The Added Value of [18F]Choline PET/CT in Low-Risk Prostate Cancer Staging: A Case Report
Abstract
1. Introduction
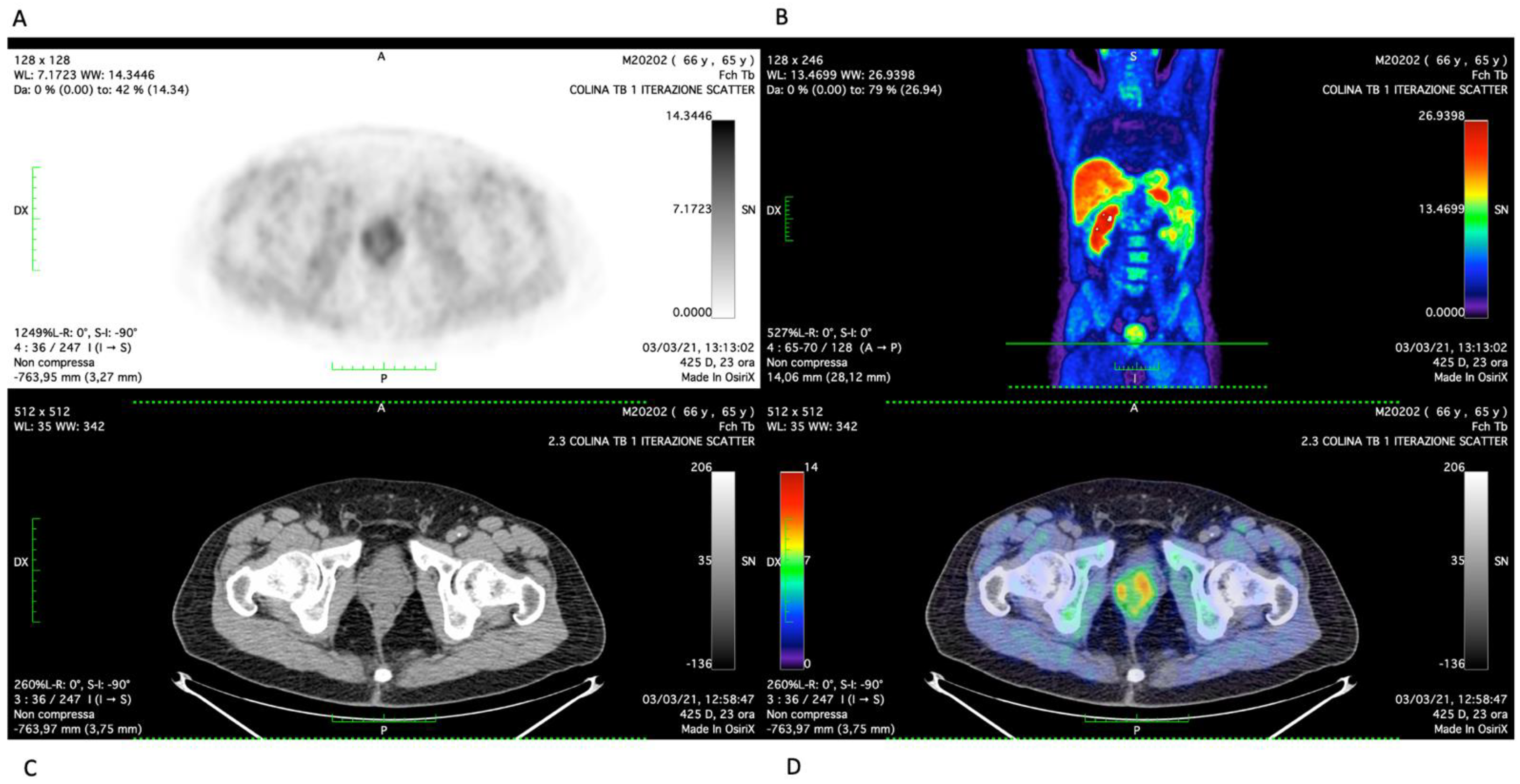
2. Case Report
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Statement of Ethics
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA A Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Fanti, S.; Minozzi, S.; Antoch, G.; Banks, I.; Briganti, A.; Carrio, I.; Chiti, A.; Clarke, N.; Eiber, M.; De Bono, J.; et al. Consensus on molecular imaging and theranostics in prostate cancer. Lancet Oncol. 2018, 19, e696–e708. [Google Scholar] [CrossRef]
- Thompson, J.; Lawrentschuk, N.; Frydenberg, M.; Thompson, L.; Stricker, P.; USANZ. The role of magnetic resonance imaging in the diagnosis and management of prostate cancer. Br. J. Urol. 2013, 112, 6–20. [Google Scholar] [CrossRef]
- Spigelman, S.S.; McNeal, J.E.; Freiha, F.S.; Stamey, T.A. Rectal Examination in Volume Determination of Carcinoma of the Prostate: Clinical and Anatomical Correlations. J. Urol. 1986, 136, 1228–1230. [Google Scholar] [CrossRef]
- Capitanio, U.; Karakiewicz, P.I.; Valiquette, L.; Perrotte, P.; Jeldres, C.; Briganti, A.; Gallina, A.; Suardi, N.; Cestari, A.; Guazzoni, G.; et al. Biopsy Core Number Represents One of Foremost Predictors of Clinically Significant Gleason Sum Upgrading in Patients With Low-risk Prostate Cancer. Urology 2009, 73, 1087–1091. [Google Scholar] [CrossRef]
- Duvnjak, P.; Schulman, A.A.; Holtz, J.N.; Huang, J.; Polascik, T.J.; Gupta, R.T. Multiparametric Prostate MR Imaging: Impact on Clinical Staging and Decision Making. Radiol. Clin. N. Am. 2018, 56, 239–250. [Google Scholar] [CrossRef]
- Schaeffer, E.; Srinivas, S.; Antonarakis, E.S.; Armstrong, A.J.; Bekelman, J.E.; Cheng, H.; D’Amico, A.V.; Davis, B.J.; Desai, N.; Dorff, T.; et al. NCCN Guidelines Insights: Prostate Cancer, Version 1.2021. J. Natl. Compr. Cancer Netw. 2021, 19, 134–143. [Google Scholar] [CrossRef]
- Trabulsi, E.J.; Rumble, R.B.; Jadvar, H.; Hope, T.; Pomper, M.; Turkbey, B.; Rosenkrantz, A.B.; Verma, S.; Margolis, D.J.; Froemming, A.; et al. Optimum Imaging Strategies for Advanced Prostate Cancer: ASCO Guideline. J. Clin. Oncol. 2020, 38, 1963–1996. [Google Scholar] [CrossRef]
- Morigi, J.J.; Stricker, P.D.; van Leeuwen, P.J.; Tang, R.; Ho, B.; Nguyen, Q.; Hruby, G.; Fogarty, G.; Jagavkar, R.; Kneebone, A.; et al. Prospective Comparison of 18F-Fluoromethylcholine Versus 68Ga-PSMA PET/CT in Prostate Cancer Patients Who Have Rising PSA After Curative Treatment and Are Being Considered for Targeted Therapy. J. Nucl. Med. 2015, 56, 1185–1190. [Google Scholar] [CrossRef]
- von Eyben, F.E.; Kairemo, K. Meta-analysis of (11)C-Choline and (18)F-Choline PET/CT for management of patients with prostate cancer. Nucl. Med. Commun. 2014, 35, 221–230. [Google Scholar] [CrossRef]
- Evangelista, L.; Guttilla, A.; Zattoni, F.; Muzzio, P.C.; Zattoni, F. Utility of Choline Positron Emission Tomography/Computed Tomography for Lymph Node Involvement Identification in Intermediate- to High-risk Prostate Cancer: A Systematic Literature Review and Meta-analysis. Eur. Urol. 2013, 63, 1040–1048. [Google Scholar] [CrossRef]
- Van den Broeck, T.; van den Bergh, R.C.N.; Briers, E.; Cornford, P.; Cumberbatch, M.; Tilki, D.; De Santis, M.; Fanti, S.; Fossati, N.; Gillessen, S.; et al. Biochemical Recurrence in Prostate Cancer: The European Association of Urology Prostate Cancer Guidelines Panel Recommendations. Eur. Urol. Focus 2020, 6, 231–234. [Google Scholar] [CrossRef]
- Linee guida Carcinoma della Prostata—AIRO, 2016. Tumori J. 2016, 102, S1–S79. [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE-Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermosifiliogr (Engl. Ed.) 2020, 112, 90–92. [Google Scholar] [CrossRef]
- Gauthé, M.; Zarca, K.; Aveline, C.; Lecouvet, F.; Balogová, S.; Cussenot, O.; Talbot, J.-N.; Durand-Zaleski, I. Comparison of 18F-sodium fluoride PET/CT, 18F-fluorocholine PET/CT and diffusion-weighted MRI for the detection of bone metastases in recurrent prostate cancer: A cost-effectiveness analysis in France. BMC Med. Imaging 2020, 20, 25. [Google Scholar] [CrossRef]
- de Feria Cardet, R.E.; Hofman, M.S.; Segard, T.; Yim, J.; Williams, S.; Francis, R.J.; Frydenberg, M.; Lawrentschuk, N.; Murphy, D.G.; De Abreu Lourenco, R. Is Prostate-specific Membrane Antigen Positron Emission Tomography/Computed Tomography Imaging Cost-effective in Prostate Cancer: An Analysis Informed by the ProPSMA Trial. Eur. Urol. 2020, 79, 413–418. [Google Scholar] [CrossRef]
- Pianou, N.K.; Stavrou, P.Z.; Vlontzou, E.; Rondogianni, P.; Exarhos, D.N.; Datseris, I.E. More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT. Hell J. Nucl Med. 2019, 22, 6–9. [Google Scholar]
- Fonti, R.; Conson, M.; Del Vecchio, S. PET/CT in radiation oncology. Semin. Oncol. 2019, 46, 202–209. [Google Scholar] [CrossRef]
- Fiorentino, A.; Laudicella, R.; Ciurlia, E.; Annunziata, S.; Lancellotta, V.; Mapelli, P.; Tuscano, C.; Caobelli, F.; Evangelista, L.; Marino, L.; et al. Positron emission tomography with computed tomography imaging (PET/CT) for the radiotherapy planning definition of the biological target volume: PART 2. Crit. Rev. Oncol. Hematol. 2019, 139, 117–124. [Google Scholar] [CrossRef]
- Gill, B.S.; Pai, S.S.; McKenzie, S.; Beriwal, S. Utility of PET for Radiotherapy Treatment Planning. PET Clin. 2015, 10, 541–554. [Google Scholar] [CrossRef]
- Alongi, P.; Laudicella, R.; Desideri, I.; Chiaravalloti, A.; Borghetti, P.; Quartuccio, N.; Fiore, M.; Evangelista, L.; Marino, L.; Caobelli, F.; et al. Positron emission tomography with computed tomography imaging (PET/CT) for the radiotherapy planning definition of the biological target volume: PART 1. Crit. Rev. Oncol. Hematol. 2019, 140, 74–79. [Google Scholar] [CrossRef]
- Shirvani, S.M.; Huntzinger, C.J.; Melcher, T.; Olcott, P.D.; Voronenko, Y.; Bartlett-Roberto, J.; Mazin, S. Biology-guided radiotherapy: Redefining the role of radiotherapy in metastatic cancer. Br. J. Radiol. 2021, 94, 20200873. [Google Scholar] [CrossRef]
- Kerkmeijer, L.G.W.; Groen, V.H.; Pos, F.J.; Haustermans, K.; Monninkhof, E.M.; Smeenk, R.J.; Kunze-Busch, M.; de Boer, J.C.J.; van der Voort van Zijp, J.; van Vulpen, M.; et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J. Clin. Oncol. 2021, 39, 787–796. [Google Scholar] [CrossRef]
- Sutinen, E.; Nurmi, M.; Roivainen, A.; Varpula, M.; Tolvanen, T.; Lehikoinen, P.; Minn, H. Kinetics of [(11)C]Choline uptake in prostate cancer: A PET Study. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 317–324. [Google Scholar] [CrossRef]
- Zhou, S.; Fu, H.; Liu, C.; Zhu, Z.; Zhang, J.; Weng, W.; Kang, J.; Liu, Q. Value of 11C-Choline PET/CT-Based Multi-Metabolic Parameter Combination in Distinguishing Early-Stage Prostate Cancer From Benign Prostate Diseases. Front. Oncol. 2021, 10, 600380. [Google Scholar] [CrossRef]
- Ghafoor, S.; Burger, I.A.; Vargas, A.H. Multimodality Imaging of Prostate Cancer. J. Nucl. Med. 2019, 60, 1350–1358. [Google Scholar] [CrossRef]
- Ferraro, D.A.; Laudicella, R.; Zeimpekis, K.; Mebert, I.; Müller, J.; Maurer, A.; Grünig, H.; Donati, O.; Sapienza, M.T.; Rueschoff, J.H.; et al. Hot needles can confirm accurate lesion sampling intraoperatively using [18F]PSMA-1007 PET/CT-guided biopsy in patients with suspected prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 1721–1730. [Google Scholar] [CrossRef]
- Laudicella, R.; Skawran, S.; Ferraro, D.A.; Mühlematter, U.J.; Maurer, A.; Grünig, H.; Rüschoff, H.J.; Rupp, N.; Donati, O.; Eberli, D.; et al. Quantitative imaging parameters to predict the local staging of prostate cancer in intermediate- to high-risk patients. Insights Imaging 2022, 13, 75. [Google Scholar] [CrossRef]
- Pepe, P.; Roscigno, M.; Pepe, L.; Panella, P.; Tamburo, M.; Marletta, G.; Savoca, F.; Candiano, G.; Cosentino, S.; Ippolito, M.; et al. Could 68Ga-PSMA PET/CT Evaluation Reduce the Number of Scheduled Prostate Biopsies in Men Enrolled in Active Surveillance Protocols? J. Clin. Med. 2022, 11, 3473. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piras, A.; Laudicella, R.; Boldrini, L.; D’Aviero, A.; Sanfratello, A.; La Rocca, A.; Scurria, S.; Salamone, G.; Alongi, P.; Angileri, T.; et al. The Added Value of [18F]Choline PET/CT in Low-Risk Prostate Cancer Staging: A Case Report. Life 2022, 12, 1728. https://doi.org/10.3390/life12111728
Piras A, Laudicella R, Boldrini L, D’Aviero A, Sanfratello A, La Rocca A, Scurria S, Salamone G, Alongi P, Angileri T, et al. The Added Value of [18F]Choline PET/CT in Low-Risk Prostate Cancer Staging: A Case Report. Life. 2022; 12(11):1728. https://doi.org/10.3390/life12111728
Chicago/Turabian StylePiras, Antonio, Riccardo Laudicella, Luca Boldrini, Andrea D’Aviero, Antonella Sanfratello, Antonino La Rocca, Salvatore Scurria, Giuseppe Salamone, Pierpaolo Alongi, Tommaso Angileri, and et al. 2022. "The Added Value of [18F]Choline PET/CT in Low-Risk Prostate Cancer Staging: A Case Report" Life 12, no. 11: 1728. https://doi.org/10.3390/life12111728
APA StylePiras, A., Laudicella, R., Boldrini, L., D’Aviero, A., Sanfratello, A., La Rocca, A., Scurria, S., Salamone, G., Alongi, P., Angileri, T., & Daidone, A. (2022). The Added Value of [18F]Choline PET/CT in Low-Risk Prostate Cancer Staging: A Case Report. Life, 12(11), 1728. https://doi.org/10.3390/life12111728







