Lithium Biological Action Mechanisms after Ischemic Stroke
Abstract
1. Introduction
2. Materials and Methods
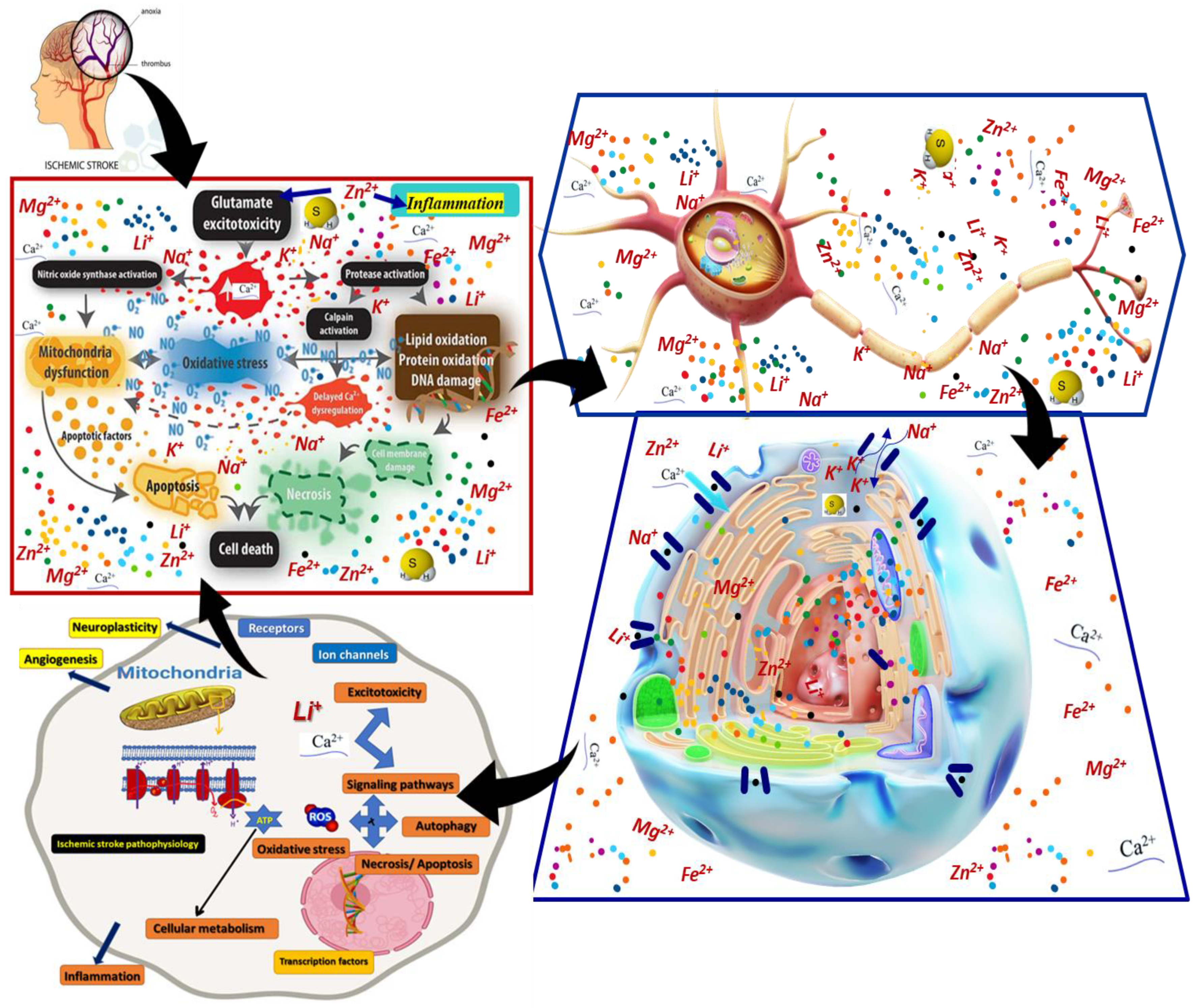
3. Ischemic Stroke Pathological Context and Lithium Interventions
4. Data on Lithium Biological Action Mechanisms after Ischemic Stroke

5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, B.; Zhang, M.; Ji, M.; Zhang, D.; Chen, B.; Gong, W.; Li, X.; Zhou, Y.; Dong, C.; Wen, G.; et al. The neuroprotective mechanism of lithium after ischaemic stroke. Commun. Biol. 2022, 5, 105. [Google Scholar] [CrossRef] [PubMed]
- Gunn, A.; Singh, A.; Diao, A.; Chen, R. Pharmacological Modulation of Autophagy for Neuroprotection in Ischaemic Stroke. J. Exp. Stroke Transl. Med. 2018, 11, 1–10. [Google Scholar] [CrossRef]
- Majdinasab, N.; Moqaddam, M.M.M.S. The effect of lithium on brain-derived neurotrophic factor—Level in patients with ischemic stroke: A clinical trial. Int. J. Hosp. Res. 2016, 5, 98–101. [Google Scholar]
- Onose, G.; Anghelescu, A.; Blendea, D.; Ciobanu, V.; Daia, C.; Firan, F.C.; Oprea, M.; Spinu, A.; Popescu, C.; Ionescu, A.; et al. Cellular and Molecular Targets for Non-Invasive, Non-Pharmacological Therapeutic/Rehabilitative Interventions in Acute Ischemic Stroke. Int. J. Mol. Sci. 2022, 23, 907. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Teoibaș-Șerban, D.; Iordache, L.; Balaurea, M.; Blendea, C.-D. Water intake meets the Water from inside the human body—Physiological, cultural, and health perspectives—Synthetic and Systematic literature review. Balneo PRM Res. J. 2021, 12, 196–209. [Google Scholar] [CrossRef]
- Munteanu, C.; Rotariu, M.; Turnea, M.; Ionescu, A.M.; Popescu, C.; Spinu, A.; Ionescu, E.V.; Oprea, C.; Țucmeanu, R.E.; Tătăranu, L.G.; et al. Main Cations and Cellular Biology of Traumatic Spinal Cord Injury. Cells 2022, 11, 2503. [Google Scholar] [CrossRef] [PubMed]
- Bulboaca, A.E.; Boarescu, P.-M.; Porfire, A.S.; Dogaru, G.; Barbalata, C.; Valeanu, M.; Munteanu, C.; Râjnoveanu, R.M.; Nicula, C.A.; Stanescu, I.C. The Effect of Nano-Epigallocatechin-Gallate on Oxidative Stress and Matrix Metalloproteinases in Experimental Diabetes Mellitus. Antioxidants 2020, 9, 172. [Google Scholar] [CrossRef]
- Chiu, C.-T.; Chuang, D.-M. Molecular actions and therapeutic potential of lithium in preclinical and clinical studies of CNS disorders. Pharmacol. Ther. 2010, 128, 281–304. [Google Scholar] [CrossRef]
- Leeds, P.R.; Yu, F.; Wang, Z.; Chiu, C.-T.; Zhang, Y.; Leng, Y.; Linares, G.R.; Chuang, D.-M. A New Avenue for Lithium: Intervention in Traumatic Brain Injury. ACS Chem. Neurosci. 2014, 5, 422–433. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Gava, F.F.; Dal-Pont, G.C.; Simões, H.L.; Damiani-Neves, M.; Andersen, M.L.; Boeck, C.R.; Quevedo, J. Effects of lithium and valproate on ERK/JNK signaling pathway in an animal model of mania induced by amphetamine. Heliyon 2019, 5, e01541. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C. Lithium Biology; Editura Balneara: Bucharest, Romania, 2013; ISBN 978-606-93550-1-5. [Google Scholar]
- Song, D.; Ji, Y.; Huang, X.; Ma, Y.; Fang, C.; Qiu, L.; Tan, X.; Chen, Y.; Wang, S.; Chang, J.; et al. Lithium attenuates blood–brain barrier damage and brain edema following intracerebral hemorrhage via an endothelial Wnt/β-catenin signaling-dependent mechanism in mice. CNS Neurosci. Ther. 2022, 28, 862–872. [Google Scholar] [CrossRef]
- Chuang, D.-M.; Wang, Z.; Chiu, C.-T. GSK-3 as a Target for Lithium-Induced Neuroprotection Against Excitotoxicity in Neuronal Cultures and Animal Models of Ischemic Stroke. Front. Mol. Neurosci. 2011, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, M.; Qassem, M.; Triantis, I.F.; Kyriacou, P.A. Advances in Therapeutic Monitoring of Lithium in the Management of Bipolar Disorder. Sensors 2022, 22, 736. [Google Scholar] [CrossRef] [PubMed]
- Kahle, K.T.; Simard, J.M.; Staley, K.J.; Nahed, B.V.; Jones, P.S.; Sun, D. Molecular Mechanisms of Ischemic Cerebral Edema: Role of Electroneutral Ion Transport. Physiology 2009, 24, 257–265. [Google Scholar] [CrossRef]
- Mohammadianinejad, S.E.; Majdinasab, N.; Sajedi, S.A.; Abdollahi, F.; Moqaddam, M.M.; Sadr, F. The effect of lithium in post-stroke motor recovery: A double-blind, placebo-controlled, randomized clinical trial. Clin. Neuropharmacol. 2014, 37, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Cramer, S.C.; Nudo, R.J. Brain Repair after Stroke; Cramer, S.C., Nudo, R.J., Eds.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Chen, H.-J.; Shen, Y.-C.; Shiao, Y.-J.; Liou, K.-T.; Hsu, W.-H.; Hsieh, P.-H.; Lee, C.-Y.; Chen, Y.-R.; Lin, Y.-L. Multiplex Brain Proteomic Analysis Revealed the Molecular Therapeutic Effects of Buyang Huanwu Decoction on Cerebral Ischemic Stroke Mice. PLoS ONE 2015, 10, e0140823. [Google Scholar] [CrossRef]
- Stebbins, G.T.; Nyenhuis, D.L.; Wang, C.; Cox, J.L.; Freels, S.; Bangen, K.; Detoledo-Morrell, L.; Sripathirathan, K.; Moseley, M.; Turner, D.A.; et al. Gray Matter Atrophy in Patients With Ischemic Stroke With Cognitive Impairment. Stroke 2008, 39, 785–793. [Google Scholar] [CrossRef]
- Forlenza, O.V.; Radanovic, M.; Talib, L.L.; Gattaz, W.F. Clinical and biological effects of long-term lithium treatment in older adults with amnestic mild cognitive impairment: Randomised clinical trial. Br. J. Psychiatry 2019, 215, 668–674. [Google Scholar] [CrossRef]
- Cui, P.; McCullough, L.D.; Hao, J. Brain to periphery in acute ischemic stroke: Mechanisms and clinical significance. Front. Neuroendocr. 2021, 63, 100932. [Google Scholar] [CrossRef]
- Pu, H.; Shi, Y.; Zhang, L.; Lu, Z.; Ye, Q.; Leak, R.K.; Xu, F.; Ma, S.; Mu, H.; Wei, Z.; et al. Protease-independent action of tissue plasminogen activator in brain plasticity and neurological recovery after ischemic stroke. Proc. Natl. Acad. Sci. USA 2019, 116, 9115–9124. [Google Scholar] [CrossRef]
- Smith, W.S.; Sung, G.; Saver, J.; Budzik, R.; Duckwiler, G.; Liebeskind, D.S.; Lutsep, H.L.; Rymer, M.M.; Higashida, R.T.; Starkman, S.; et al. Mechanical thrombectomy for acute ischemic stroke: Final results of the multi MERCI trial. Stroke 2008, 39, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Chu, T.-H.; Wu, W. Lithium enhances proliferation and neuronal differentiation of neural progenitor cells in vitro and after transplantation into the adult rat spinal cord. Exp. Neurol. 2007, 206, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.X.; Haupt, B.M.; Bähr, M.; Tatenhorst, L.; Doeppner, T.R. Treating cerebral ischemia: Novel therapeutic strategies from experimental stroke research. In Cereb. Ischemia; Exon Publications: Brisbane, Australia, 2021; pp. 165–186. [Google Scholar]
- Ji, Y.-B.; Gao, Q.; Tan, X.-X.; Huang, X.-W.; Ma, Y.-Z.; Fang, C.; Wang, S.-N.; Qiu, L.-H.; Cheng, Y.-X.; Guo, F.-Y.; et al. Lithium alleviates blood-brain barrier breakdown after cerebral ischemia and reperfusion by upregulating endothelial Wnt/β-catenin signaling in mice. Neuropharmacology 2021, 186, 108474. [Google Scholar] [CrossRef] [PubMed]
- Doeppner, T.R.; Kaltwasser, B.; Sanchez-Mendoza, E.H.; Caglayan, A.B.; Bähr, M.; Hermann, D.M. Lithium-induced neuroprotection in stroke involves increased miR-124 expression, reduced RE1-silencing transcription factor abundance and decreased protein deubiquitination by GSK3β inhibition-independent pathways. J. Cereb. Blood Flow Metab. 2017, 37, 914–926. [Google Scholar] [CrossRef]
- Zhu, H.; Poon, W.; Liu, Y.; Leung, G.K.-K.; Wong, Y.; Feng, Y.; Ng, S.C.P.; Tsang, K.S.; Sun, D.T.F.; Yeung, D.K.; et al. Phase I–II Clinical Trial Assessing Safety and Efficacy of Umbilical Cord Blood Mononuclear Cell Transplant Therapy of Chronic Complete Spinal Cord Injury. Cell Transplant. 2016, 25, 1925–1943. [Google Scholar] [CrossRef]
- Kazemi, H.; Noori-Zadeh, A.; Darabi, F.R.S. Lithium prevents cell apoptosis through autophagy induction. Bratisl. Lek. Listy 2018, 119, 234–239. [Google Scholar] [CrossRef]
- Lyoo, I.K.; Dager, S.R.; E Kim, J.; Yoon, S.J.; Friedman, S.; Dunner, D.L.; Renshaw, P.F. Lithium-Induced Gray Matter Volume Increase As a Neural Correlate of Treatment Response in Bipolar Disorder: A Longitudinal Brain Imaging Study. Neuropsychopharmacology 2010, 35, 1743–1750. [Google Scholar] [CrossRef]
- Tong, M.; He, Z.; Lin, X.; Zhou, Y.; Wang, Q.; Zheng, Z.; Chen, J.; Xu, H.; Tian, N. Lithium chloride contributes to blood–spinal cord barrier integrity and functional recovery from spinal cord injury by stimulating autophagic flux. Biochem. Biophys. Res. Commun. 2018, 495, 2525–2531. [Google Scholar] [CrossRef]
- Bosche, B.; Molcanyi, M.; Rej, S.; Doeppner, T.R.; Obermann, M.; Müller, D.J.; Das, A.; Hescheler, J.; Macdonald, R.L.; Noll, T.; et al. Low-Dose Lithium Stabilizes Human Endothelial Barrier by Decreasing MLC Phosphorylation and Universally Augments Cholinergic Vasorelaxation Capacity in a Direct Manner. Front. Physiol. 2016, 7, 593. [Google Scholar] [CrossRef]
- Onose, G.; Anghelescu, A.; Blendea, C.D.; Ciobanu, V.; Daia, C.O.; Firan, F.C.; Munteanu, C.; Oprea, M.; Spinu, A.; Popescu, C. Non-invasive, non-pharmacological/bio-technological interventions towards neurorestoration upshot after ischemic stroke, in adults—Systematic, synthetic, literature review. Front. Biosci. 2021, 26, 1204. [Google Scholar] [CrossRef]
- Castillo, J.; Dávalos, A.; Marrugat, J.; Noya, M. Timing for Fever-Related Brain Damage in Acute Ischemic Stroke. Stroke 1998, 29, 2455–2460. [Google Scholar] [CrossRef] [PubMed]
- Yoo, B.-K.; Kwon, K.; Lee, E.R.; Kim, S.-W.; Yu, K. Hypothermia Regulates Insulin-like Growth Factor 1 Gene Expression in PC12 Cells. Biomed. Sci. Lett. 2017, 23, 39–43. [Google Scholar] [CrossRef]
- Bathina, S.; Das, U.N. Brain-derived neurotrophic factor and its clinical implications. Arch. Med. Sci. 2015, 11, 1164–1178. [Google Scholar] [CrossRef] [PubMed]
- Aleksić, D.; Aksić, M.; Divac, N.; Radonjić, V.; Filipović, B.; Jakovčevski, I. Thermomineral water promotes axonal sprouting but does not reduce glial scar formation in a mouse model of spinal cord injury. Neural Regen. Res. 2014, 9, 2174–2181. [Google Scholar] [PubMed]
- Chen, P.-H.; Tsai, S.-Y.; Pan, C.-H.; Chang, C.-K.; Su, S.-S.; Chen, C.-C.; Kuo, C.-J. Mood stabilisers and risk of stroke in bipolar disorder. Br. J. Psychiatry 2019, 215, 409–414. [Google Scholar] [CrossRef]
- Jakobsson, E.; Argüello-Miranda, O.; Chiu, S.-W.; Fazal, Z.; Kruczek, J.; Nunez-Corrales, S.; Pandit, S.; Pritchet, L. Towards a Unified Understanding of Lithium Action in Basic Biology and its Significance for Applied Biology. J. Membr. Biol. 2017, 250, 587–604. [Google Scholar] [CrossRef]
- Yang, M.L.; Li, J.J.; So, K.F.; Chen, J.Y.H.; Cheng, W.S.; Wu, J.; Wang, Z.M.; Gao, F.; Young, W. Efficacy and safety of lithium carbonate treatment of chronic spinal cord injuries: A double-blind, randomized, placebo-controlled clinical trial. Spinal Cord 2012, 50, 141–146. [Google Scholar] [CrossRef]
- Young, W. Review of Lithium Effects on Brain and Blood. Cell Transplant. 2009, 18, 951–975. [Google Scholar] [CrossRef]
- Doeppner, T.R.; Haupt, M.; Bähr, M. Lithium beyond psychiatric indications: The reincarnation of a new old drug. Neural Regen. Res. 2021, 16, 2383–2387. [Google Scholar] [CrossRef] [PubMed]
- Ramli, F.F.; Cowen, P.J.; Godlewska, B.R. The Potential Use of Ebselen in Treatment-Resistant Depression. Pharmaceuticals 2022, 15, 485. [Google Scholar] [CrossRef]
- Haupt, M.; Zechmeister, B.; Bosche, B.; Lieschke, S.; Zheng, X.; Zhang, L.; Venkataramani, V.; Jin, F.; Hein, K.; Weber, M.S.; et al. Lithium enhances post-stroke blood-brain barrier integrity, activates the MAPK/ERK1/2 pathway and alters immune cell migration in mice. Neuropharmacology 2020, 181, 108357. [Google Scholar] [CrossRef]
- Zhang, L.-Q.; Zhang, W.-M.; Deng, L.; Xu, Z.-X.; Lan, W.-B.; Lin, J.-H. Transplantation of a Peripheral Nerve with Neural Stem Cells Plus Lithium Chloride Injection Promote the Recovery of Rat Spinal Cord Injury. Cell Transplant. 2018, 27, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, O.V.; De-Paula, V.J.R.; Diniz, B.S.O. Neuroprotective effects of lithium: Implications for the treatment of Alzheimer’s disease and related neurodegenerative disorders. ACS Chem. Neurosci. 2014, 5, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Khayachi, A.; Ase, A.; Liao, C.; Kamesh, A.; Kuhlmann, N.; Schorova, L.; Chaumette, B.; Dion, P.; Alda, M.; Séguéla, P.; et al. Chronic lithium treatment alters the excitatory/inhibitory balance of synaptic networks and reduces mGluR5–PKC signalling in mouse cortical neurons. J. Psychiatry Neurosci. 2021, 46, E402–E414. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, S.; Hough, C.J.; Chuang, D.-M. Chronic lithium treatment robustly protects neurons in the central nervous system against excitotoxicity by inhibiting N-methyl-D-aspartate receptor-mediated calcium influx. Proc. Natl. Acad. Sci. USA 1998, 95, 2642–2647. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.-W.; Chuang, D.-M. Long term lithium treatment suppresses p53 and Bax expression but increases Bcl-2 expression: A prominent role in neuroprotection against excitotoxicity. J. Biol. Chem. 1999, 274, 6039–6042. [Google Scholar] [CrossRef]
- Mora, A.; Sabio, G.; Polo, R.-A.G.; Cuenda, A.; Alessi, D.; Alonso, J.C.; Fuentes, J.M.; Soler, G.; Centeno, F. Lithium inhibits caspase 3 activation and dephosphorylation of PKB and GSK3 induced by K+ deprivation in cerebellar granule cells. J. Neurochem. 2001, 78, 199–206. [Google Scholar] [CrossRef]
- Chalecka-Franaszek, E.; Chuang, D.-M. Lithium activates the serine/threonine kinase Akt-1 and suppresses glutamate-induced inhibition of Akt-1 activity in neurons. Proc. Natl. Acad. Sci. USA 1999, 96, 8745–8750. [Google Scholar] [CrossRef] [PubMed]
- Lazzara, C.A.; Kim, Y.-H. Potential application of lithium in Parkinson’s and other neurodegenerative diseases. Front. Neurosci. 2015, 9, 403. [Google Scholar] [CrossRef]
- Ciftci, E.; Karacay, R.; Caglayan, A.; Altunay, S.; Ates, N.; Altintas, M.O.; Doeppner, T.R.; Yulug, B.; Kilic, E. Neuroprotective effect of lithium in cold- induced traumatic brain injury in mice. Behav. Brain Res. 2020, 392, 112719. [Google Scholar] [CrossRef]
- Pan, Y.; Short, J.L.; Newman, S.A.; Choy, K.H.; Tiwari, D.; Yap, C.; Senyschyn, D.; Banks, W.A.; Nicolazzo, J.A. Cognitive benefits of lithium chloride in APP/PS1 mice are associated with enhanced brain clearance of β-amyloid. Brain Behav. Immun. 2018, 70, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Wang, Z.; Tchantchou, F.; Chiu, C.-T.; Zhang, Y.; Chuang, D.-M. Lithium Ameliorates Neurodegeneration, Suppresses Neuroinflammation, and Improves Behavioral Performance in a Mouse Model of Traumatic Brain Injury. J. Neurotrauma 2012, 29, 362–374. [Google Scholar] [CrossRef] [PubMed]
- Palmos, A.B.; Duarte, R.R.R.; Smeeth, D.M.; Hedges, E.C.; Nixon, D.F.; Thuret, S.; Powell, T.R. Lithium treatment and human hippocampal neurogenesis. Transl. Psychiatry 2021, 11, 555. [Google Scholar] [CrossRef] [PubMed]
- Stoica, S.I.; Bleotu, C.; Ciobanu, V.; Ionescu, A.M.; Albadi, I.; Onose, G.; Munteanu, C. Considerations about Hypoxic Changes in Neuraxis Tissue Injuries and Recovery. Biomedicines 2022, 10, 481. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Chou, F.H.-C.; Tsai, K.-Y.; Su, C.-Y.; Shen, S.-P.; Chung, T.-C. The Incidence and Relative Risk of Stroke among Patients with Bipolar Disorder: A Seven-Year Follow-Up Study. PLoS ONE 2013, 8, e73037. [Google Scholar] [CrossRef]
- Lan, C.-C.; Liu, C.-C.; Lin, C.-H.; Lan, T.-Y.; McInnis, M.G.; Chan, C.-H.; Lan, T.-H. A reduced risk of stroke with lithium exposure in bipolar disorder: A population-based retrospective cohort study. Bipolar Disord. 2015, 17, 705–714. [Google Scholar] [CrossRef]
- Rajah, G.B.; Ding, Y. Experimental neuroprotection in ischemic stroke: A concise review. Neurosurg. Focus 2017, 42, E2. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, J.; Ahn, M.; Shin, T. Lithium ameliorates rat spinal cord injury by suppressing glycogen synthase kinase-3β and activating heme oxygenase-1. Anat. Cell Biol. 2017, 50, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.; Munteanu, D.; Cinteza, D. GSK-3β expression after treatment of glial cells with lithium and Maria lithium mineral water from Malnaş-Băi. Maedica J. Clin. Med. 2009, 4, 106–113. [Google Scholar]
- Xu, J.; Culman, J.; Blume, A.; Brecht, S.; Gohlke, P. Chronic Treatment With a Low Dose of Lithium Protects the Brain Against Ischemic Injury by Reducing Apoptotic Death. Stroke 2003, 34, 1287–1292. [Google Scholar] [CrossRef]
- He, X.-J.; Wang, F.; Zhao, Y.-J.; Qiao, H.; Liu, D.-F.; Li, J.; Li, J.-X.; Chang, S.-E.; Lu, T.; Li, F.-T.; et al. Lithium promotes recovery after spinal cord injury. Neural Regen. Res. 2022, 17, 1324. [Google Scholar] [CrossRef]
- Jong Youl, K.; Masahito, K.; Midori, Y. Innate inflammatory responses in stroke: Mechanisms and potential therapeutic targets. Curr. Med. Chem. 2014, 21, 2076–2097. [Google Scholar]
- Schepetkin, I.A.; Chernysheva, G.A.; Aliev, O.I.; Kirpotina, L.N.; Smol’Yakova, V.I.; Osipenko, A.N.; Plotnikov, M.B.; Kovrizhina, A.R.; Khlebnikov, A.I.; Plotnikov, E.V.; et al. Neuroprotective Effects of the Lithium Salt of a Novel JNK Inhibitor in an Animal Model of Cerebral Ischemia–Reperfusion. Biomedicines 2022, 10, 2119. [Google Scholar] [CrossRef] [PubMed]
- Bristot, G.; Ascoli, B.M.; Scotton, E.; Géa, L.P.; Pfaffenseller, B.; Kauer-Sant’Anna, M. Effects of lithium on inflammatory and neurotrophic factors after an immune challenge in a lisdexamfetamine animal model of mania. Braz. J. Psychiatry 2019, 41, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Boyko, M.; Nassar, A.; Kaplanski, J.; Zlotnik, A.; Sharon-Granit, Y.; Azab, A.N. Effects of Acute Lithium Treatment on Brain Levels of Inflammatory Mediators in Poststroke Rats. BioMed Res. Int. 2015, 2015, 916234. [Google Scholar] [CrossRef] [PubMed]
- Kerr, F.; Bjedov, I.; Sofola-Adesakin, O. Molecular Mechanisms of Lithium Action: Switching the Light on Multiple Targets for Dementia Using Animal Models. Front. Mol. Neurosci. 2018, 11, 297. [Google Scholar] [CrossRef]
- He, X.; Li, Y.; Deng, B.; Lin, A.; Zhang, G.; Ma, M.; Wang, Y.; Yang, Y.; Kang, X. The PI3K/AKT signalling pathway in inflammation, cell death and glial scar formation after traumatic spinal cord injury: Mechanisms and therapeutic opportunities. Cell Prolif. 2022, 55, e13275. [Google Scholar] [CrossRef] [PubMed]
- Waelput, W.; Broekaert, D.; Vandekerckhove, J.; Brouckaert, P.; Tavernier, J.; Libert, C. A Mediator Role For Metallothionein in Tumor Necrosis Factor–induced Lethal Shock. J. Exp. Med. 2001, 194, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Stoica, S.I.; Onose, G.; Hoteteu, M.; Munteanu, C. Effects of ethanol and deferoxamine on rat primary glial cell cultures, in regard with ischemia induced by traumatic spinal cord injury. Balneo PRM Res. J. 2022, 13, 502. [Google Scholar] [CrossRef]
- Liao, H.-Y.; Wang, Z.-Q.; Ran, R.; Zhou, K.-S.; Ma, C.-W.; Zhang, H.-H. Biological Functions and Therapeutic Potential of Autophagy in Spinal Cord Injury. Front. Cell Dev. Biol. 2021, 9, 761273. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Fan, M.; Jin, W.; Li, W.A.; Jia, Y.; Dong, Y.; Jiang, X.; Xu, J.; Meng, N.; Lv, P. Lithium chloride ameliorated spatial cognitive impairment through activating mTOR phosphorylation and inhibiting excessive autophagy in the repeated cerebral ischemia-reperfusion mouse model. Exp. Ther. Med. 2020, 20, 109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; He, X.-J.; Wang, F.; Zhai, X.; Li, X.-H. Lithium promotes recovery of neurological function after spinal cord injury by inducing autophagy. Neural Regen. Res. 2018, 13, 2191–2199. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Hao, Y.; Sun, L.; Zhao, Y.; Zheng, X.; Song, L. The dual roles of autophagy and the GPCRs-mediating autophagy signaling pathway after cerebral ischemic stroke. Mol. Brain 2022, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Senatorov, V.V.; Chen, R.-W.; Chuang, D.-M. Postinsult treatment with lithium reduces brain damage and facilitates neurological recovery in a rat ischemia/reperfusion model. Proc. Natl. Acad. Sci. USA 2003, 100, 6210–6215. [Google Scholar] [CrossRef] [PubMed]
- Gitlin, M. Lithium side effects and toxicity: Prevalence and management strategies. Int. J. Bipolar Disord. 2016, 4, 1–10. [Google Scholar] [CrossRef]
- Leung, A.M. Lithium Use Is Associated with an Increased Risk of Hypothyroidism. Clin. Thyroidol. 2015, 278, 315–316. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Shaw, S.; Kamsteeg, E.-J.; Vandewalle, A.; Deen, P.M. Development of Lithium-Induced Nephrogenic Diabetes Insipidus Is Dissociated from Adenylyl Cyclase Activity. J. Am. Soc. Nephrol. 2006, 17, 1063–1072. [Google Scholar] [CrossRef]
- Keleş Altun, İ.; Kılıç, N.; Yıldızoğlu, E.; Atagün, M.İ. Lithium Associated Side Effects and Neurotoxicity: Is Lithium Neurotoxicity Related to Iron Deposition? Psikiyatr Guncel Yaklasimlar. Curr. Approaches Psychiatry 2019, 11, 141–153. [Google Scholar]
- Orleans, R.A.; Dubin, M.J.; Kast, K.A. The effect of a therapeutic lithium level on a stroke-related cerebellar tremor. BMJ Case Rep. 2018, 2018, bcr2017222920. [Google Scholar] [CrossRef]
- Rybakowski, J.K. Challenging the Negative Perception of Lithium and Optimizing Its Long-Term Administration. Front. Mol. Neurosci. 2018, 11, 349. [Google Scholar] [CrossRef] [PubMed]
- Rybakowski, J.K.; Permoda-Osip, A.; Borkowska, A. Response to prophylactic lithium in bipolar disorder may be associated with a preservation of executive cognitive functions. Eur. Neuropsychopharmacol. 2009, 19, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Underwood, B.R.; Jones, P.B.; Lewis, J.R.; Cardinal, R.N. Association between lithium use and the incidence of dementia and its subtypes: A retrospective cohort study. PLoS Med. 2022, 19, e1003941. [Google Scholar] [CrossRef]
- Ferrie, L.; Young, A.H.; McQuade, R. Effect of lithium and lithium withdrawal on potassium-evoked dopamine release and tyrosine hydroxylase expression in the rat. Int. J. Neuropsychopharmacol. 2006, 9, 729–735. [Google Scholar] [CrossRef] [PubMed]
- Alda, M. Lithium in the treatment of bipolar disorder: Pharmacology and pharmacogenetics. Mol. Psychiatry 2016, 20, 661–670. [Google Scholar] [CrossRef]
- Saxena, A.; Scaini, G.; Bavaresco, D.V.; Leite, C.; Valvassoria, S.S.; Carvalho, A.F.; Quevedo, J. Role of Protein Kinase C in Bipolar Disorder: A Review of the Current Literature. Complex Psychiatry 2017, 3, 108–124. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.F.; Los, G.V.; Hokin, L.E. Lithium stimulates glutamate “release” and inositol 1,4,5-trisphosphate accumulation via activation of the N-methyl-D-aspartate receptor in monkey and mouse cerebral cortex slices. Proc. Natl. Acad. Sci. USA 1994, 91, 8358–8362. [Google Scholar] [CrossRef] [PubMed]
- Rijal, S.; Jang, S.; Park, S.; Han, S. Lithium Enhances the GABAergic Synaptic Activities on the Hypothalamic Preoptic Area (hPOA) Neurons. Int. J. Mol. Sci. 2021, 22, 3908. [Google Scholar] [CrossRef]
- Böer, U.; Eglins, J.; Krause, D.; Schnell, S.; Schöfl, C.; Knepel, W. Enhancement by lithium of cAMP-induced CRE/CREB-directed gene transcription conferred by TORC on the CREB basic leucine zipper domain. Biochem. J. 2007, 408, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Brudvig, J.J.; Weimer, J.M. X MARCKS the spot: Myristoylated alanine-rich C kinase substrate in neuronal function and disease. Front. Cell. Neurosci. 2015, 9, 407. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Liang, Y.; Chen, F.; Wang, H.; Zhu, G. The effect of lithium chloride on the motor function of spinal cord injury–controlled rat and the relevant mechanism. Eur. J. Inflamm. 2019, 17, 2058739219852855. [Google Scholar] [CrossRef]
- Fallah, E.; Arman, S.; Najafi, M.; Shayegh, B. Effect of Tamoxifen and Lithium on Treatment of Acute Mania Symptoms in Children and Adolescents. Iran. J. Child Neurol. 2016, 10, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Freland, L.; Beaulieu, J.-M. Inhibition of GSK3 by lithium, from single molecules to signaling networks. Front. Mol. Neurosci. 2012, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.M.; Tracy, D. Lithium: The pharmacodynamic actions of the amazing ion. Ther. Adv. Psychopharmacol. 2013, 3, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Snitow, M.E.; Bhansali, R.S.; Klein, P.S. Lithium and Therapeutic Targeting of GSK-3. Cells 2021, 10, 255. [Google Scholar] [CrossRef]
- Hongisto, V.; Smeds, N.; Brecht, S.; Herdegen, T.; Courtney, M.; Coffey, E.T. Lithium Blocks the c-Jun Stress Response and Protects Neurons via Its Action on Glycogen Synthase Kinase 3. Mol. Cell. Biol. 2003, 23, 6027–6036. [Google Scholar] [CrossRef]
- Takahashi, T.; Steinberg, G.K.; Zhao, H. Lithium treatment reduces brain injury induced by focal ischemia with partial reperfusion and the protective mechanisms dispute the importance of akt activity. Aging Dis. 2012, 3, 226–233. [Google Scholar]
- Bearden, C.E.; Thompson, P.; Dalwani, M.; Hayashi, K.M.; Lee, A.D.; Nicoletti, M.; Trakhtenbroit, M.; Glahn, D.C.; Brambilla, P.; Sassi, R.B.; et al. Greater Cortical Gray Matter Density in Lithium-Treated Patients with Bipolar Disorder. Biol. Psychiatry 2007, 62, 7–16. [Google Scholar] [CrossRef]
- Wise, T.; Radua, J.; Via, E.; Cardoner, N.; Abe, O.; Adams, T.M.; Amico, F.; Cheng, Y.; Cole, J.H.; de Azevedo Marques Périco, C.; et al. Common and distinct patterns of grey-matter volume alteration in major depression and bipolar disorder: Evidence from voxel-based meta-analysis. Mol. Psychiatry 2017, 22, 1455–1463. [Google Scholar] [CrossRef]
- Hajek, T.; Kopecek, M.; Höschl, C.; Alda, M. Smaller hippocampal volumes in patients with bipolar disorder are masked by exposure to lithium: A meta-analysis. J. Psychiatry Neurosci. 2012, 37, 333–343. [Google Scholar] [CrossRef]
- Benedetti, F.; Poletti, S.; Radaelli, D.; Locatelli, C.; Pirovano, A.; Lorenzi, C.; Vai, B.; Bollettini, I.; Falini, A.; Smeraldi, E.; et al. Lithium and GSK-3β promoter gene variants influence cortical gray matter volumes in bipolar disorder. Psychopharmacology 2015, 232, 1325–1336. [Google Scholar] [CrossRef]
- Bora, E.; Fornito, A.; Yücel, M.; Pantelis, C. Voxelwise Meta-Analysis of Gray Matter Abnormalities in Bipolar Disorder. Biol. Psychiatry 2010, 67, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.R.; Herrmann, N.; Scott, C.J.M.; Black, S.E.; Swartz, R.H.; Hopyan, J.; Lanctôt, K.L. Lithium Carbonate in a Poststroke Population: Exploratory Analyses of Neuroanatomical and Cognitive Outcomes. J. Clin. Psychopharmacol. 2019, 39, 67–71. [Google Scholar] [CrossRef] [PubMed]
- Necus, J.; Sinha, N.; Smith, F.E.; Thelwall, P.E.; Flowers, C.J.; Taylor, P.N.; Blamire, A.M.; Cousins, D.A.; Wang, Y. White matter microstructural properties in bipolar disorder in relationship to the spatial distribution of lithium in the brain. J. Affect. Disord. 2019, 253, 224–231. [Google Scholar] [CrossRef]
- Sun, Y.R. Lithium Carbonate in a Post-Stroke Population: Preliminary Analyses of Neuroanatomical and Neuropsychiatric Outcomes, and Associations with BDNF from a Pilot Study. 2017. Available online: https://hdl.handle.net/1807/79365 (accessed on 2 October 2022).
- Kempton, M.J.; Geddes, J.R.; Ettinger, U.; Williams, S.C.R.; Grasby, P.M. Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch. Gen. Psychiatry 2008, 65, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.J.; Bebchuk, J.M.; Wilds, I.B.; Chen, G.; Menji, H.K. Lithium-induced increase in human brain grey matter. Lancet 2000, 356, 1241–1242. [Google Scholar] [CrossRef]
- Anand, A.; Nakamura, K.; Spielberg, J.M.; Cha, J.; Karne, H.; Hu, B. Integrative analysis of lithium treatment associated effects on brain structure and peripheral gene expression reveals novel molecular insights into mechanism of action. Transl. Psychiatry 2020, 10, 103. [Google Scholar] [CrossRef]
- Beaulieu, J.-M. A role for Akt and glycogen synthase kinase-3 as integrators of dopamine and serotonin neurotransmission in mental health. J. Psychiatry Neurosci. 2012, 37, 7–16. [Google Scholar] [CrossRef]
- Van Woerkom, A.E. A fully integrated new paradigm for lithium’s mode of action—Lithium utilizes latent cellular fail-safe mechanisms. Neuropsychiatr. Dis. Treat. 2017, 13, 275–302. [Google Scholar] [CrossRef]
- Zaworski, J.; Delannoy, P.Y.; Boussekey, N.; Thellier, D.; Georges, H.; Leroy, O. Lithium: One drug, five complications. J. Intensive Care. 2017, 5, 70. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munteanu, C.; Rotariu, M.; Turnea, M.; Tătăranu, L.G.; Dogaru, G.; Popescu, C.; Spînu, A.; Andone, I.; Ionescu, E.V.; Țucmeanu, R.E.; et al. Lithium Biological Action Mechanisms after Ischemic Stroke. Life 2022, 12, 1680. https://doi.org/10.3390/life12111680
Munteanu C, Rotariu M, Turnea M, Tătăranu LG, Dogaru G, Popescu C, Spînu A, Andone I, Ionescu EV, Țucmeanu RE, et al. Lithium Biological Action Mechanisms after Ischemic Stroke. Life. 2022; 12(11):1680. https://doi.org/10.3390/life12111680
Chicago/Turabian StyleMunteanu, Constantin, Mariana Rotariu, Marius Turnea, Ligia Gabriela Tătăranu, Gabriela Dogaru, Cristina Popescu, Aura Spînu, Ioana Andone, Elena Valentina Ionescu, Roxana Elena Țucmeanu, and et al. 2022. "Lithium Biological Action Mechanisms after Ischemic Stroke" Life 12, no. 11: 1680. https://doi.org/10.3390/life12111680
APA StyleMunteanu, C., Rotariu, M., Turnea, M., Tătăranu, L. G., Dogaru, G., Popescu, C., Spînu, A., Andone, I., Ionescu, E. V., Țucmeanu, R. E., Oprea, C., Țucmeanu, A., Cseppento, C. N., Silișteanu, S. C., & Onose, G. (2022). Lithium Biological Action Mechanisms after Ischemic Stroke. Life, 12(11), 1680. https://doi.org/10.3390/life12111680








