Myocardial Viability Testing in the Management of Ischemic Heart Failure
Abstract
1. Introduction
2. Material and Methods
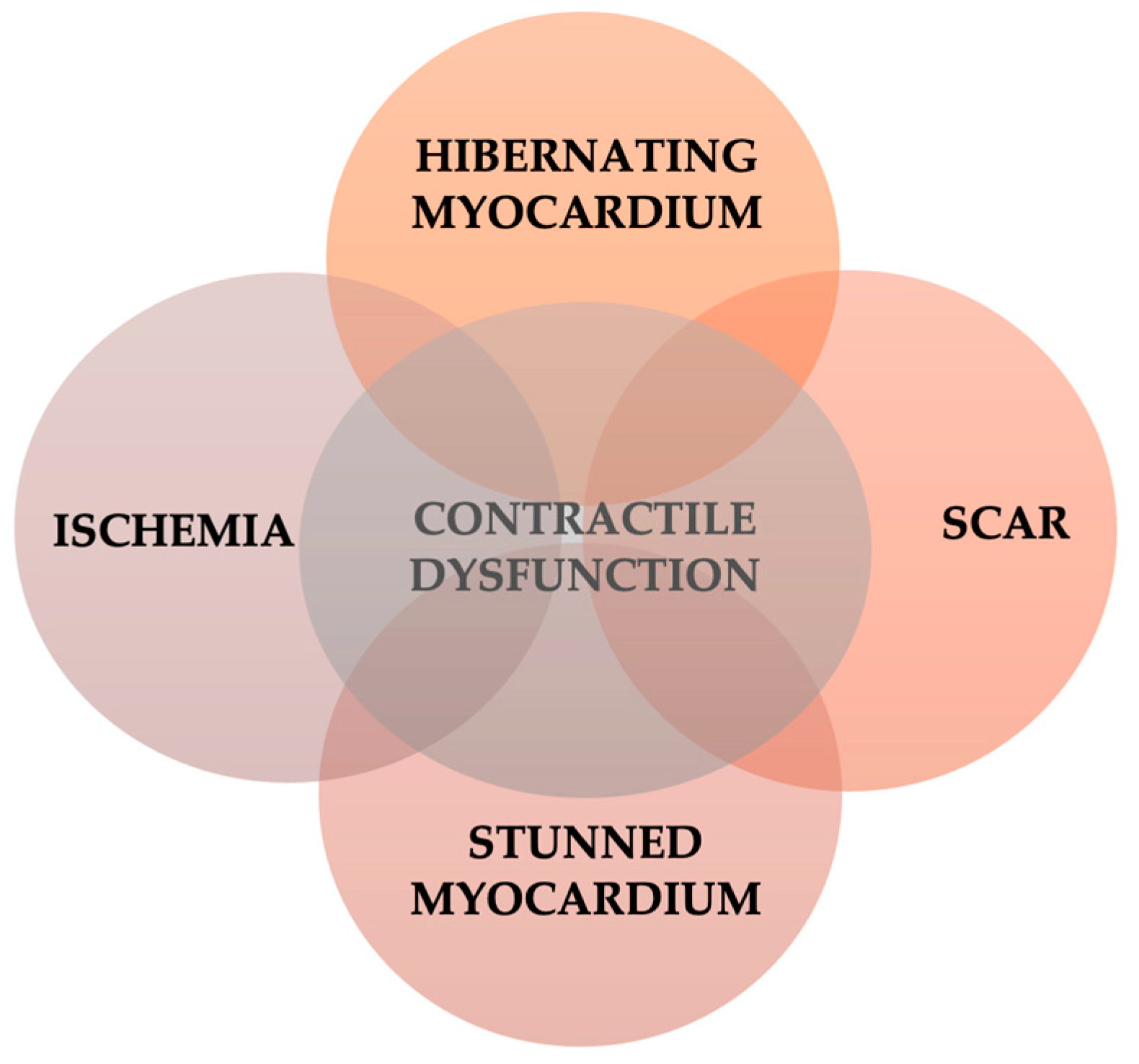
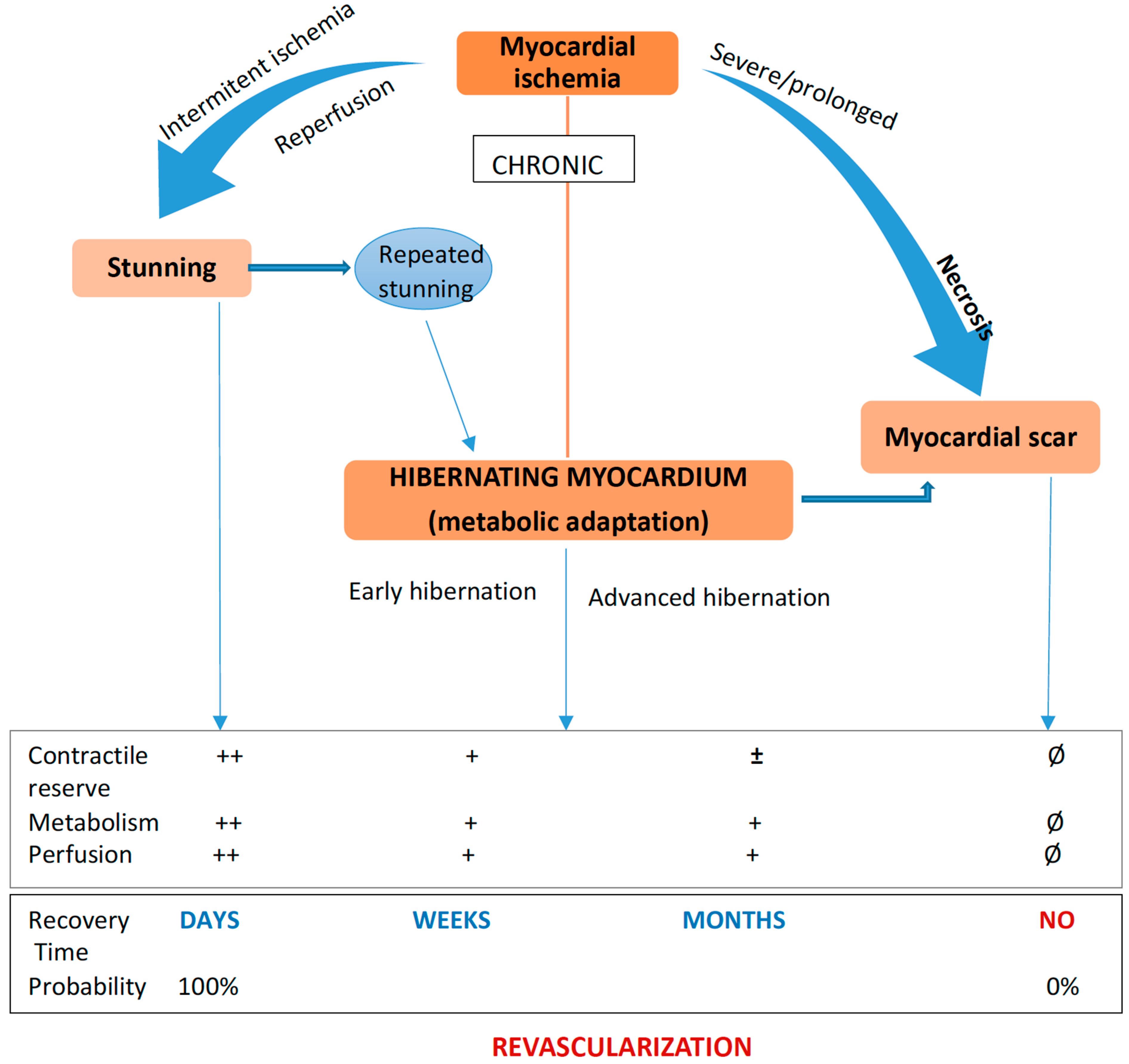
3. Viable Myocardium: Stunned and Hibernating
4. Methods for Myocardial Viability Assessment
4.1. Markers of Myocardial Viability and Methods of Assessment
4.2. Methods for Myocardial Viability Assessment
4.2.1. Echocardiography
4.2.2. Gated Single-Photon Emission Computerized Tomography (SPECT)
4.2.3. Positron Emission Tomography (PET)
4.2.4. Cardiac Magnetic Resonance Imaging (MRI)
4.2.5. Cardiac Computed Tomography
4.2.6. Coronary Angiography
4.2.7. Electrocardiography
5. Myocardial Viability Related Studies
6. Hibernating Myocardium and Prognosis in Ischemic Heart Failure
6.1. Revascularization and Prognosis
6.2. Interaction between Myocardial Ischemia and Viability
6.3. Pharmacological Therapy and Prognosis in Ischemic Heart Failure
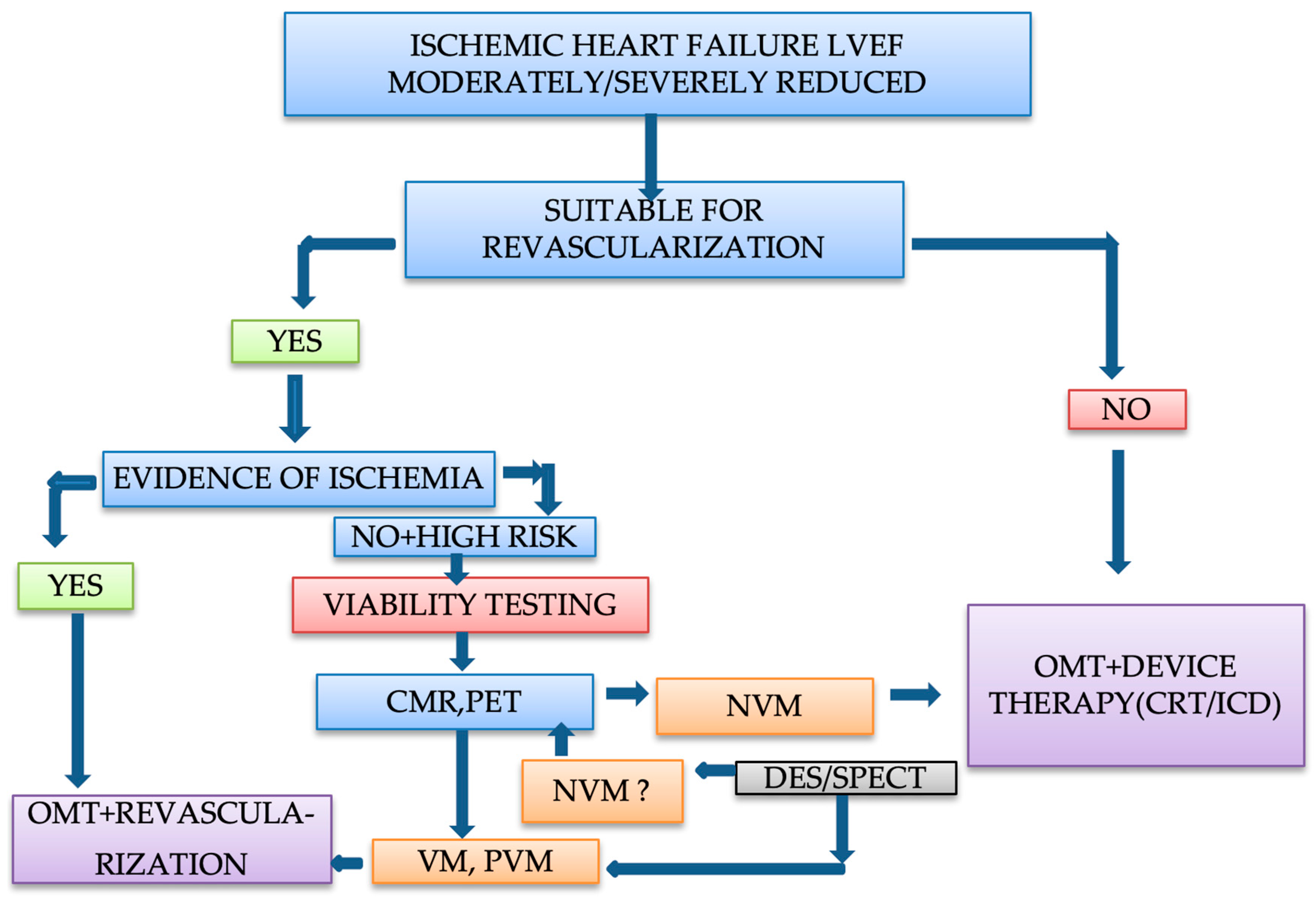
7. Clinical Approach and Management of Patients with Ischemic Heart Failure
8. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Travin, M.I.; Bergmann, S.R. Assessment of myocardial viability. Semin. Nucl. Med. 2005, 35, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Dimagli, A.; Benedetto, U. Surgical revascularization of hibernating myocardium: The known and the unknown. Vessel Plus 2021, 5, 13. [Google Scholar] [CrossRef]
- Bhat, A.; Gan, G.C.H.; Tan, T.C.; Hsu, C.; Denniss, A.R. Myocardial Viability: From Proof of Concept to Clinical Practice. Cardiol. Res. Pract. 2016, 2016, 1020818. [Google Scholar] [CrossRef] [PubMed]
- Gerber, B.L.; Rousseau, M.F.; Ahn, S.A.; de Waroux, J.-B.L.P.; Pouleur, A.-C.; Phlips, T.; Vancraeynest, D.; Pasquet, A.; Vanoverschelde, J.-L.J. Prognostic Value of Myocardial Viability by Delayed-Enhanced Magnetic Resonance in Patients with Coronary Artery Disease and Low Ejection Fraction: Impact of Revascularization Therapy. J. Am. Coll. Cardiol. 2012, 59, 825–835. [Google Scholar] [CrossRef]
- Beller, G.A. Noninvasive assessment of myocardial viability. Am. Coll. Cardiol. 2000, 343, 1488–1490. [Google Scholar] [CrossRef]
- Cahill, T.J.; Kharbanda, R.K. Viability testing to guide myocardial revascularisation in patients with heart failure. Indian J. Thorac. Cardiovasc. Surg. 2018, 34, 206–212. [Google Scholar] [CrossRef]
- Allman, K.C.; Shaw, L.J.; Hachamovitch, R.; Udelson, J.E. Myocardial viability testing and impact of revascularization on prognosis in patients with coronary artery disease and left ventricular dysfunction: A meta-analysis. J. Am. Coll. Cardiol. 2002, 39, 1151–1158. [Google Scholar] [CrossRef]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Giombolini, C.; Ragni, T.; Marino, P.N.; Reboldi, G.; Ambrosio, G. Patients with Hibernating Myocardium Show Altered Left Ventricular Volumes and Shape, Which Revert after Revascularization: Evidence That Dyssynergy Might Directly Induce Cardiac Remodeling. J. Am. Coll. Cardiol. 2006, 47, 969–977. [Google Scholar] [CrossRef]
- van Deursen, V.M.; Urso, R.; Laroche, C.; Damman, K.; Dahlström, U.; Tavazzi, L.; Maggioni, A.P.; Voors, A.A. Co-morbidities in patients with heart failure: An analysis of the European Heart Failure Pilot Survey. Eur. J. Heart Fail. 2014, 16, 103–111. [Google Scholar] [CrossRef]
- Topkara, V.K.; Cheema, F.H.; Kesavaramanujam, S.; Mercando, M.L.; Cheema, A.F.; Namerow, P.B.; Argenziano, M.; Naka, Y.; Oz, M.C.; Esrig, B.C. Coronary Artery Bypass Grafting in Patients with Low Ejection Fraction. Circulation 2005, 112, I-344–I-350. [Google Scholar] [CrossRef]
- Gössl, M.; Faxon, D.P.; Bell, M.R.; Holmes, D.R.; Gersh, B.J. Complete versus incomplete revascularization with coronary artery bypass graft or percutaneous intervention in stable coronary artery disease. Circ. Cardiovasc. Interv. 2012, 5, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Garcia, M.J.; Kwong, R.Y.; Scherrer-Crosbie, M.; Taub, C.C.; Blankstein, R.; Lima, J.; Bonow, R.O.; Eshtehardi, P.; Bois, J.P. State of the Art: Imaging for Myocardial Viability: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Imaging 2020, 13, e000053. [Google Scholar] [CrossRef] [PubMed]
- Cahill, T.J.; Kharbanda, R.K. Heart failure after myocardial infarction in the era of primary percutaneous coronary intervention: Mechanisms, incidence and identification of patients at risk. World J. Cardiol. 2017, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Babes, E.E.; Bustea, C.; Behl, T.; Abdel-Daim, M.M.; Nechifor, A.C.; Stoicescu, M.; Brisc, C.M.; Moisi, M.; Gitea, D.; Iovanovici, D.C.; et al. Acute coronary syndromes in diabetic patients, outcome, revascularization, and antithrombotic therapy. Biomed. Pharmacother. 2022, 148, 112772. [Google Scholar] [CrossRef]
- Babes, E.E.; Zaha, D.C.; Tit, D.M.; Nechifor, A.C.; Bungau, S.; Andronie-Cioara, F.L.; Behl, T.; Stoicescu, M.; Munteanu, M.A.; Rus, M.; et al. Value of Hematological and Coagulation Parameters as Prognostic Factors in Acute Coronary Syndromes. Diagnostics 2021, 11, 850. [Google Scholar] [CrossRef]
- Heusch, G.; Sipido, K.R. Myocardial Hibernation. Circ. Res. 2004, 94, 1005–1007. [Google Scholar] [CrossRef]
- Panza, J.A.; Chrzanowski, L.; Bonow, R.O. Myocardial Viability Assessment Before Surgical Revascularization in Ischemic Cardiomyopathy: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 78, 1068–1077. [Google Scholar] [CrossRef]
- Ryan, M.; Morgan, H.; Chiribiri, A.; Nagel, E.; Cleland, J.; Perera, D. Myocardial viability testing: All STICHed up, or about to be REVIVED? Eur. Heart J. 2021, 43, 118–126. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Takashi, E.; Ashraf, M. Pathologic Assessment of Myocardial Cell Necrosis and Apoptosis after Ischemia and Reperfusion with Molecular and Morphological Markers. J. Mol. Cell. Cardiol. 2000, 32, 209–224. [Google Scholar] [CrossRef]
- Kloner, R.A.; Bolli, R.; Marban, E.; Reinlib, L.; Braunwald, E. Medical and Cellular Implications of Stunning, Hibernation, and Preconditioning. Circulation 1998, 97, 1848–1867. [Google Scholar] [CrossRef] [PubMed]
- Rahimtoola, S.H. The hibernating myocardium. Am. Heart J. 1989, 117, 211–221. [Google Scholar] [CrossRef]
- Katikireddy, C.K.; Samim, A. Myocardial viability assessment and utility in contemporary management of ischemic cardiomyopathy. Clin. Cardiol. 2022, 45, 152–161. [Google Scholar] [CrossRef] [PubMed]
- Shah, B.N.; Khattar, R.S.; Senior, R. The hibernating myocardium: Current concepts, diagnostic dilemmas, and clinical challenges in the post-STICH era. Eur. Heart J. 2013, 34, 1323–1336. [Google Scholar] [CrossRef]
- Vanoverschelde, J.L.; Wijns, W.; Depré, C.; Essamri, B.; Heyndrickx, G.R.; Borgers, M.; Bol, A.; Melin, J.A. Mechanisms of chronic regional postischemic dysfunction in humans. New insights from the study of noninfarcted collateral-dependent myocardium. Circulation 1993, 87, 1513–1523. [Google Scholar] [CrossRef]
- Ahmed, C.M.; Sultan, M.A.U. The Context of StichTrial: Viable or not viable Does it matter before revascularization? Univers. Heart J. 2021, 17, 79–80. [Google Scholar] [CrossRef]
- Page, B.J.; Banas, M.D.; Suzuki, G.; Weil, B.R.; Young, R.F.; Fallavollita, J.A.; Palka, B.A.; Canty, J.M. Revascularization of chronic hibernating myocardium stimulates myocyte proliferation and partially reverses chronic adaptations to ischemia. J. Am. Coll. Cardiol. 2015, 65, 684–697. [Google Scholar] [CrossRef]
- Carli, M.F.D. Assessment of myocardial viability after myocardial infarction. J. Nucl. Cardiol. 2002, 9, 229–235. [Google Scholar] [CrossRef]
- Rahimtoola, S.H.; Canna, G.L.; Ferrari, R. Hibernating myocardium: Another piece of the puzzle falls into place. J. Am. Coll. Cardiol. 2006, 47, 978–980. [Google Scholar] [CrossRef]
- LJ, J.B.; Ruiz-Salmerón, R. Assessment of myocardial viability in patients before revascularization. Rev. Esp. Cardiol. 2003, 56, 721–733. [Google Scholar]
- Almeida, A.G.; Carpenter, J.-P.; Cameli, M.; Donal, E.; Dweck, M.R.; Flachskampf, F.A.; Maceira, A.M.; Muraru, D.; Neglia, D.; Pasquet, A.; et al. Multimodality imaging of myocardial viability: An expert consensus document from the European Association of Cardiovascular Imaging (EACVI). Eur. Heart J. Cardiovasc. Imaging 2021, 22, e97–e125. [Google Scholar] [CrossRef]
- Allman, K.C. Noninvasive assessment myocardial viability: Current status and future directions. J. Nucl. Cardiol. 2013, 20, 618–637. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.F.L.; Bax, J.J.; Poldermans, D.; Elhendy, A.; Ferrari, R.; Rahimtoola, S.H. Hibernating Myocardium: Diagnosis and Patient Outcomes. Curr. Probl. Cardiol. 2007, 32, 375–410. [Google Scholar] [CrossRef] [PubMed]
- LaCanna, G.; Rahimtoola, S.H.; Visioli, O.; Giubbini, R.; Alfieri, O.; Zognio, M.; Milan, E.; Ceconi, C.; Gargano, M.; LoRusso, R.; et al. Sensitivity, specificity, and predictive accuracies of non-invasive tests, singly and in combination, for diagnosis of hibernating myocardium. Eur. Heart J. 2000, 21, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Cwajg, J.M.; Cwajg, E.; Nagueh, S.F.; He, Z.-X.; Qureshi, U.; Olmos, L.I.; Quinones, M.A.; Verani, M.S.; Winters, W.L.; Zoghbi, W.A. End-diastolic wall thickness as a predictor of recovery of function in myocardial hibernation: Relation to rest-redistribution Tl-201 tomography and dobutamine stress echocardiography. J. Am. College Cardiol. 2000, 35, 1152–1161. [Google Scholar] [CrossRef]
- Bax, J.J.; Schinkel, A.F.L.; Boersma, E.; Elhendy, A.; Rizzello, V.; Maat, A.; Roelandt, J.R.T.C.; Wall, E.E.V.D.; Poldermans, D. Extensive Left Ventricular Remodeling Does Not Allow Viable Myocardium to Improve in Left Ventricular Ejection Fraction after Revascularization and Is Associated with Worse Long-Term Prognosis. Circulation 2004, 110, II-18–II-22. [Google Scholar] [CrossRef]
- Sawada, S. Dobutamine Echocardiography for Detection of Viable Myocardium in Ischemic Cardiomyopathy. Echocardiography 2000, 17, 69–77. [Google Scholar] [CrossRef]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e48. [Google Scholar] [CrossRef]
- Cornel, J.H.; Bax, J.J.; Elhendy, A.; Maat, A.P.W.M.; Kimman, G.-J.P.; Geleijnse, M.L.; Rambaldi, R.; Boersma, E.; Fioretti, P.M. Biphasic Response to Dobutamine Predicts Improvement of Global Left Ventricular Function after Surgical Revascularization in Patients with Stable Coronary Artery Disease: Implications of Time Course of Recovery on Diagnostic Accuracy. J. Am. Coll. Cardiol. 1998, 31, 1002–1010. [Google Scholar] [CrossRef]
- Löffler, A.I.; Kramer, C.M. Myocardial Viability Testing to Guide Coronary Revascularization. Interv. Cardiol. Clin. 2018, 7, 355–365. [Google Scholar] [CrossRef]
- Katikireddy, C.K.; Mann, N.; Brown, D.; Tosh, A.V.; Stergiopoulos, K. Evaluation of myocardial ischemia and viability by noninvasive cardiac imaging. Expert Rev. Cardiovasc. Ther. 2012, 10, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Bonow, R.O.; Castelvecchio, S.; Panza, J.A.; Berman, D.S.; Velazquez, E.J.; Michler, R.E.; She, L.; Holly, T.A.; Desvigne-Nickens, P.; Kosevic, D.; et al. Severity of Remodeling, Myocardial Viability, and Survival in Ischemic LV Dysfunction after Surgical Revascularization. JACC Cardiovasc. Imaging 2015, 8, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, M.; Liang, D.H.; Brown, P.; Gessford, E.; Schnittger, I. Contrast echocardiography is superior to tissue harmonics for assessment of left ventricular function in mechanically ventilated patients. Am. Heart J. 2000, 140, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Kornbluth, M.; Liang, D.H.; Paloma, A.; Schnittger, I. Native Tissue Harmonic Imaging Improves Endocardial Border Definition and Visualization of Cardiac Structures. J. Am. Soc. Echocardiogr. 1998, 11, 693–701. [Google Scholar] [CrossRef]
- Bax, J.J.; Wijns, W.; Cornel, J.H.; Visser, F.C.; Boersma, E.; Fioretti, P.M. Accuracy of currently available techniques for prediction of functional recovery after revascularization in patients with left ventricular dysfunction due to chronic coronary artery disease: Comparison of pooled data. J. Am. Coll. Cardiol. 1997, 30, 1451–1460. [Google Scholar] [CrossRef]
- Rizzello, V.; Poldermans, D.; Bax, J. Assessment of myocardial viability in chronic ischemic heart disease: Current status. Q. J. Nucl. Med. Mol. Imaging 2005, 49, 81. [Google Scholar]
- Pantea-Roșan, L.R.; Bungau, S.G.; Radu, A.-F.; Pantea, V.A.; Moisi, M.I.; Vesa, C.M.; Behl, T.; Nechifor, A.C.; Babes, E.E.; Stoicescu, M. A Narrative Review of the Classical and Modern Diagnostic Methods of the No-Reflow Phenomenon. Diagnostics 2022, 12, 932. [Google Scholar] [CrossRef]
- Wei, K. Assessment of Myocardial Viability Using Myocardial Contrast Echocardiography. Echocardiography 2005, 22, 85–94. [Google Scholar] [CrossRef]
- Shimoni, S.; Frangogiannis, N.G.; Aggeli, C.J.; Shan, K.; Quinones, M.A.; Espada, R.; Letsou, G.V.; Lawrie, G.M.; Winters, W.L.; Reardon, M.J.; et al. Microvascular Structural Correlates of Myocardial Contrast Echocardiography in Patients with Coronary Artery Disease and Left Ventricular Dysfunction. Circulation 2002, 106, 950–956. [Google Scholar] [CrossRef]
- Korosoglou, G.; Hansen, A.; Hoffend, J.; Gavrilovic, G.; Wolf, D.; Zehelein, J.; Haberkorn, U.; Kuecherer, H. Comparison of real-time myocardial contrast echocardiography for the assessment of myocardial viability with fluorodeoxyglucose-18 positron emission tomography and dobutamine stress echocardiography. Am. J. Cardiol. 2004, 94, 570–576. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Vaduganathan, P.; Ali, N.; Blaustein, A.; Verani, M.S.; Winters, W.L.; Zoghbi, W.A. Identification of Hibernating Myocardium: Comparative Accuracy of Myocardial Contrast Echocardiography, Rest-Redistribution Thallium-201 Tomography and Dobutamine Echocardiography. J. Am. Coll. Cardiol. 1997, 29, 985–993. [Google Scholar] [CrossRef]
- Roes, S.D.; Mollema, S.A.; Lamb, H.J.; van der Wall, E.E.; de Roos, A.; Bax, J.J. Validation of Echocardiographic Two-Dimensional Speckle Tracking Longitudinal Strain Imaging for Viability Assessment in Patients with Chronic Ischemic Left Ventricular Dysfunction and Comparison with Contrast-Enhanced Magnetic Resonance Imaging. Am. J. Cardiol. 2009, 104, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Schinkel, A.F.; Poldermans, D.; Elhendy, A.; Bax, J.J. Assessment of myocardial viability in patients with heart failure. J. Nucl. Med. 2007, 48, 1135–1146. [Google Scholar] [CrossRef] [PubMed]
- Dilsizian, V.; Rocco, T.P.; Freedman, N.M.T.; Leon, M.B.; Bonow, R.O. Enhanced Detection of Ischemic but Viable Myocardium by the Reinjection of Thallium after Stress-Redistribution Imaging. N. Engl. J. Med. 1990, 323, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Mazur, W.; Williams, K.A.; Kalra, D.K. Myocardial viability–State of the art: Is it still relevant and how to best assess it with imaging? Trends Cardiovasc. Med. 2018, 28, 24–37. [Google Scholar] [CrossRef]
- Dakik, H.A.; Howell, J.F.; Lawrie, G.M.; Espada, R.; Weilbaecher, D.G.; He, Z.-X.; Mahmarian, J.J.; Verani, M.S. Assessment of Myocardial Viability with 99mTc-Sestamibi Tomography Before Coronary Bypass Graft Surgery. Circulation 1997, 96, 2892–2898. [Google Scholar] [CrossRef] [PubMed]
- Maes, A.F.; Borgers, P.M.; Flameng, W.; Nuyts, J.L.; van de Werf, F.; Ausma, J.J.; Sergeant, P.; Mortelmans, L.A. Assessment of Myocardial Viability in Chronic Coronary Artery Disease Using Technetium-99m Sestamibi SPECT: Correlation with Histologic and Positron Emission Tomographic Studies and Functional Follow-Up. J. Am. Coll. Cardiol. 1997, 29, 62–68. [Google Scholar] [CrossRef]
- Wagner, A.; Mahrholdt, H.; Holly, T.A.; Elliott, M.D.; Regenfus, M.; Parker, M.; Klocke, F.J.; Bonow, R.O.; Kim, R.J.; Judd, R.M. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: An imaging study. Lancet 2003, 361, 374–379. [Google Scholar] [CrossRef]
- Ma, H.; Li, S.; Wu, Z.; Liu, J.; Liu, H.; Guo, X. Comparison of 99mTc-N-DBODC5 and 99mTc-MIBI of Myocardial Perfusion Imaging for Diagnosis of Coronary Artery Disease. BioMed Res. Int. 2013, 2013, 145427. [Google Scholar] [CrossRef][Green Version]
- Khalaf, S.; Chamsi-Pasha, M.; Al-Mallah, M.H. Assessment of myocardial viability by PET. Curr. Opin. Cardiol. 2019, 34, 466–472. [Google Scholar] [CrossRef]
- Berman, D.S.; Hachamovitch, R.; Shaw, L.J.; Friedman, J.D.; Hayes, S.W.; Thomson, L.E.; Fieno, D.S.; Germano, G.; Slomka, P.; Wong, N.D. Roles of nuclear cardiology, cardiac computed tomography, and cardiac magnetic resonance: Assessment of patients with suspected coronary artery disease. J. Nucl. Med. 2006, 47, 74–82. [Google Scholar] [PubMed]
- Romero, J.; Xue, X.; Gonzalez, W.; Garcia, M.J. CMR Imaging Assessing Viability in Patients with Chronic Ventricular Dysfunction Due to Coronary Artery Disease: A Meta-Analysis of Prospective Trials. JACC Cardiovasc. Imaging 2012, 5, 494–508. [Google Scholar] [CrossRef] [PubMed]
- Rogers, W.J.; Kramer, C.M.; Geskin, G.; Hu, Y.-L.; Theobald, T.M.; Vido, D.A.; Petruolo, S.; Reichek, N. Early Contrast-Enhanced MRI Predicts Late Functional Recovery after Reperfused Myocardial Infarction. Circulation 1999, 99, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.C.; Zerhouni, E.A.; Judd, R.M.; Lugo-Olivieri, C.H.; Barouch, L.A.; Schulman, S.P.; Blumenthal, R.S.; Lima, J.A.C. Prognostic Significance of Microvascular Obstruction by Magnetic Resonance Imaging in Patients with Acute Myocardial Infarction. Circulation 1998, 97, 765–772. [Google Scholar] [CrossRef]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.-L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The Use of Contrast-Enhanced Magnetic Resonance Imaging to Identify Reversible Myocardial Dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef]
- Gutberlet, M.; Fröhlich, M.; Mehl, S.; Amthauer, H.; Hausmann, H.; Meyer, R.; Siniawski, H.; Ruf, J.; Plotkin, M.; Denecke, T.; et al. Myocardial viability assessment in patients with highly impaired left ventricular function: Comparison of delayed enhancement, dobutamine stress MRI, end-diastolic wall thickness, and TI201-SPECT with functional recovery after revascularization. Eur. Radiol. 2005, 15, 872–880. [Google Scholar] [CrossRef]
- Choi, K.M.; Kim, R.J.; Gubernikoff, G.; Vargas, J.D.; Parker, M.; Judd, R.M. Transmural Extent of Acute Myocardial Infarction Predicts Long-Term Improvement in Contractile Function. Circulation 2001, 104, 1101–1107. [Google Scholar] [CrossRef]
- Gerber, B.L.; Rochitte, C.E.; Bluemke, D.A.; Melin, J.A.; Crosille, P.; Becker, L.C.; Lima, J.A.C. Relation Between Gd-DTPA Contrast Enhancement and Regional Inotropic Response in the Periphery and Center of Myocardial Infarction. Circulation 2001, 104, 998–1004. [Google Scholar] [CrossRef]
- Souto, A.L.M.; Souto, R.M.; Teixeira, I.C.R.; Nacif, M.S. Myocardial viability on cardiac magnetic resonance. Arq. Bras. Cardiol. 2017, 108, 458–469. [Google Scholar] [CrossRef]
- Hillenbrand, H.B.; Kim, R.J.; Parker, M.A.; Fieno, D.S.; Judd, R.M. Early Assessment of Myocardial Salvage by Contrast-Enhanced Magnetic Resonance Imaging. Circulation 2000, 102, 1678–1683. [Google Scholar] [CrossRef]
- Klein, C.; Nekolla, S.G.; Bengel, F.M.; Momose, M.; Sammer, A.; Haas, F.; Schnackenburg, B.; Delius, W.; Mudra, H.; Wolfram, D.; et al. Assessment of Myocardial Viability with Contrast-Enhanced Magnetic Resonance Imaging. Circulation 2002, 105, 162–167. [Google Scholar] [CrossRef] [PubMed]
- Baer, F.M.; Theissen, P.; Schneider, C.A.; Voth, E.; Sechtem, U.; Schicha, H.; Erdmann, E. Dobutamine Magnetic Resonance Imaging Predicts Contractile Recovery of Chronically Dysfunctional Myocardium after Successful Revascularization. J. Am. Coll. Cardiol. 1998, 31, 1040–1048. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Jin, H.; Deng, S.; Ren, D.; Greiser, A.; Fu, C.; Gao, H.; Zeng, M. Role of Myocardial Extracellular Volume Fraction Measured with Magnetic Resonance Imaging in the Prediction of Left Ventricular Functional Outcome after Revascularization of Chronic Total Occlusion of Coronary Arteries. Korean J. Radiol. 2019, 20, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Kidambi, A.; Motwani, M.; Uddin, A.; Ripley, D.P.; McDiarmid, A.K.; Swoboda, P.P.; Broadbent, D.A.; Musa, T.A.; Erhayiem, B.; Leader, J.; et al. Myocardial Extracellular Volume Estimation by CMR Predicts Functional Recovery Following Acute MI. JACC Cardiovasc. Imaging 2017, 10, 989–999. [Google Scholar] [CrossRef] [PubMed]
- Stathogiannis, K.; Mor-Avi, V.; Rashedi, N.; Lang, R.M.; Patel, A.R. Regional myocardial strain by cardiac magnetic resonance feature tracking for detection of scar in ischemic heart disease. Magn. Reson. Imaging 2020, 68, 190–196. [Google Scholar] [CrossRef]
- Israel, O.; Weiler-Sagie, M.; Rispler, S.; Bar-Shalom, R.; Frenkel, A.; Keidar, Z.; Bar-Shalev, A.; Strauss, H.W. PET/CT quantitation of the effect of patient-related factors on cardiac 18F-FDG uptake. J. Nucl. Med. 2007, 48, 234–239. [Google Scholar]
- Panza, J.A.; Ellis, A.M.; Al-Khalidi, H.R.; Holly, T.A.; Berman, D.S.; Oh, J.K.; Pohost, G.M.; Sopko, G.; Chrzanowski, L.; Mark, D.B.; et al. Myocardial Viability and Long-Term Outcomes in Ischemic Cardiomyopathy. N. Engl. J. Med. 2019, 381, 739–748. [Google Scholar] [CrossRef]
- Erthal, F.; Wiefels, C.; Promislow, S.; Kandolin, R.; Stadnick, E.; Mielniczuk, L.; Ruddy, T.; Small, G.; Beanlands, R. Myocardial viability: From PARR-2 to IMAGE HF-current evidence and future directions. Int. J. Cardiovasc. Sci. 2019, 32, 70–83. [Google Scholar] [CrossRef]
- Lardo, A.C.; Cordeiro, M.A.; Silva, C.; Amado, L.C.; George, R.T.; Saliaris, A.P.; Schuleri, K.H.; Fernandes, V.R.; Zviman, M.; Nazarian, S. Contrast-enhanced multidetector computed tomography viability imaging after myocardial infarction: Characterization of myocyte death, microvascular obstruction, and chronic scar. Circulation 2006, 113, 394–404. [Google Scholar] [CrossRef]
- Beleslin, B.; Ostojic, M.; Djordjevic-Dikic, A.; Vukcevic, V.; Stojkovic, S.; Nedeljkovic, M.; Stankovic, G.; Orlic, D.; Milic, N.; Stepanovic, J.; et al. The value of fractional and coronary flow reserve in predicting myocardial recovery in patients with previous myocardial infarction. Eur. Heart J. 2008, 29, 2617–2624. [Google Scholar] [CrossRef]
- Fearon, W.F.; Shah, M.; Ng, M.; Brinton, T.; Wilson, A.; Tremmel, J.A.; Schnittger, I.; Lee, D.P.; Vagelos, R.H.; Fitzgerald, P.J.; et al. Predictive Value of the Index of Microcirculatory Resistance in Patients with ST-Segment Elevation Myocardial Infarction. J. Am. Coll. Cardiol. 2008, 51, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.-S.; Yoon, M.-H.; Tahk, S.-J.; Yang, H.-M.; Choi, B.-J.; Choi, S.-Y.; Sheen, S.-S.; Hwang, G.-S.; Kang, S.-J.; Shin, J.-H. Usefulness of the index of microcirculatory resistance for invasively assessing myocardial viability immediately after primary angioplasty for anterior myocardial infarction. Eur. Heart J. 2009, 30, 2854–2860. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taneja, A.K.; Hayat, S.; Swinburn, J.; Senior, R. Usefulness of Q waves on ECG for the prediction of contractile reserve after acute myocardial infarction. Int. J. Cardiol. 2010, 145, 265–266. [Google Scholar] [CrossRef] [PubMed]
- Al-Mohammad, A.; Norton, M.Y.; Mahy, I.R.; Patel, J.C.; Welch, A.E.; Mikecz, P.; Walton, S. Can the surface electrocardiogram be used to predict myocardial viability? Heart 1999, 82, 663. [Google Scholar] [CrossRef] [PubMed]
- Nakano, A.; Lee, J.-D.; Shimizu, H.; Tsuchida, T.; Yonekura, Y.; Ishii, Y.; Ueda, T. Reciprocal ST-segment depression associated with exercise-induced ST-segment elevation indicates residual viability after myocardial infarction. J. Am. Coll. Cardiol. 1999, 33, 620–626. [Google Scholar] [CrossRef]
- Loeb, H.S.; Friedman, N.C. Normalization of Abnormal T-waves During Stress Testing does not Identify Patients with Reversible Perfusion Defects. Clin. Cardiol. 2007, 30, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Shim, W.J.; Jung, S.W.; Pak, H.N.; Lee, S.J.; Song, W.H.; Kim, Y.H.; Seo, H.S.; Oh, D.J.; Ro, Y.M. Relationship between T-wave normalization on exercise ECG and myocardial functional recovery in patients with acute myocardial infarction. Korean J. Intern. Med. 2002, 17, 122. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018, 40, 87–165. [Google Scholar] [CrossRef]
- Narula, J.; Dawson, M.S.; Singh, B.K.; Amanullah, A.; Acio, E.R.; Chaudhry, F.A.; Arani, R.B.; Iskandrian, A.E. Noninvasive characterization of stunned, hibernating, remodeled and nonviable myocardium in ischemic cardiomyopathy. J. Am. Coll. Cardiol. 2000, 36, 1913–1919. [Google Scholar] [CrossRef]
- Inaba, Y.; Chen, J.A.; Bergmann, S.R. Quantity of viable myocardium required to improve survival with revascularization in patients with ischemic cardiomyopathy: A meta-analysis. J. Nucl. Cardiol. 2010, 17, 646–654. [Google Scholar] [CrossRef]
- Glaveckaite, S.; Valeviciene, N.; Palionis, D.; Puronaite, R.; Serpytis, P.; Laucevicius, A. Prediction of long-term segmental and global functional recovery of hibernating myocardium after revascularisation based on low dose dobutamine and late gadolinium enhancement cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2014, 16, 83. [Google Scholar] [CrossRef] [PubMed]
- Rizzello, V.; Poldermans, D.; Biagini, E.; Schinkel, A.F.L.; Boersma, E.; Boccanelli, A.; Marwick, T.; Roelandt, J.R.T.C.; Bax, J.J. Prognosis of patients with ischaemic cardiomyopathy after coronary revascularisation: Relation to viability and improvement in left ventricular ejection fraction. Heart 2009, 95, 1273–1277. [Google Scholar] [CrossRef] [PubMed]
- Farina, G.R.; Ferreira, I.; Aguadé-Bruix, S.; Castell-Conesa, J.; Igual, A.; Candell-Riera, J. Analysis of the number of patients needed to treat by coronary revascularization in relation to the presence of myocardial viability in gated SPECT images: A prospective cohort study from a nuclear cardiology unit. Rev. Esp. Med. Nucl. 2009, 28, 6–10. [Google Scholar]
- Crişan, S.; Petrescu, L.; Lazăr, M.A.; Văcărescu, C.; Nicola, A.-R.; Cozma, D.; Mornoş, C.; Luca, C.T. Reduced ejection fraction heart failure–new data from multicenter studies and national registries regarding general and elderly populations: Hopes and disappointments. Clin. Interv. Aging 2018, 13, 651. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.F.; Marwick, T.H.; Flores, D.R.; Jaber, W.A.; Brunken, R.C.; Cerqueira, M.D.; Hachamovitch, R. Identification of Therapeutic Benefit from Revascularization in Patients with Left Ventricular Systolic Dysfunction. Circ. Cardiovasc. Imaging 2013, 6, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Pagley, P.R.; Beller, G.A.; Watson, D.D.; Gimple, L.W.; Ragosta, M. Improved Outcome after Coronary Bypass Surgery in Patients with Ischemic Cardiomyopathy and Residual Myocardial Viability. Circulation 1997, 96, 793–800. [Google Scholar] [CrossRef] [PubMed]
- Beanlands, R.S.B.; Nichol, G.; Huszti, E.; Humen, D.; Racine, N.; Freeman, M.; Gulenchyn, K.Y.; Garrard, L.; deKemp, R.; Guo, A.; et al. F-18-Fluorodeoxyglucose Positron Emission Tomography Imaging-Assisted Management of Patients with Severe Left Ventricular Dysfunction and Suspected Coronary Disease: A Randomized, Controlled Trial (PARR-2). J. Am. Coll. Cardiol. 2007, 50, 2002–2012. [Google Scholar] [CrossRef] [PubMed]
- Ardle, B.M.; Shukla, T.; Nichol, G.; deKemp, R.A.; Bernick, J.; Guo, A.; Lim, S.P.; Davies, R.A.; Haddad, H.; Duchesne, L. Long-term follow-up of outcomes with F-18-fluorodeoxyglucose positron emission tomography imaging–assisted management of patients with severe left ventricular dysfunction secondary to coronary disease. Circ. Cardiovasc. Imaging 2016, 9, e004331. [Google Scholar] [CrossRef]
- Abraham, A.; Nichol, G.; Williams, K.A.; Guo, A.; deKemp, R.A.; Garrard, L.; Davies, R.A.; Duchesne, L.; Haddad, H.; Chow, B.; et al. 18F-FDG PET Imaging of Myocardial Viability in an Experienced Center with Access to 18F-FDG and Integration with Clinical Management Teams: The Ottawa-FIVE Substudy of the PARR 2 Trial. J. Nucl. Med. 2010, 51, 567. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Deja, M.A.; Jain, A.; Sopko, G.; Marchenko, A.; Ali, I.S.; Pohost, G.; Gradinac, S.; Abraham, W.T.; et al. Coronary-Artery Bypass Surgery in Patients with Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1607–1616. [Google Scholar] [CrossRef]
- Bonow, R.O.; Maurer, G.; Lee, K.L.; Holly, T.A.; Binkley, P.F.; Desvigne-Nickens, P.; Drozdz, J.; Farsky, P.S.; Feldman, A.M.; Doenst, T.; et al. Myocardial Viability and Survival in Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2011, 364, 1617–1625. [Google Scholar] [CrossRef] [PubMed]
- Bax, J.J.; Visser, F.C.; Poldermans, D.; Elhendy, A.; Cornel, J.H.; Boersma, E.; van Lingen, A.; Fioretti, P.M.; Visser, C.A. Time course of functional recovery of stunned and hibernating segments after surgical revascularization. Circulation 2001, 104, I-314–I-318. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; Petrie, M.C.; Greenwood, J.P.; O’Kane, P.D.; Evans, R.; Sculpher, M.; McDonagh, T.; Gershlick, A.; de Belder, M.; et al. Percutaneous Revascularization for Ischemic Ventricular Dysfunction: Rationale and Design of the REVIVED-BCIS2 Trial: Percutaneous Coronary Intervention for Ischemic Cardiomyopathy. JACC Heart Fail. 2018, 6, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Calvert, M.; Freemantle, N.; Arrow, Y.; Ball, S.G.; Bonser, R.S.; Chattopadhyay, S.; Norell, M.S.; Pennell, D.J.; Senior, R. The Heart Failure Revascularisation Trial (HEART). Eur. J. Heart Fail. 2011, 13, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Crean, A.; Khan, S.N.; Davies, L.C.; Coulden, R.; Dutka, D.P. Assessment of Myocardial scar; comparison Between 18F-FDG peT, cMR and 99Tc-sestamibi. Clin. Med. Cardiol. 2009, 3, 69–76. [Google Scholar] [CrossRef]
- Arai, A.E. The cardiac magnetic resonance (CMR) approach to assessing myocardial viability. J. Nucl. Cardiol. 2011, 18, 1095–1102. [Google Scholar] [CrossRef]
- Kühl, H.P.; Lipke, C.S.A.; Krombach, G.A.; Katoh, M.; Battenberg, T.F.; Nowak, B.; Heussen, N.; Buecker, A.; Schaefer, W.M. Assessment of reversible myocardial dysfunction in chronic ischaemic heart disease: Comparison of contrast-enhanced cardiovascular magnetic resonance and a combined positron emission tomography–single photon emission computed tomography imaging protocol. Eur. Heart J. 2006, 27, 846–853. [Google Scholar] [CrossRef]
- Kassab, K.; Kattoor, A.J.; Doukky, R. Ischemia and viability testing in new-onset heart failure. Curr. Cardiol. Rep. 2020, 22, 1–14. [Google Scholar] [CrossRef]
- Canty, J.M.; Suzuki, G.; Banas, M.D.; Verheyen, F.; Borgers, M.; Fallavollita, J.A. Hibernating Myocardium. Circ. Res. 2004, 94, 1142–1149. [Google Scholar] [CrossRef]
- Yan, A.T.; Shayne, A.J.; Brown, K.A.; Gupta, S.N.; Chan, C.W.; Luu, T.M.; Carli, M.F.D.; Reynolds, H.G.; Stevenson, W.G.; Kwong, R.Y. Characterization of the Peri-Infarct Zone by Contrast-Enhanced Cardiac Magnetic Resonance Imaging Is a Powerful Predictor of Post–Myocardial Infarction Mortality. Circulation 2006, 114, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Azevedo, C.F.; Cheng, A.; Gupta, S.N.; Bluemke, D.A.; Foo, T.K.; Gerstenblith, G.; Weiss, R.G.; Marbán, E.; Tomaselli, G.F.; et al. Infarct Tissue Heterogeneity by Magnetic Resonance Imaging Identifies Enhanced Cardiac Arrhythmia Susceptibility in Patients with Left Ventricular Dysfunction. Circulation 2007, 115, 2006–2014. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, Y.; Yang, X.; Geng, B.; Liu, Y.; Shang, X.; Liu, J.; Lan, X.; Dong, N. Limited prognostic value of myocardial viability assessment in patients with coronary artery diseases and severe left ventricular dysfunction. J. Thoracic Dis. 2018, 10, 2249–2255. [Google Scholar] [CrossRef] [PubMed]
- Orlandini, A.; Castellana, N.; Pascual, A.; Botto, F.; Bahit, M.C.; Chacon, C.; Diaz, M.L.; Diaz, R. Myocardial viability for decision-making concerning revascularization in patients with left ventricular dysfunction and coronary artery disease: A meta-analysis of non-randomized and randomized studies. Int. J. Cardiol. 2015, 182, 494–499. [Google Scholar] [CrossRef] [PubMed]
- Davoudi, F.; Miyashita, S.; Yoo, T.K.; Imahira, U.; Kimmelstiel, C.; Huggins, G.S.; Downey, B.C. Do patients with non-viable myocardium from ischemic cardiomyopathy benefit from revascularization? A systematic review and meta-analysis. Cardiovasc. Revasc. Med. 2022, 1–6. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, E.; Mielniczuk, L.M.; Wells, G.A.; deKemp, R.A.; Klein, R.; Coyle, D.; Ardle, B.M.; Paterson, I.; White, J.A.; Arnold, M.; et al. Alternative Imaging Modalities in Ischemic Heart Failure (AIMI-HF) IMAGE HF Project I-A: Study protocol for a randomized controlled trial. Trials 2013, 14, 218. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A. Stunned and Hibernating Myocardium: Where Are We Nearly 4 Decades Later? J. Am. Heart Assoc. 2020, 9, e015502. [Google Scholar] [CrossRef]
- Rahimi, K.; Banning, A.P.; Cheng, A.S.H.; Pegg, T.J.; Karamitsos, T.D.; Channon, K.M.; Darby, S.; Taggart, D.P.; Neubauer, S.; Selvanayagam, J.B. Prognostic value of coronary revascularisation-related myocardial injury: A cardiac magnetic resonance imaging study. Heart 2009, 95, 1937. [Google Scholar] [CrossRef]
- Bondarenko, O.; Beek, A.M.; Twisk, J.W.R.; Visser, C.A.; van Rossum, A.C. Time course of functional recovery after revascularization of hibernating myocardium: A contrast-enhanced cardiovascular magnetic resonance study. Eur. Heart J. 2008, 29, 2000–2005. [Google Scholar] [CrossRef]
- Perry, A.S.; Mann, D.L.; Brown, D.L. Improvement of ejection fraction and mortality in ischaemic heart failure. Heart 2021, 107, 326–331. [Google Scholar] [CrossRef]
- Pellikka, P.A.; She, L.; Holly, T.A.; Lin, G.; Varadarajan, P.; Pai, R.G.; Bonow, R.O.; Pohost, G.M.; Panza, J.A.; Berman, D.S.; et al. Variability in Ejection Fraction Measured By Echocardiography, Gated Single-Photon Emission Computed Tomography, and Cardiac Magnetic Resonance in Patients with Coronary Artery Disease and Left Ventricular Dysfunction. JAMA Netw. Open 2018, 1, e181456. [Google Scholar] [CrossRef] [PubMed]
- Carson, P.; Wertheimer, J.; Miller, A.; O’Connor, C.M.; Pina, I.L.; Selzman, C.; Sueta, C.; She, L.; Greene, D.; Lee, K.L.; et al. The STICH Trial (Surgical Treatment for Ischemic Heart Failure): Mode-of-Death Results. JACC Heart Fail. 2013, 1, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Mark, D.B.; Knight, J.D.; Velazquez, E.J.; Wasilewski, J.; Howlett, J.G.; Smith, P.K.; Spertus, J.A.; Rajda, M.; Yadav, R.; Hamman, B.L. Quality-of-life outcomes with coronary artery bypass graft surgery in ischemic left ventricular dysfunction: A randomized trial. Ann. Intern. Med. 2014, 161, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Stewart, R.A.H.; Szalewska, D.; She, L.; Lee, K.L.; Drazner, M.H.; Lubiszewska, B.; Kosevic, D.; Ruengsakulrach, P.; Nicolau, J.C.; Coutu, B.; et al. Exercise Capacity and Mortality in Patients with Ischemic Left Ventricular Dysfunction Randomized to Coronary Artery Bypass Graft Surgery or Medical Therapy: An Analysis From the STICH Trial (Surgical Treatment for Ischemic Heart Failure). JACC Heart Fail. 2014, 2, 335–343. [Google Scholar] [CrossRef]
- Doenst, T.; Haverich, A.; Serruys, P.; Bonow, R.O.; Kappetein, P.; Falk, V.; Velazquez, E.; Diegeler, A.; Sigusch, H. PCI and CABG for Treating Stable Coronary Artery Disease: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2019, 73, 964–976. [Google Scholar] [CrossRef]
- Curtis, J.P.; Sokol, S.I.; Wang, Y.; Rathore, S.S.; Ko, D.T.; Jadbabaie, F.; Portnay, E.L.; Marshalko, S.J.; Radford, M.J.; Krumholz, H.M. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J. Am. Coll. Cardiol. 2003, 42, 736–742. [Google Scholar] [CrossRef]
- Cleland, J.G.; Hindricks, G.; Petrie, M. The shocking lack of evidence for implantable cardioverter defibrillators for heart failure; with or without cardiac resynchronization. Eur. Heart J. 2019, 40, 2128–2130. [Google Scholar] [CrossRef]
- Disertori, M.; Rigoni, M.; Pace, N.; Casolo, G.; Masè, M.; Gonzini, L.; Lucci, D.; Nollo, G.; Ravelli, F. Myocardial fibrosis assessment by LGE is a powerful predictor of ventricular tachyarrhythmias in ischemic and nonischemic LV dysfunction: A meta-analysis. JACC Cardiovasc. Imaging 2016, 9, 1046–1055. [Google Scholar] [CrossRef]
- Halliday, B.P.; Gulati, A.; Ali, A.; Guha, K.; Newsome, S.; Arzanauskaite, M.; Vassiliou, V.S.; Lota, A.; Izgi, C.; Tayal, U.; et al. Association Between Midwall Late Gadolinium Enhancement and Sudden Cardiac Death in Patients with Dilated Cardiomyopathy and Mild and Moderate Left Ventricular Systolic Dysfunction. Circulation 2017, 135, 2106–2115. [Google Scholar] [CrossRef]
- Acosta, J.; Fernández-Armenta, J.; Borràs, R.; Anguera, I.; Bisbal, F.; Martí-Almor, J.; Tolosana, J.M.; Penela, D.; Andreu, D.; Soto-Iglesias, D.; et al. Scar Characterization to Predict Life-Threatening Arrhythmic Events and Sudden Cardiac Death in Patients with Cardiac Resynchronization Therapy: The GAUDI-CRT Study. JACC Cardiovasc. Imaging 2018, 11, 561–572. [Google Scholar] [CrossRef]
- Meo, M.; Bonizzi, P.; Bear, L.R.; Cluitmans, M.; Abell, E.; Haïssaguerre, M.; Bernus, O.; Dubois, R. Body surface mapping of ventricular repolarization heterogeneity: An ex-vivo multiparameter study. Front. Physiol. 2020, 11, 933. [Google Scholar] [CrossRef] [PubMed]
- Aung, M.; Mihir, P.; John, S.; David, H.-S. Impact of chronic total coronary occlusion revascularisation on infarct-related myocardial scars responsible for recurrent ventricular tachycardia. EuroIntervention 2021, 16, 1204–1206. [Google Scholar]
- van Dongen, I.M.; Kolk, M.Z.; Elias, J.; Meijborg, V.M.; Coronel, R.; de Bakker, J.M.; Claessen, B.E.; Delewi, R.; Ouweneel, D.M.; Scheunhage, E.M. The effect of revascularization of a chronic total coronary occlusion on electrocardiographic variables. A sub-study of the EXPLORE trial. J. Electrocardiol. 2018, 51, 906–912. [Google Scholar] [CrossRef] [PubMed]
- Carluccio, E.; Biagioli, P.; Alunni, G.; Murrone, A.; Leonelli, V.; Pantano, P.; Vincenti, G.; Giombolini, C.; Ragni, T.; Reboldi, G. Effect of revascularizing viable myocardium on left ventricular diastolic function in patients with ischaemic cardiomyopathy. Eur. Heart J. 2009, 30, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Mashayekhi, K.; Nührenberg, T.G.; Toma, A.; Gick, M.; Ferenc, M.; Hochholzer, W.; Comberg, T.; Rothe, J.; Valina, C.M.; Löffelhardt, N. A randomized trial to assess regional left ventricular function after stent implantation in chronic total occlusion: The REVASC trial. JACC Cardiovasc. Interv. 2018, 11, 1982–1991. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Y.; Petrie, M.C.; Jolicoeur, E.M.; Madhavan, M.V.; Velazquez, E.J.; Moses, J.W.; Lansky, A.J.; Stone, G.W. PCI in Patients with Heart Failure: Current Evidence, Impact of Complete Revascularization, and Contemporary Techniques to Improve Outcomes. J. Soc. Cardiovasc. Angiogr. Interv. 2022, 1, 100020. [Google Scholar] [CrossRef]
- McDiarmid, A.K.; Loh, H.; Nikitin, N.; Cleland, J.G.; Ball, S.G.; Greenwood, J.P.; Plein, S.; Sparrow, P. Predictive power of late gadolinium enhancement for myocardial recovery in chronic ischaemic heart failure: A HEART sub-study. ESC Heart Fail. 2014, 1, 146–153. [Google Scholar] [CrossRef][Green Version]
- Hamburger, R.F. Left ventricular dysfunction in ischemic heart disease. Cardiovasc. Innov. Appl. 2019, 3, 297–303. [Google Scholar] [CrossRef]
- Bleeker, G.B.; Schalij, M.J.; Molhoek, S.G.; Holman, E.R.; Verwey, H.F.; Steendijk, P.; van der Wall, E.E.; Bax, J.J. Frequency of left ventricular dyssynchrony in patients with heart failure and a narrow QRS complex. Am. J. Cardiol. 2005, 95, 140–142. [Google Scholar] [CrossRef]
- Roemer, S.; Jaglan, A.; Santos, D.; Umland, M.; Jain, R.; Tajik, A.J.; Khandheria, B.K. The Utility of Myocardial Work in Clinical Practice. J. Am. Soc. Echocardiogr. 2021, 34, 807–818. [Google Scholar] [CrossRef]
- Sammut, E.; Zarinabad, N.; Wesolowski, R.; Morton, G.; Chen, Z.; Sohal, M.; Carr-White, G.; Razavi, R.; Chiribiri, A. Feasibility of high-resolution quantitative perfusion analysis in patients with heart failure. J. Cardiovasc. Magn. Reson. 2015, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pasquet, A.S.; Robert, A.; D’Hondt, A.-M.; Dion, R.; Melin, J.A.; Vanoverschelde, J.-L.J. Prognostic value of myocardial ischemia and viability in patients with chronic left ventricular ischemic dysfunction. Circulation 1999, 100, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Sawada, S.G.; Lewis, S.J.; Foltz, J.; Ando, A.; Khouri, S.; Kaser, S.; Gradus-Pizlo, I.; Gill, W.; Fineberg, N.; Segar, D. Usefulness of rest and low-dose dobutamine wall motion scores in predicting survival and benefit from revascularization in patients with ischemic cardiomyopathy. Am. J. Cardiol. 2002, 89, 811–816. [Google Scholar] [CrossRef]
- Gupta, A.; Harrington, M.; Albert, C.M.; Bajaj, N.S.; Hainer, J.; Morgan, V.; Bibbo, C.F.; Bravo, P.E.; Osborne, M.T.; Dorbala, S. Myocardial scar but not ischemia is associated with defibrillator shocks and sudden cardiac death in stable patients with reduced left ventricular ejection fraction. JACC Clin. Electrophysiol. 2018, 4, 1200–1210. [Google Scholar] [CrossRef]
- Lopes, R.D.; Alexander, K.P.; Stevens, S.R.; Reynolds, H.R.; Stone, G.W.; Piña, I.L.; Rockhold, F.W.; Elghamaz, A.; Lopez-Sendon, J.L.; Farsky, P.S. Initial invasive versus conservative management of stable ischemic heart disease in patients with a history of heart failure or left ventricular dysfunction: Insights from the ISCHEMIA trial. Circulation 2020, 142, 1725–1735. [Google Scholar] [CrossRef] [PubMed]
- Hachamovitch, R.; Soman, P. ISCHEMIA trial: Are we still fighting the last war? Circ. Cardiovasc. Imaging 2021, 14, e012319. [Google Scholar] [CrossRef]
- Cleland, J.G.F.; Pennell, D.J.; Ray, S.G.; Coats, A.J.; Macfarlane, P.W.; Murray, G.D.; Mule, J.D.; Vered, Z.; Lahiri, A. Myocardial viability as a determinant of the ejection fraction response to carvedilol in patients with heart failure (CHRISTMAS trial): Randomised controlled trial. Lancet 2003, 362, 14–21. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- McMurray, J.; Packer, M.; Desai, A.; Gong, J.; Lefkowitz, M.; Rizkala, A.; Rouleau, J.; Shi, V.; Solomon, S.; Swedberg, K. PARADIGM-HF Investigators and Committees. 36 Drugs Targeting RAAS in the Treatment of Hypertension Angiotensin-neprilysin inhibition versus enalapril in heart failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef]
- McMurray, J.J.V.; Solomon, S.D.; Inzucchi, S.E.; Køber, L.; Kosiborod, M.N.; Martinez, F.A.; Ponikowski, P.; Sabatine, M.S.; Anand, I.S.; Bělohlávek, J.; et al. Dapagliflozin in Patients with Heart Failure and Reduced Ejection Fraction. N. Engl. J. Med. 2019, 381, 1995–2008. [Google Scholar] [CrossRef]
- Kosiborod, M.N.; Jhund, P.S.; Docherty, K.F.; Diez, M.; Petrie, M.C.; Verma, S.; Nicolau, J.C.; Merkely, B.; Kitakaze, M.; DeMets, D.L. Effects of dapagliflozin on symptoms, function, and quality of life in patients with heart failure and reduced ejection fraction: Results from the DAPA-HF trial. Circulation 2020, 141, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Deb, S.; Wijeysundera, H.C.; Ko, D.T.; Tsubota, H.; Hill, S.; Fremes, S.E. Coronary Artery Bypass Graft Surgery vs Percutaneous Interventions in Coronary Revascularization: A Systematic Review. JAMA 2013, 310, 2086–2095. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 ACC/AHA/HFSA Guideline for the Management of Heart Failure. J. Card. Fail. 2022, 28, e1–e167. [Google Scholar] [CrossRef] [PubMed]
- Li, D.L.; Kronenberg, M.W. Myocardial perfusion and viability imaging in coronary artery disease: Clinical value in diagnosis, prognosis, and therapeutic guidance. Am. J. Med. 2021, 134, 968–975. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P. Initial invasive or conservative strategy for stable coronary disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Kazakauskaite, E.; Vajauskas, D.; Unikaite, R.; Jonauskiene, I.; Virbickiene, A.; Zaliaduonyte, D.; Lapinskas, T.; Jurkevicius, R. Comparative analysis of myocardial viability multimodality imaging in patients with previous myocardial infarction and symptomatic heart failure. Medicina 2022, 58, 368. [Google Scholar] [CrossRef]
- Ngu, J.M.C.; Ruel, M.; Sun, L.Y. Left ventricular function recovery after revascularization: Comparative effects of percutaneous coronary intervention and coronary artery bypass grafting. Curr. Opin. Cardiol. 2018, 33, 633–637. [Google Scholar] [CrossRef]
- Velazquez, E.J.; Lee, K.L.; Jones, R.H.; Al-Khalidi, H.R.; Hill, J.A.; Panza, J.A.; Michler, R.E.; Bonow, R.O.; Doenst, T.; Petrie, M.C.; et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N. Engl. J. Med. 2016, 374, 1511–1520. [Google Scholar] [CrossRef]
- Pantea-Roșan, L.R.; Pantea, V.A.; Bungau, S.; Tit, D.M.; Behl, T.; Vesa, C.M.; Bustea, C.; Moleriu, R.D.; Rus, M.; Popescu, M.I.; et al. No-Reflow after PPCI—A Predictor of Short-Term Outcomes in STEMI Patients. J. Clin. Med. 2020, 9, 2956. [Google Scholar] [CrossRef]
- Members, W.C.; Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M. 2021 ACC/AHA/SCAI guideline for coronary artery revascularization: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar]
- Yang, T.; Lu, M.-J.; Sun, H.-S.; Tang, Y.; Pan, S.-W.; Zhao, S.-H. Myocardial scar identified by magnetic resonance imaging can predict left ventricular functional improvement after coronary artery bypass grafting. PLoS ONE 2013, 8, e81991. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.Y.; Gaudino, M.; Chen, R.J.; Eddeen, A.B.; Ruel, M. Long-term outcomes in patients with severely reduced left ventricular ejection fraction undergoing percutaneous coronary intervention vs coronary artery bypass grafting. JAMA Cardiol. 2020, 5, 631–641. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babes, E.E.; Tit, D.M.; Bungau, A.F.; Bustea, C.; Rus, M.; Bungau, S.G.; Babes, V.V. Myocardial Viability Testing in the Management of Ischemic Heart Failure. Life 2022, 12, 1760. https://doi.org/10.3390/life12111760
Babes EE, Tit DM, Bungau AF, Bustea C, Rus M, Bungau SG, Babes VV. Myocardial Viability Testing in the Management of Ischemic Heart Failure. Life. 2022; 12(11):1760. https://doi.org/10.3390/life12111760
Chicago/Turabian StyleBabes, Elena Emilia, Delia Mirela Tit, Alexa Florina Bungau, Cristiana Bustea, Marius Rus, Simona Gabriela Bungau, and Victor Vlad Babes. 2022. "Myocardial Viability Testing in the Management of Ischemic Heart Failure" Life 12, no. 11: 1760. https://doi.org/10.3390/life12111760
APA StyleBabes, E. E., Tit, D. M., Bungau, A. F., Bustea, C., Rus, M., Bungau, S. G., & Babes, V. V. (2022). Myocardial Viability Testing in the Management of Ischemic Heart Failure. Life, 12(11), 1760. https://doi.org/10.3390/life12111760








