. Resuscitation 2010, 81, 1479–1487. (In English) [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Yu, R.-G.; Yin, N.-N.; Zhou, J.-X. Combination of extracorporeal membrane oxygenation and continuous renal replacement therapy in critically ill patients: A systematic review. Crit. Care 2014, 18, 675. (In English) [Google Scholar] [CrossRef] [PubMed]
- Ellouze, O.; Vuillet, M.; Perrot, J.; Grosjean, S.; Missaoui, A.; Aho, S.; Malapert, G.; Bouhemad, B.; Bouchot, O.; Girard, C. Comparable outcome of out-of-hospital cardiac arrest and in-hospital cardiac arrest treated with extracorporeal life support. Artif. Organs 2017, 42, 15–21. (In English) [Google Scholar] [CrossRef] [PubMed]
- Neugarten, J.; Golestaneh, L.; Kolhe, N.V. Sex differences in acute kidney injury requiring dialysis. BMC Nephrol. 2018, 19, 131. (In English) [Google Scholar] [CrossRef]
- Han, S.-S.; Kim, H.J.; Lee, S.J.; Kim, W.J.; Hong, Y.; Lee, H.-Y.; Song, S.-Y.; Jung, H.H.; Ahn, H.S.; Ahn, I.M.; et al. Effects of renal replacement therapy in patients receiving extracorporeal membrane oxygenation: A meta-analysis. Ann. Thorac. Surg. 2015, 100, 1485–1495. (In English) [Google Scholar] [CrossRef]
- Ryu, J.-A.; Cho, Y.H.; Sung, K.; Choi, S.H.; Yang, J.H.; Choi, J.-H.; Lee, D.-S.; Yang, J.-H. Predictors of neurological outcomes after successful extracorporeal cardiopulmonary resuscitation. BMC Anesthesiol. 2015, 15, 26. (In English) [Google Scholar] [CrossRef]
- Blankstein, R.; Ward, R.P.; Arnsdorf, M.; Jones, B.; Lou, Y.-B.; Pine, M. Female gender is an independent predictor of operative mortality after coronary artery bypass graft surgery. Circulation 2005, 112, I323–I327. (In English) [Google Scholar] [CrossRef]
- Kaestner, F.; Rapp, D.; Trudzinski, F.; Olewczynska, N.; Wagenpfeil, S.; Langer, F.; Flaig, M.; Wilkens, H.; Bals, R.; Klingele, M.; et al. High serum bilirubin levels, nt-pro-bnp, and lactate predict mortality in long-term, severely ill respiratory ECMO patients. ASAIO J. 2018, 64, 232–237. (In English) [Google Scholar] [CrossRef]
- Steinhorn, R.H.; Isham-Schopf, B.; Smith, C.; Green, T.P. Hemolysis during long-term extracorporeal membrane oxygenation. J. Pediatr. 1989, 115, 625–630. (In English) [Google Scholar] [CrossRef]
- Lee, S.H.; Chung, C.H.; Lee, J.W.; Jung, S.H.; Choo, S.J. Factors predicting early- and long-term survival in patients undergoing extracorporeal membrane oxygenation (ECMO). J. Card. Surg. 2012, 27, 255–263. (In English) [Google Scholar] [CrossRef]
- Hermann, A.; Schellongowski, P.; Bojic, A.; Robak, O.; Buchtele, N.; Staudinger, T. ECMO without anticoagulation in patients with disease-related severe thrombocytopenia: Feasible but futile? Artif. Organs 2019, 43, 1077–1084. (In English) [Google Scholar] [CrossRef] [PubMed]
- Jiritano, F.; Serraino, G.F.; Cate, H.T.; Fina, D.; Matteucci, M.; Mastroroberto, P.; Lorusso, R. Platelets and extra-corporeal membrane oxygenation in adult patients: A systematic review and meta-analysis. Intensiv. Care Med. 2020, 46, 1154–1169. (In English) [Google Scholar] [CrossRef] [PubMed]
- Mazzeffi, M.; Tanaka, K. Platelets and ECMO: Should we worry about count, function, or both? Intensiv. Care Med. 2016, 42, 1199–1200. (In English) [Google Scholar] [CrossRef] [PubMed]
- Mazzeffi, M.; Rabin, J.; Deatrick, K.; Krause, E.; Madathil, R.; Grazioli, A.; Bathula, A.; Jackson, B.; Taylor, B.; Plazak, M. Platelet transfusion and in-hospital mortality in veno-arterial extracorporeal membrane oxygenation patients. ASAIO J. 2021, 68, 1249–1255. (In English) [Google Scholar] [CrossRef] [PubMed]
- Mazzeffi, M.A.; Tanaka, K.; Roberts, A.; Rector, R.; Menaker, J.; Kon, Z.; Deatrick, K.B.; Kaczorowski, D.; Griffith, B.; Herr, D. Bleeding, thrombosis, and transfusion with two heparin anticoagulation protocols in venoarterial ECMO patients. J. Cardiothorac. Vasc. Anesthesia 2019, 33, 1216–1220. (In English) [Google Scholar] [CrossRef]
- Chen, Z.; Mondal, N.; Zheng, S.; Koenig, S.C.; Slaughter, M.S.; Griffith, B.P.; Wu, Z.J. High shear induces platelet dysfunction leading to enhanced thrombotic propensity and diminished hemostatic capacity. Platelets 2017, 30, 112–119. (In English) [Google Scholar] [CrossRef]
- Djordjevic, I.; Deppe, A.-C.; Sabashnikov, A.; Kuhn, E.; Eghbalzadeh, K.; Merkle, J.; Gerfer, S.; Gaisendrees, C.; Ivanov, B.; Moellenbeck, L.; et al. Concomitant ECMO and IABP support in postcardiotomy cardiogenic shock patients. Heart Lung Circ. 2021, 30, 1533–1539. (In English) [Google Scholar] [CrossRef]
- Djordjevic, I.; Eghbalzadeh, K.; Sabashnikov, A.; Deppe, A.; Kuhn, E.; Merkle, J.; Weber, C.; Ivanov, B.; Ghodsizad, A.; Rustenbach, C.; et al. Central vs peripheral venoarterial ECMO in postcardiotomy cardiogenic shock. J. Card. Surg. 2020, 35, 1037–1042. (In English) [Google Scholar] [CrossRef]
- Djordjevic, I.; Sabashnikov, A.; Deppe, A.C.; Kuhn, E.; Eghbalzadeh, K.; Merkle, J.; Maier, J.; Weber, C.; Azizov, F.; Sindhu, D.; et al. Risk factors associated with 30-day mortality for out-of-center ECMO support: Experience from the newly launched ECMO retrieval service. J. Artif. Organs 2019, 22, 110–117. (In English) [Google Scholar] [CrossRef]
- Djordjevic, I.; Ivanov, B.; Sabashnikov, A.; Gaisendrees, C.; Gerfer, S.; Suhr, L.; Avgeridou, S.; Merkle-Storms, J.; Mihaylova, M.; Eghbalzadeh, K.; et al. Impact of obesity on in-hospital outcomes in veno-arterial ECMO patients. Hear. Lung Circ. 2022, 31, 1393–1398. (In English) [Google Scholar] [CrossRef]

| Male (n = 62) | Female (n = 25) | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD | 54.3 ± 13.8 | 54.7 ± 13.7 | 0.976 |
| BMI (kg/m2), mean ± SD | 27.2 ± 6.2 | 28.1 ± 8.0 | 0.370 |
| EuroSCORE II (%), mean ± SD | 7 ± 3 | 5 ± 3 | 0.390 |
| Distance to patient (km), mean ± SD | 22.8 ± 24.6 | 30.4 ± 25.9 | 0.841 |
| Central ECMO, n (%) | 4 (6.7%) | 0 (0.0%) | 0.253 |
| Peripheral ECMO, n (%) | 58 (93.3%) | 25 (100%) | 0.248 |
| Implantation technique, PP, n (%) | 50 (89.3%) | 25 (100%) | 0.117 |
| Arterial canula (Fr.), mean ± SD | 17.7 ± 1.2 | 17.0 ± 1.1 | 0.020 |
| Venous canula (Fr.), mean ± SD | 22.1 ± 1.2 | 21.6 ± 1.1 | 0.151 |
| DPC canula (Fr.), mean ± SD | 7.2 ± 0.7 | 6.8 ± 0.8 | 0.797 |
| eCPR, n (%) | 18 (30.0%) | 7 (30.4%) | 0.584 |
| Initial ECMO flow, L/m, mean ± SD | 4.0 ± 1.8 | 3.7 ± 2.3 | 0.770 |
| ECMO duration, h, mean ± SD | 90.4 ± 83.8 | 100.3 ± 82.6 | 0.947 |
| Inotropic support, n (%) | 51 (82.2%) | 18 (72.0%) | 0.696 |
| IABP, n (%) | 6 (10.3%) | 0 (0.0%) | 0.125 |
| Impella CP®, n (%) | 2 (3.4%) | 0 (0.0%) | 0.515 |
| ECMO weaning, n (%) | 24 (42.1%) | 14 (58.3%) | 0.137 |
| RBC, n, mean ± SD | 19.0 ± 20.5 | 18.2 ± 16.9 | 0.646 |
| FFP, n, mean ± SD | 10.1 ± 13.9 | 7.3 ± 9.5 | 0.207 |
| Platelets, n, mean ± SD | 1.8 ± 2.6 | 1.5 ± 1.8 | 0.302 |
| Male (n = 62) | Female (n = 25) | p-Value | |
|---|---|---|---|
| MAP (mmHg), mean ± SD | 57.7 ± 21.7 | 56.6 ± 23.9 | 0.349 |
| CVP (mmHg), mean ± SD | 10.0 ± 6.0 | 13.2 ± 7.9 | 0.730 |
| SvO2 (%), mean ± SD | 76.5 ± 13.0 | 68.1 ± 25.2 | 0.004 |
| pO2 (mmHg), mean ± SD | 151 ± 110 | 200 ± 149 | 0.115 |
| pCO2 (mmHg), mean ± SD | 41.2 ± 13.4 | 48.8 ± 32.5 | <0.001 |
| pH, mean ± SD | 7.2 ± 0.4 | 7.2 ± 0.1 | 0.842 |
| FiO2 (%), mean ± SD | 79.5 ± 25.5 | 75.0 ± 33.6 | 0.133 |
| Urea (mg/dL), mean ± SD | 80.6 ± 57.1 | 57.3 ± 35.5 | 0.068 |
| Creatinine (mg/dL), mean ± SD | 2.5 ± 1.9 | 1.5 ± 0.7 | 0.043 |
| Lactate (mmol/L), mean ± SD | 9.9 ± 6.9 | 9.6 ± 7.3 | 0.491 |
| Bilirubin (mg/dL), mean ± SD | 1.8 ± 3.7 | 0.7 ± 0.5 | 0.033 |
| AST (U/L), mean ± SD | 974 ± 1492 | 1744 ± 3079 | 0.004 |
| ALT (U/L), mean ± SD | 610 ± 1063 | 1110 ± 2075 | 0.004 |
| Hb (g/dL), mean ± SD | 11.1 ± 2.7 | 10.1 ± 3.3 | 0.193 |
| Hct (%), mean ± SD | 34.1 ± 7.6 | 30.4 ± 8.3 | 0.497 |
| WBC (109/L), mean ± SD | 5.3 ± 8.8 | 8.0 ± 10.8 | 0.066 |
| Platelets (109/L), mean ± SD | 50.4 ± 87.7 | 103.8 ± 158.6 | <0.001 |
| CRP (mg/L), mean ± SD | 68.4 ± 91.2 | 76.7 ± 108.9 | 0.483 |
| Na (mmol/L), mean ± SD | 142.1 ± 6.7 | 140.6 ± 5.7 | 0.160 |
| K (mmol/L), mean ± SD | 4.6 ± 0.8 | 4.4 ± 0.7 | 0.673 |
| aPTT (s), mean ± SD | 76.0 ± 38.5 | 90.7 ± 38.1 | 0.294 |
| Male (n = 62) | Female (n = 25) | p-Value | |
|---|---|---|---|
| MAP (mmHg), mean ± SD | 66.9 ± 11.2 | 64.8 ± 10.4 | 0.505 |
| CVP (mmHg), mean ± SD | 13.3 ± 9.8 | 9.8 ± 3.4 | 0.357 |
| SvO2 (%), mean ± SD | 73.3 ± 6.8 | 75.7 ± 7.6 | 0.994 |
| pO2 (mmHg), mean ± SD | 138.4 ± 69.8 | 117.1 ± 28.6 | 0.027 |
| pCO2 (mmHg), mean ± SD | 39.6 ± 5.3 | 38.2 ± 5.4 | 0.964 |
| pH, mean ± SD | 7.4 ± 0.1 | 7.4 ± 0.08 | 0.874 |
| FiO2 (%), mean ± SD | 60.8 ± 48.0 | 44.1 ± 23.1 | 0.284 |
| Urea (mg/dL), mean ± SD | 76.2 ± 42.9 | 55.7 ± 27.1 | 0.035 |
| Creatinine (mg/dL), mean ± SD | 2.7 ± 3.3 | 2.2 ± 2.1 | 0.830 |
| Lactate (mmol/L), mean ± SD | 4.6 ± 4.8 | 3.2 ± 2.8 | 0.304 |
| Bilirubin (mg/dL), mean ± SD | 3.4 ± 4.5 | 1.8 ± 1.2 | 0.027 |
| AST (U/L), mean ± SD | 1978 ± 3292 | 1889 ± 3011 | 0.751 |
| ALT (U/L), mean ± SD | 877 ± 1440 | 720 ± 1080 | 0.381 |
| Hb (g/dL), mean ± SD | 10.2 ± 1.2 | 10.6 ± 1.6 | 0.874 |
| Hct (%), mean ± SD | 28.8 ± 3.6 | 28.4 ± 3.4 | 0.679 |
| WBC (109/L), mean ± SD | 3.7 ± 6.9 | 6.2 ± 7.6 | 0.246 |
| Platelet(109/L), mean ± SD | 30.3 ± 57.3 | 67.0 ± 101.3 | <0.001 |
| CRP (mg/L), mean ± SD | 111.9 ± 83.6 | 128.5 ± 91.3 | 0.952 |
| Na (mmol/L), mean ± SD | 144.1 ± 4.3 | 142.6 ± 5.0 | 0.781 |
| K (mmol/L), mean ± SD | 5.8 ± 0.7 | 4.6 ± 0.4 | 0.243 |
| aPTT (s), mean ± SD | 61.7 ± 29.1 | 63.9 ± 22.2 | 0.280 |
| Male (n = 62) | Female (n = 25) | p-Value | |
|---|---|---|---|
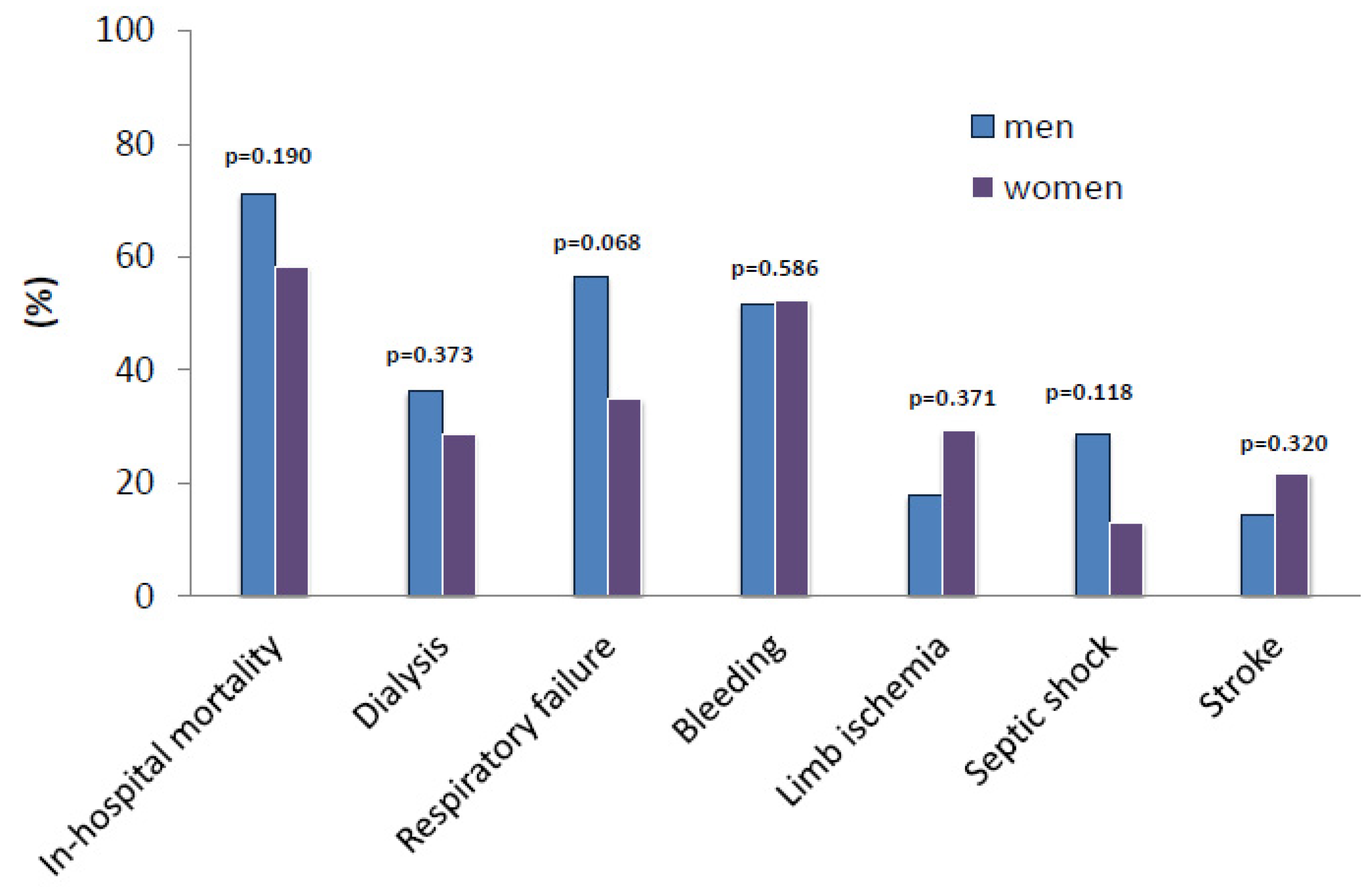
| Stroke, n (%) | 8 (14.5%) | 5 (21.7%) | 0.320 |
| Thromboembolic events, n (%) | 12 (21.8%) | 6 (26.1%) | 0.446 |
| Bleeding, n (%) | 29 (51.8%) | 12 (52.2%) | 0.586 |
| Limb ischemia, n (%) | 10 (17.9%) | 7 (29.2%) | 0.371 |
| Limb ischemia requiring intervention, n (%) | 5 (8.9%) | 4 (16.7%) | 0.441 |
| Respiratory failure, n (%) | 31 (56.4%) | 8 (34.8%) | 0.068 |
| Hepatic failure, n (%) | 19 (34.5%) | 6 (26.1%) | 0.326 |
| Renal failure, n (%) | 34 (61.8%) | 11 (47.8%) | 0.187 |
| Dialysis, n (%) | 17 (36.2%) | 6 (28.6%) | 0.373 |
| Oxygenator failure, n (%) | 1 (1.9%) | (0.0%) | 0.701 |
| SIRS, n (%) | 22 (40.0%) | 6 (26.1%) | 0.182 |
| Septic shock, n (%) | 16 (28.6%) | 3 (13.0%) | 0.118 |
| ICU stay (days), mean ± SD | 9 ± 11 | 10 ± 13 | 0.901 |
| Hospital stay (days), mean ± SD | 10 ± 12 | 11 ± 13 | 0.909 |
| Mortality rate (in-hospital), n (%) | 42 (71.2%) | 14 (58.3%) | 0.190 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krasivskyi, I.; Ivanov, B.; Vehrenberg, J.; Eghbalzadeh, K.; Gerfer, S.; Gaisendrees, C.; Kuhn, E.; Sabashnikov, A.; Mader, N.; Djordjevic, I.; et al. Sex-Related Differences in Short-Term Outcomes after Mobile VA-ECMO Implantation: Five-Year Experience of an ECMO Retrieval Program. Life 2022, 12, 1746. https://doi.org/10.3390/life12111746
Krasivskyi I, Ivanov B, Vehrenberg J, Eghbalzadeh K, Gerfer S, Gaisendrees C, Kuhn E, Sabashnikov A, Mader N, Djordjevic I, et al. Sex-Related Differences in Short-Term Outcomes after Mobile VA-ECMO Implantation: Five-Year Experience of an ECMO Retrieval Program. Life. 2022; 12(11):1746. https://doi.org/10.3390/life12111746
Chicago/Turabian StyleKrasivskyi, Ihor, Borko Ivanov, Johannes Vehrenberg, Kaveh Eghbalzadeh, Stephen Gerfer, Christopher Gaisendrees, Elmar Kuhn, Anton Sabashnikov, Navid Mader, Ilija Djordjevic, and et al. 2022. "Sex-Related Differences in Short-Term Outcomes after Mobile VA-ECMO Implantation: Five-Year Experience of an ECMO Retrieval Program" Life 12, no. 11: 1746. https://doi.org/10.3390/life12111746
APA StyleKrasivskyi, I., Ivanov, B., Vehrenberg, J., Eghbalzadeh, K., Gerfer, S., Gaisendrees, C., Kuhn, E., Sabashnikov, A., Mader, N., Djordjevic, I., & Wahlers, T. (2022). Sex-Related Differences in Short-Term Outcomes after Mobile VA-ECMO Implantation: Five-Year Experience of an ECMO Retrieval Program. Life, 12(11), 1746. https://doi.org/10.3390/life12111746









