Let-7 microRNAs Are Possibly Associated with Perineural Invasion in Colorectal Cancer by Targeting IGF Axis
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. KRAS/BRAF Mutation Detection and MSI Evaluation
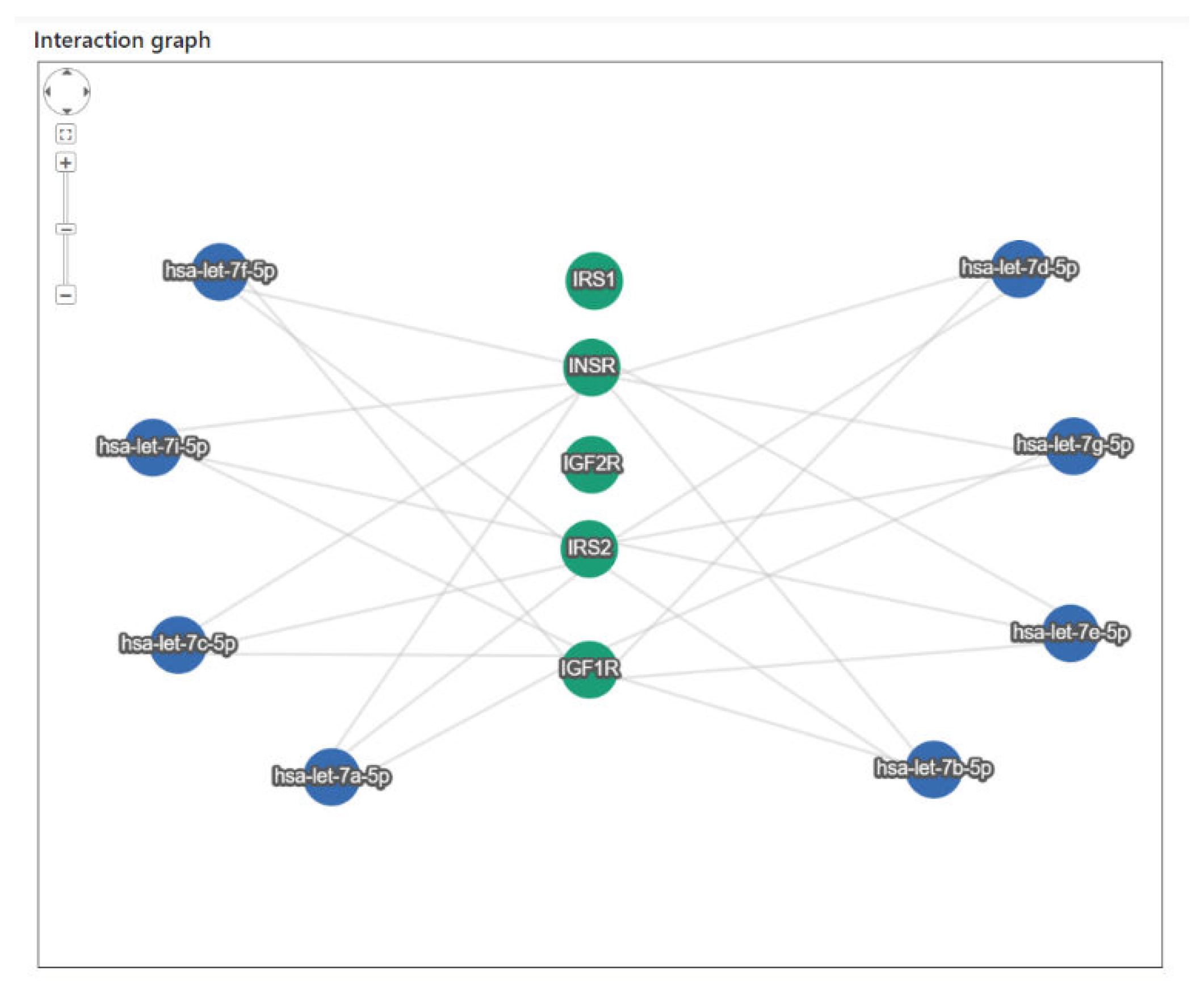
2.3. In Silico let-7 miRNAs and IGF1 Signaling Pathway Interaction
2.4. miRNAs Expression Analysis
2.5. Statistical Analysis
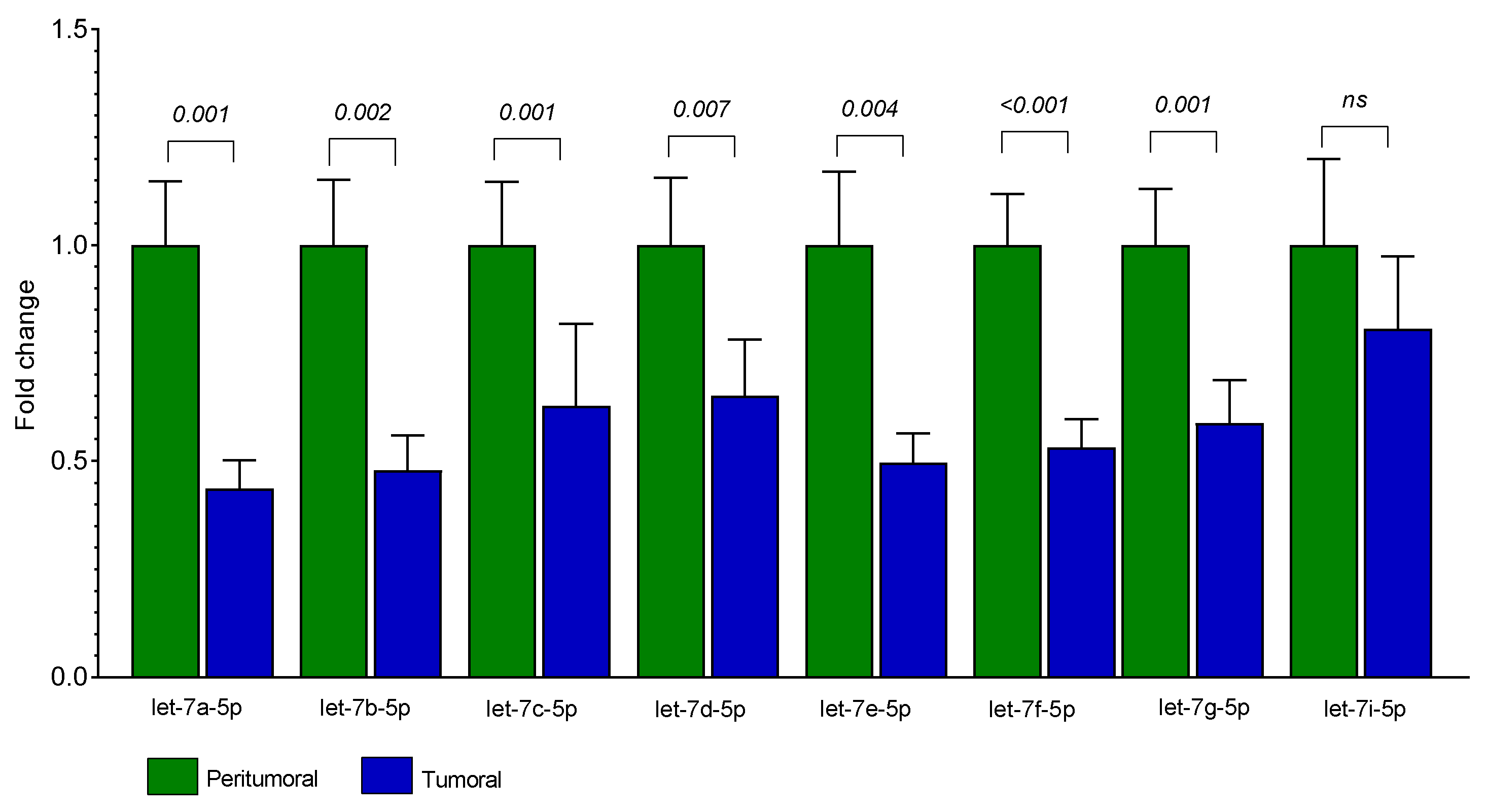
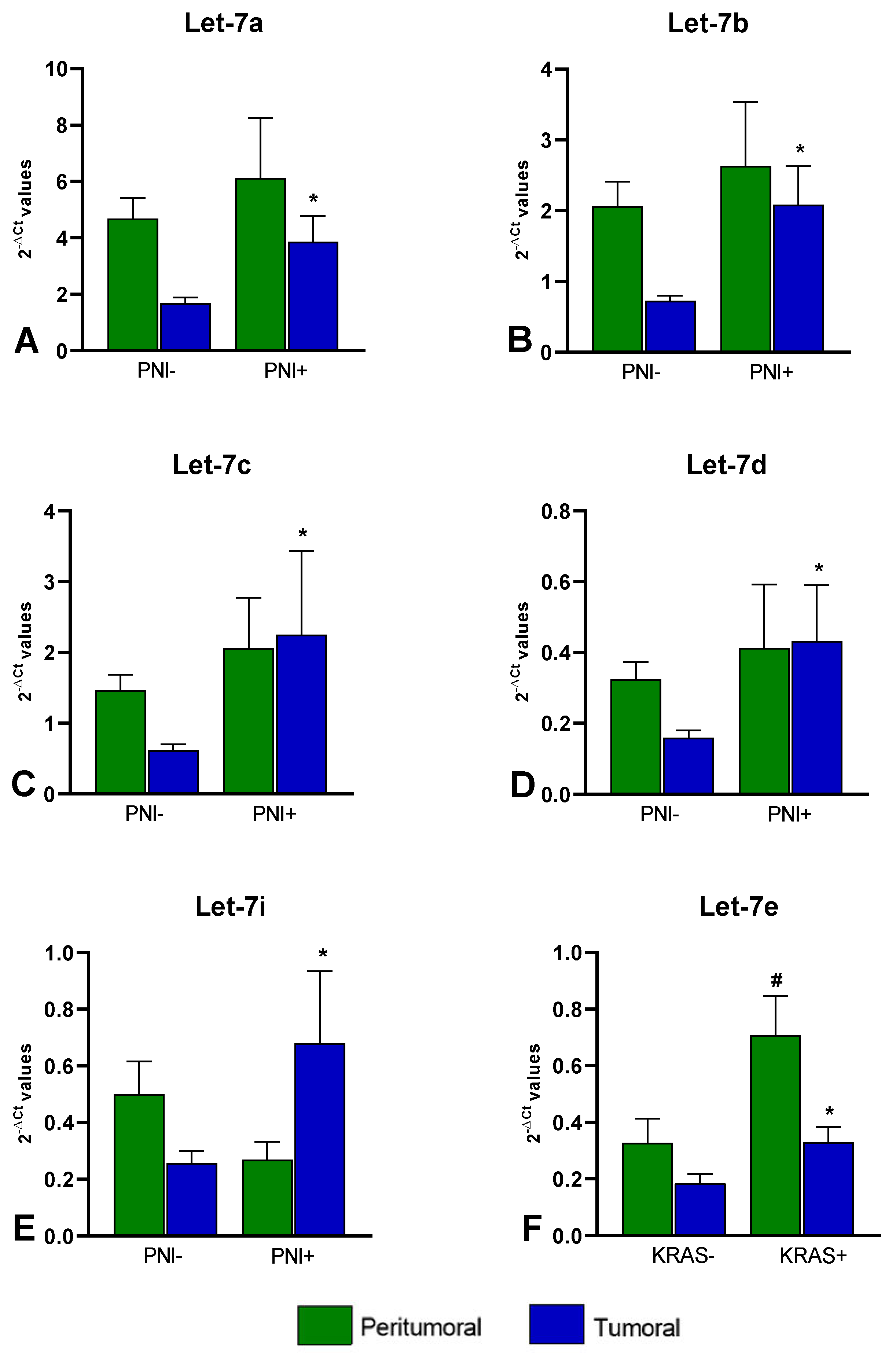
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siddle, K. Signalling by insulin and IGF receptors: Supporting acts and new players. J. Mol. Endocrinol. 2011, 47, R1–R10. [Google Scholar] [CrossRef]
- Brahmkhatri, V.P.; Prasanna, C.; Atreya, H.S. Insulin-like growth factor system in cancer: Novel targeted therapies. Biomed Res. Int. 2015, 2015, 538019. [Google Scholar] [CrossRef] [PubMed]
- Nwabo Kamdje, A.H.; Seke Etet, P.F.; Kipanyula, M.J.; Vecchio, L.; Tagne Simo, R.; Njamnshi, A.K.; Lukong, K.E.; Mimche, P.N. Insulin-like growth factor-1 signaling in the tumor microenvironment: Carcinogenesis, cancer drug resistance, and therapeutic potential. Front. Endocrinol. 2022, 13, 927390. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, X.; Du, L.-L.; Wang, Y.; Liu, D.-B.; Han, C.-Z.; Jing, J.-X.; Zhao, X.-W.; Xu, X.-Q. Possible roles of insulin, IGF-1 and IGFBPs in initiation and progression of colorectal cancer. World J. Gastroenterol. 2014, 20, 1608–1613. [Google Scholar] [CrossRef]
- Allard, J.B.; Duan, C. IGF-Binding Proteins: Why Do They Exist and Why Are There So Many? Front. Endocrinol. 2018, 9, 117. [Google Scholar] [CrossRef]
- Sridhar, S.S.; Goodwin, P.J. Insulin-insulin-like growth factor axis and colon cancer. J. Clin. Oncol. 2009, 27, 165–167. [Google Scholar] [CrossRef]
- Kasprzak, A. Insulin-Like Growth Factor 1 (IGF-1) Signaling in Glucose Metabolism in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 6434. [Google Scholar] [CrossRef]
- Naguib, R.; Abouegylah, M.; Sharkawy, S.; Fayed, A.A.; Naguib, H. Evaluation of Serum Levels of Insulin-Like Growth Factor 1 and Insulin-Like Growth Factor-Binding Protein 3 in Patients With Colorectal Cancer: A Case-Control Study. Cureus 2021, 13, e19881. [Google Scholar] [CrossRef]
- Peters, G.; Gongoll, S.; Langner, C.; Mengel, M.; Piso, P.; Klempnauer, J.; Rüschoff, J.; Kreipe, H.; von Wasielewski, R. IGF-1R, IGF-1 and IGF-2 expression as potential prognostic and predictive markers in colorectal-cancer. Virchows Arch. 2003, 443, 139–145. [Google Scholar] [CrossRef]
- Shiratsuchi, I.; Akagi, Y.; Kawahara, A.; Kinugasa, T.; Romeo, K.; Yoshida, T.; Ryu, Y.; Gotanda, Y.; Kage, M.; Shirouzu, K. Expression of IGF-1 and IGF-1R and their relation to clinicopathological factors in colorectal cancer. Anticancer Res. 2011, 31, 2541–2545. [Google Scholar]
- Nosho, K.; Yamamoto, H.; Taniguchi, H.; Adachi, Y.; Yoshida, Y.; Arimura, Y.; Endo, T.; Hinoda, Y.; Imai, K. Interplay of insulin-like growth factor-II, insulin-like growth factor-I, insulin-like growth factor-I receptor, COX-2, and matrix metalloproteinase-7, play key roles in the early stage of colorectal carcinogenesis. Clin. Cancer Res. 2004, 10, 7950–7957. [Google Scholar] [CrossRef]
- Heckl, S.M.; Pellinghaus, M.; Behrens, H.-M.; Krüger, S.; Schreiber, S.; Röcken, C. Questioning the IGF1 receptor’s assigned role in CRC—A case for rehabilitation? BMC Cancer 2020, 20, 704. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, N.; Oshima, T.; Yoshihara, K.; Aoyama, T.; Hayashi, T.; Yamada, T.; Sato, T.; Shiozawa, M.; Yoshikawa, T.; Morinaga, S.; et al. Clinicopathological significance and impact on outcomes of the gene expression levels of IGF-1, IGF-2 and IGF-1R, IGFBP-3 in patients with colorectal cancer: Overexpression of the IGFBP-3 gene is an effective predictor of outcomes in patients with colorec. Oncol. Lett. 2017, 13, 3958–3966. [Google Scholar] [CrossRef] [PubMed]
- Chirshev, E.; Oberg, K.C.; Ioffe, Y.J.; Unternaehrer, J.J. Let-7 as biomarker, prognostic indicator, and therapy for precision medicine in cancer. Clin. Transl. Med. 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pan, W.; Shen, Y.; Chen, Z.; Zhang, L.; Zhang, Y.; Luo, Q.; Ying, X. IGF1/IGF1R and microRNA let-7e down-regulate each other and modulate proliferation and migration of colorectal cancer cells. Cell Cycle 2018, 17, 1212–1219. [Google Scholar] [CrossRef] [PubMed]
- Knijn, N.; Mogk, S.C.; Teerenstra, S.; Simmer, F.; Nagtegaal, I.D. Perineural Invasion is a Strong Prognostic Factor in Colorectal Cancer: A Systematic Review. Am. J. Surg. Pathol. 2016, 40, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Yao, Z.; Wu, W.; Ju, J.; Xu, Y.; Liu, Y. Perineural Invasion Is a Strong Prognostic Factor but Not a Predictive Factor of Response to Adjuvant Chemotherapy in Node-Negative Colon Cancer. Front. Oncol. 2021, 11, 663154. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Xian, H.-C.; Dai, L.; Tang, Y.-L.; Liang, X.-H. MicroRNAs: Emerging driver of cancer perineural invasion. Cell Biosci. 2021, 11, 117. [Google Scholar] [CrossRef]
- Cionca, F.L.; Dobre, M.; Dobrea, C.M.; Iosif, C.I.; Comănescu, M.V.; Ardeleanu, C.M. Mutational status of KRAS and MMR genes in a series of colorectal carcinoma cases. Rom. J. Morphol. Embryol. 2018, 59, 121–129. [Google Scholar] [PubMed]
- Kern, F.; Aparicio-Puerta, E.; Li, Y.; Fehlmann, T.; Kehl, T.; Wagner, V.; Ray, K.; Ludwig, N.; Lenhof, H.-P.; Meese, E.; et al. miRTargetLink 2.0-interactive miRNA target gene and target pathway networks. Nucleic Acids Res. 2021, 49, W409–W416. [Google Scholar] [CrossRef] [PubMed]
- Belfiore, A.; Malaguarnera, R. Insulin receptor and cancer. Endocr. Relat. Cancer 2011, 18, R125–R147. [Google Scholar] [CrossRef]
- Yuan, J.; Yin, Z.; Tao, K.; Wang, G.; Gao, J. Function of insulin-like growth factor 1 receptor in cancer resistance to chemotherapy. Oncol. Lett. 2018, 15, 41–47. [Google Scholar] [CrossRef]
- Massimino, M.; Sciacca, L.; Parrinello, N.L.; Scalisi, N.M.; Belfiore, A.; Vigneri, R.; Vigneri, P. Insulin Receptor Isoforms Differently Regulate Cell Proliferation and Apoptosis in the Ligand-Occupied and Unoccupied State. Int. J. Mol. Sci. 2021, 22, 8729. [Google Scholar] [CrossRef]
- Vigneri, P.G.; Tirrò, E.; Pennisi, M.S.; Massimino, M.; Stella, S.; Romano, C.; Manzella, L. The Insulin/IGF System in Colorectal Cancer Development and Resistance to Therapy. Front. Oncol. 2015, 5, 230. [Google Scholar] [CrossRef] [PubMed]
- Andres, S.F.; Simmons, J.G.; Mah, A.T.; Santoro, M.A.; Van Landeghem, L.; Lund, P.K. Insulin receptor isoform switching in intestinal stem cells, progenitors, differentiated lineages and tumors: Evidence that IR-B limits proliferation. J. Cell Sci. 2013, 126, 5645–5656. [Google Scholar] [CrossRef]
- Li, J.; Tang, Q.; Dong, W.; Wang, Y. CircBACH1/let-7a-5p axis enhances the proliferation and metastasis of colorectal cancer by upregulating CREB5 expression. J. Gastrointest. Oncol. 2020, 11, 1186–1199. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.-Z.; Shan, G.-F.; Yang, H.; Zha, J.; Wang, L.; Chen, J.-M.; Zhang, X.-S. Cisatracurium regulates the CXCR4/let-7a-5p axis to inhibit colorectal cancer progression by suppressing TGF-β/SMAD2/3 signalling. Chem. Biol. Interact. 2021, 339, 109424. [Google Scholar] [CrossRef] [PubMed]
- Yabasin, I.B.; Lu, Z.; Yu, J.-C.; Wen, Q. Cisatracurium-induced proliferation impairment and death of colorectal cancer cells, HCT116 is mediated by p53 dependent intrinsic apoptotic pathway in vitro. Biomed. Pharmacother. 2017, 91, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Ozcan, O.; Kara, M.; Yumrutas, O.; Bozgeyik, E.; Bozgeyik, I.; Celik, O.I. MTUS1 and its targeting miRNAs in colorectal carcinoma: Significant associations. Tumour Biol. 2016, 37, 6637–6645. [Google Scholar] [CrossRef] [PubMed]
- Bahnassy, A.A.; Salem, S.E.; El-Sayed, M.; Khorshid, O.; Abdellateif, M.S.; Youssef, A.S.; Mohanad, M.; Hussein, M.; Zekri, A.-R.N.; Ali, N.M. MiRNAs as molecular biomarkers in stage II egyptian colorectal cancer patients. Exp. Mol. Pathol. 2018, 105, 260–271. [Google Scholar] [CrossRef]
- Marinović, S.; Škrtić, A.; Catela Ivković, T.; Poljak, M.; Kapitanović, S. Regulation of KRAS protein expression by miR-544a and KRAS-LCS6 polymorphism in wild-type KRAS sporadic colon adenocarcinoma. Hum. Cell 2021, 34, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-P.; Huang, C.-C.; Yeh, K.-T.; Ke, T.-W.; Wei, P.-L.; Yang, J.-R.; Cheng, Y.-W. Down-regulation of let-7a-5p predicts lymph node metastasis and prognosis in colorectal cancer: Implications for chemotherapy. Surg. Oncol. 2016, 25, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Vishnubalaji, R.; Hamam, R.; Abdulla, M.-H.; Mohammed, M.A.V.; Kassem, M.; Al-Obeed, O.; Aldahmash, A.; Alajez, N.M. Genome-wide mRNA and miRNA expression profiling reveal multiple regulatory networks in colorectal cancer. Cell Death Dis. 2015, 6, e1614. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Kim, G.; Kwak, S.G.; Baek, D.W.; Kang, B.W.; Kim, H.J.; Park, S.Y.; Park, J.S.; Choi, G.-S.; Hur, K.; et al. Predictive Value of Circulating miRNAs in Lymph Node Metastasis for Colon Cancer. Genes 2021, 12, 176. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Lin, Y.; Liu, X.; Chen, J.; Shen, B. Biomarker Discovery for the Carcinogenic Heterogeneity Between Colon and Rectal Cancers Based on lncRNA-Associated ceRNA Network Analysis. Front. Oncol. 2020, 10, 535985. [Google Scholar] [CrossRef]
- Vojtechova, Z.; Zavadil, J.; Klozar, J.; Grega, M.; Tachezy, R. Comparison of the miRNA expression profiles in fresh frozen and formalin-fixed paraffin-embedded tonsillar tumors. PLoS ONE 2017, 12, e0179645. [Google Scholar] [CrossRef]
- Zhou, X.-G.; Huang, X.-L.; Liang, S.-Y.; Tang, S.-M.; Wu, S.-K.; Huang, T.-T.; Mo, Z.-N.; Wang, Q.-Y. Identifying miRNA and gene modules of colon cancer associated with pathological stage by weighted gene co-expression network analysis. OncoTargets Ther. 2018, 11, 2815–2830. [Google Scholar] [CrossRef]
- Ni, Y.; Lu, C.; Wang, W.; Gao, W.; Yu, C. circBANP promotes colorectal cancer growth and metastasis via sponging let-7d-5p to modulate HMGA1/Wnt/β-catenin signaling. Mol. Ther. Oncolytics 2021, 21, 119–133. [Google Scholar] [CrossRef]
- Choo, K.B.; Soon, Y.L.; Nguyen, P.N.N.; Hiew, M.S.Y.; Huang, C.-J. MicroRNA-5p and -3p co-expression and cross-targeting in colon cancer cells. J. Biomed. Sci. 2014, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xu, K.; Gu, D.; He, L.; Xie, L.; Chen, Z.; Fan, Z.; Zhu, L.; Du, M.; Chu, H.; et al. Genetic variants in RPA1 associated with the response to oxaliplatin-based chemotherapy in colorectal cancer. J. Gastroenterol. 2019, 54, 939–949. [Google Scholar] [CrossRef]
- Feng, Y.; Liu, X.; Han, Y.; Chen, M.; Zhang, L.; Hu, Y.; Chen, L.; Chen, G.; Li, N. Rotundic Acid Regulates the Effects of Let-7f-5p on Caco2 Cell Proliferation. Anticancer Agents Med. Chem. 2021, 21, 902–909. [Google Scholar] [CrossRef]
- de Brito, B.L.; Lourenço, S.V.; Damascena, A.S.; Kowalski, L.P.; Soares, F.A.; Coutinho-Camillo, C.M. Expression of stem cell-regulating miRNAs in oral cavity and oropharynx squamous cell carcinoma. J. Oral Pathol. Med. 2016, 45, 647–654. [Google Scholar] [CrossRef]
- Kolenda, T.; Guglas, K.; Teresiak, A.; Bliźniak, R.; Lamperska, K. Low let-7d and high miR-205 expression levels positively influence HNSCC patient outcome. J. Biomed. Sci. 2019, 26, 17. [Google Scholar] [CrossRef]
| Features | CRC Patients (N = 25) |
|---|---|
| Age (mean ± SD; min-max) | 65.56 ± 11.53 (30–83) |
| Sex (N; %) | F (13; 52%); M (12; 48%) |
| Family history of cancer (N; %) | 6; 24% |
| Moderate alcohol consumption (N; %) | 12; 48% |
| Localization (N; %) | Ascending colon (5; 20%) Transverse colon (2; 8%) Descending colon (3; 12%) Sigmoid (5; 20%) RSJ (6; 24%) Rectum (4; 16%) |
| Grade (N; %) | G1 (4; 16 %) G2 (14; 56%) G3 (7; 28%) |
| TNM (N) | T2 N0 M0 (N = 1); T2 N1 M0 (N = 1); T2 N2 M0 (N = 1); T3 N0 M0 (N = 10); T3 N1 M0 (N = 5); T3 N2 M0 (N = 3); T4 N0 M0 (N = 2); T4 N1 M0 (N = 1); T4 N2 M0 (N = 1); |
| Histologic subtype (N; %) | Adenocarcinoma (21; 84%) Mucinous (4; 16%) |
| Lymphovascular invasion (N; %) | 9; 36% |
| Perineural invasion (N; %) | 6; 24% |
| Microsatellite instability (N; %) | 2; 8% |
| KRAS/BRAF mutations | |
| KRAS codon 12 (N; %) | 9; 36% |
| KRAS codon 13 (N; %) | 3; 12% |
| BRAF codon 600 (N; %) | 2; 8% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niculae, A.M.; Dobre, M.; Herlea, V.; Manuc, T.E.; Trandafir, B.; Milanesi, E.; Hinescu, M.E. Let-7 microRNAs Are Possibly Associated with Perineural Invasion in Colorectal Cancer by Targeting IGF Axis. Life 2022, 12, 1638. https://doi.org/10.3390/life12101638
Niculae AM, Dobre M, Herlea V, Manuc TE, Trandafir B, Milanesi E, Hinescu ME. Let-7 microRNAs Are Possibly Associated with Perineural Invasion in Colorectal Cancer by Targeting IGF Axis. Life. 2022; 12(10):1638. https://doi.org/10.3390/life12101638
Chicago/Turabian StyleNiculae, Andrei Marian, Maria Dobre, Vlad Herlea, Teodora Ecaterina Manuc, Bogdan Trandafir, Elena Milanesi, and Mihail Eugen Hinescu. 2022. "Let-7 microRNAs Are Possibly Associated with Perineural Invasion in Colorectal Cancer by Targeting IGF Axis" Life 12, no. 10: 1638. https://doi.org/10.3390/life12101638
APA StyleNiculae, A. M., Dobre, M., Herlea, V., Manuc, T. E., Trandafir, B., Milanesi, E., & Hinescu, M. E. (2022). Let-7 microRNAs Are Possibly Associated with Perineural Invasion in Colorectal Cancer by Targeting IGF Axis. Life, 12(10), 1638. https://doi.org/10.3390/life12101638






