Influence of Different Types of Retinal Cameras on the Performance of Deep Learning Algorithms in Diabetic Retinopathy Screening
Abstract
1. Introduction
2. Materials and Methods
3. Statistical Analysis
4. Results
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, N.H.; Shaw, J.E.; Karuranga, S.; Huang, Y.; da Rocha Fernandes, J.D.; Ohlrogge, A.W.; Malanda, B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018, 138, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Resnikoff, S.; Lansingh, V.C.; Washburn, L.; Felch, W.; Gauthier, T.M.; Taylor, H.R.; Eckert, K.; Parke, D.; Wiedemann, P. Estimated number of ophthalmologists worldwide (International Council of Ophthalmology update): Will we meet the needs? Br. J. Ophthalmol. 2020, 104, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Rogers, T.W.; Gonzalez-Bueno, J.; Garcia Franco, R.; Lopez Star, E.; Méndez Marín, D.; Vassallo, J.; Lansingh, V.C.; Trikha, S.; Jaccard, N. Evaluation of an AI system for the detection of diabetic retinopathy from images captured with a handheld portable fundus camera: The MAILOR AI study. Eye 2021, 35, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Sarao, V.; Veritti, D.; Lanzetta, P. Automated diabetic retinopathy detection with two different retinal imaging devices using artificial intelligence: A comparison study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2647–2654. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.; Cheung, C.Y.; Lim, G.; Tan, G.S.; Quang, N.D.; Gan, A.; Hamzah, H.; Garcia-Franco, R.; San Yeo, I.Y.; Lee, S.Y.; et al. Development and validation of a deep learning system for diabetic retinopathy and related eye diseases using retinal images from multiethnic populations with diabetes. JAMA 2017, 318, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.Y.; Bressler, N.M. Artificial intelligence with deep learning technology looks into diabetic retinopathy screening. JAMA 2016, 316, 2366–2367. [Google Scholar] [CrossRef] [PubMed]
- Abràmoff, M.D.; Lavin, P.T.; Birch, M.; Shah, N.; Folk, J.C. Pivotal trial of an autonomous AI-based diagnostic system for detection of diabetic retinopathy in primary care offices. NPJ Digit Med. 2018, 3, 39. [Google Scholar] [CrossRef] [PubMed]
- Ipp, E.; Liljenquist, D.; Bode, B.; Shah, V.N.; Silverstein, S.; Regillo, C.D.; Lim, J.I.; Sadda, S.; Domalpally, A.; Gray, G.; et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA Netw. Open. 2021, 4, e2134254. [Google Scholar] [CrossRef] [PubMed]
- Ruamviboonsuk, P.; Krause, J.; Chotcomwongse, P.; Sayres, R.; Raman, R.; Widner, K.; Campana, B.J.; Phene, S.; Hemarat, K.; Tadarati, M.; et al. Deep learning versus human graders for classifying diabetic retinopathy severity in a nationwide screening program. NPJ Digit. Med. 2019, 2, 25. [Google Scholar]
- Gulshan, V.; Peng, L.; Coram, M.; Stumpe, M.C.; Wu, D.; Narayanaswamy, A.; Venugopalan, S.; Widner, K.; Madams, T.; Cuadros, J.; et al. Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. JAMA 2016, 316, 2402–2410. [Google Scholar] [CrossRef] [PubMed]
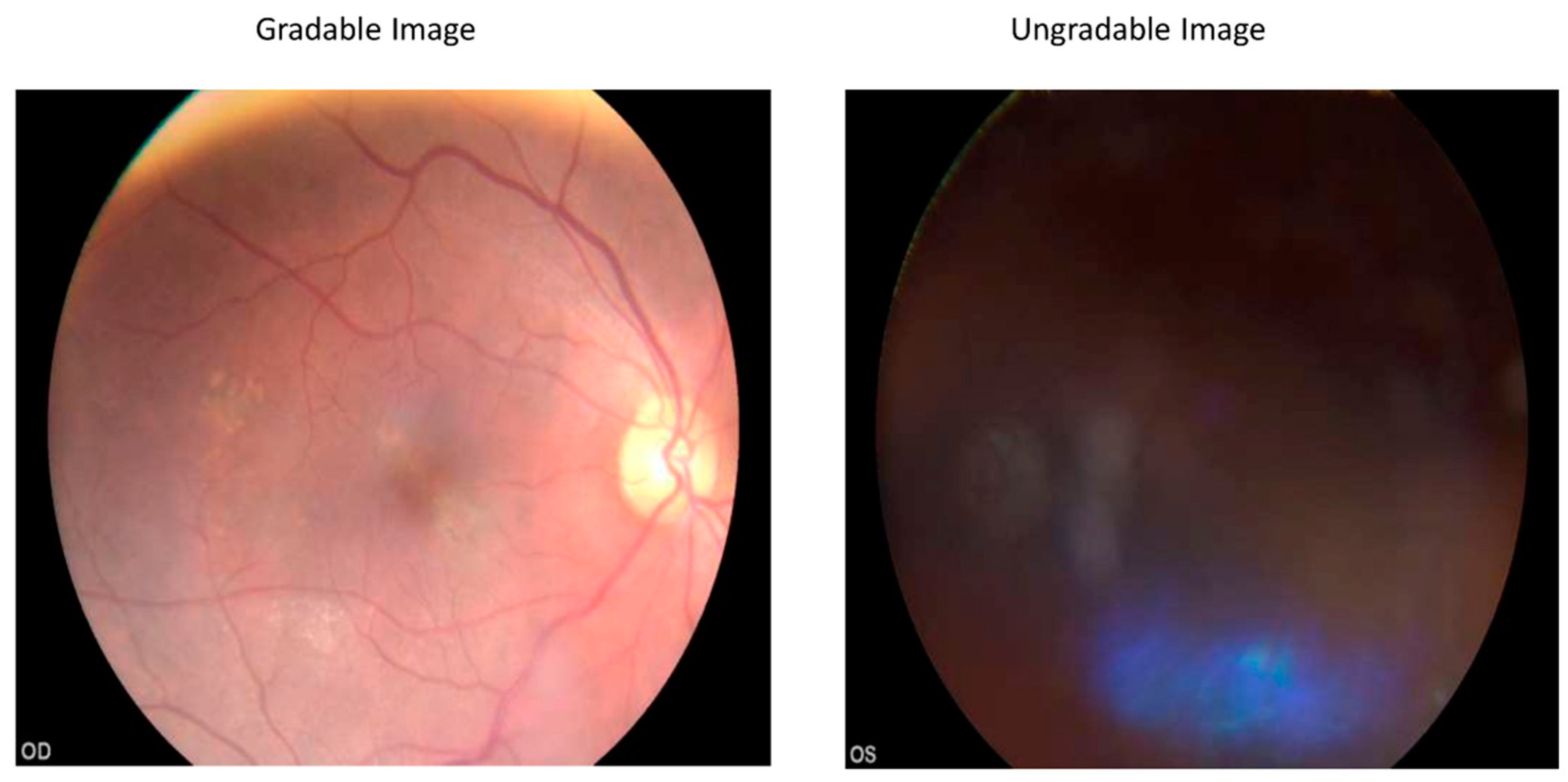
| Specifications | Kowa Nonmyd α-DⅢ | Kowa Nonmyd 7 | Kowa Nonmyd WX | Kowa VX 10 α | Kowa VX 20 | Nidek AFC 210 |
|---|---|---|---|---|---|---|
| Field angle | 45°/30° | 45°/20° | Normal: 45° Stereo: 34° (20° × 27°) SP:45° | - | - | 45° |
| Internal fixation target | Normal: 3 positions (central, disc, macula); Mosaic: 9 positions | 4 fixed dots switching type | Central, Disc, Macula, Mosaic: 8 positions | 4 fixed dots right or left eye switching (Non-mydriatic mode) | Central, Disc, Macula, Peripheral | LED (70 points) |
| Minimum pupil size | 3.5 mm | 4.0 mm (small pupil mode: 3.7 mm) | Normal mode: 4.0 mm, SP mode: 3.5 mm, Stereo mode: 4.0 mm | Non-mydriatic mode- 4.00 mm; Mydriatic mode- 5.5 mm; Small pupil- 4.00 mm | Non-mydriatic mode- 4.00 mm; Mydriatic mode- 5.5 mm; Small pupil- 4.00/3.5 mm | 4.0 mm (Small pupil: 3.7 mm) |
| Focusing | Split luminous bars coincidence | Split luminous bars coincidence | Split luminous bars coincidence | Point matching method (ON/OFF switch) | Split luminous bars coincidence | Infrared split bright target coincidence |
| Compensation range of examined eye | Without compensation: −15D to +13D; With—compensation: −32D to −12D; With + compensation: +10D to +40D | Without compensation: −15D to +13D; With—compensation: −33D to −11D; With + compensation: +10D to +40D | Without compensation: −12D to +13D; With—compensation: −32D to −10D; With + compensation: +10D to +35D | Without compensation −12D to +13D; With—compensation: −10D to −32D; With + compensation: +10D to +35D | Without compensation −12D to +13D; With—compensation: −10D to −32D; With + compensation: +10D to +35D | Total: −33D to +35D; With minus dioptric lens: −33D to −7D; With no dioptric lens: −12D to +15D; With plus dioptric lens: +11D to +35D |
| Parameter | All | Gradable Images | Ungradable Images | p-Value |
|---|---|---|---|---|
| No of subjects | 4588 | 3816 | 772 | - |
| Age, mean ± SD | 59.19 ± 11.52 | 57.81 ± 11.17 | 66.01 ± 10.76 | <0.001 |
| Gender, n (%) | ||||
| Male | 1524 (33.2) | 1282 (33.6) | 242 (31.3) | <0.001 |
| Female | 3063 (66.8) | 2533 (66.4) | 530 (68.7) | <0.001 |
| HbA1c, (%) | 5.56 ± 3.70 | 5.51 ± 3.37 | 5.80 ± 3.55 | <0.001 |
| Serum FBS, (mg/dL) | 117.61 ± 77.17 | 117.21 ± 77.15 | 119.61 ± 77.27 | 0.431 |
| Serum LDL, (mg/dL) | 79.93 ± 57.68 | 80.39 ± 58.43 | 77.62 ± 53.77 | 0.001 |
| Visual acuity (logMAR), median(IQR) | ||||
| Right eye | 0.17 (0.39) | 0.17 (0.30) | 0.30 (0.37) | <0.001 |
| Left eye | 0.17 (0) | 0.17 (0) | 0.30 (0) | <0.001 |
| Both eyes | 0.17 (0.30) | 0.17 (0.35) | 0.30 (0.34) | <0.001 |
| Ophthalmologist (N%) | Human Graders (N%) | DL (N%) | |
|---|---|---|---|
| No DR | 12,648 (82.39) | 13,225 (86.15) | 12,376 (80.62) |
| Mild NPDR | 1172 (7.63) | 811 (5.28) | 647 (4.21) |
| Moderate NPDR | 1239 (8.07) | 1081 (7.04) | 1456 (9.48) |
| Severe NPDR | 94 (0.61) | 78 (0.51) | 402 (2.62) |
| PDR | 198 (1.29) | 156 (1.02) | 170 (1.11) |
| Camera Name | Camera Code | Resolution (Megapixel) | Gradable Images N = 15,351 n (%) | Ungradable Images N = 4057 n (%) |
|---|---|---|---|---|
| Kowa Nonmyd | 1 | 2 | 1383 (9.0) | 1039 (25.6) |
| Kowa Nonmyd α-DⅢ | 3 | 8 | 3241 (21.1) | 775 (19.1) |
| Kowa Nonmyd 7 | 4A | 10 | 1895 (12.3) | 344 (8.5) |
| Kowa Nonmyd WX | 5 | 12 | 733 (4.8) | 45 (1.1) |
| Kowa VX 10 α | 6 | 12.3 | 728 (4.7) | 118 (2.9) |
| Kowa VX 20 | 7 | 15 | 3133 (20.4) | 409 (10.1) |
| Kowa Nonmyd 7 | 4B | 16 | 1249 (8.1) | 493 (12.2) |
| Nidek AFC 210 | 8 | 18 | 2989 (19.5) | 834 (20.6) |
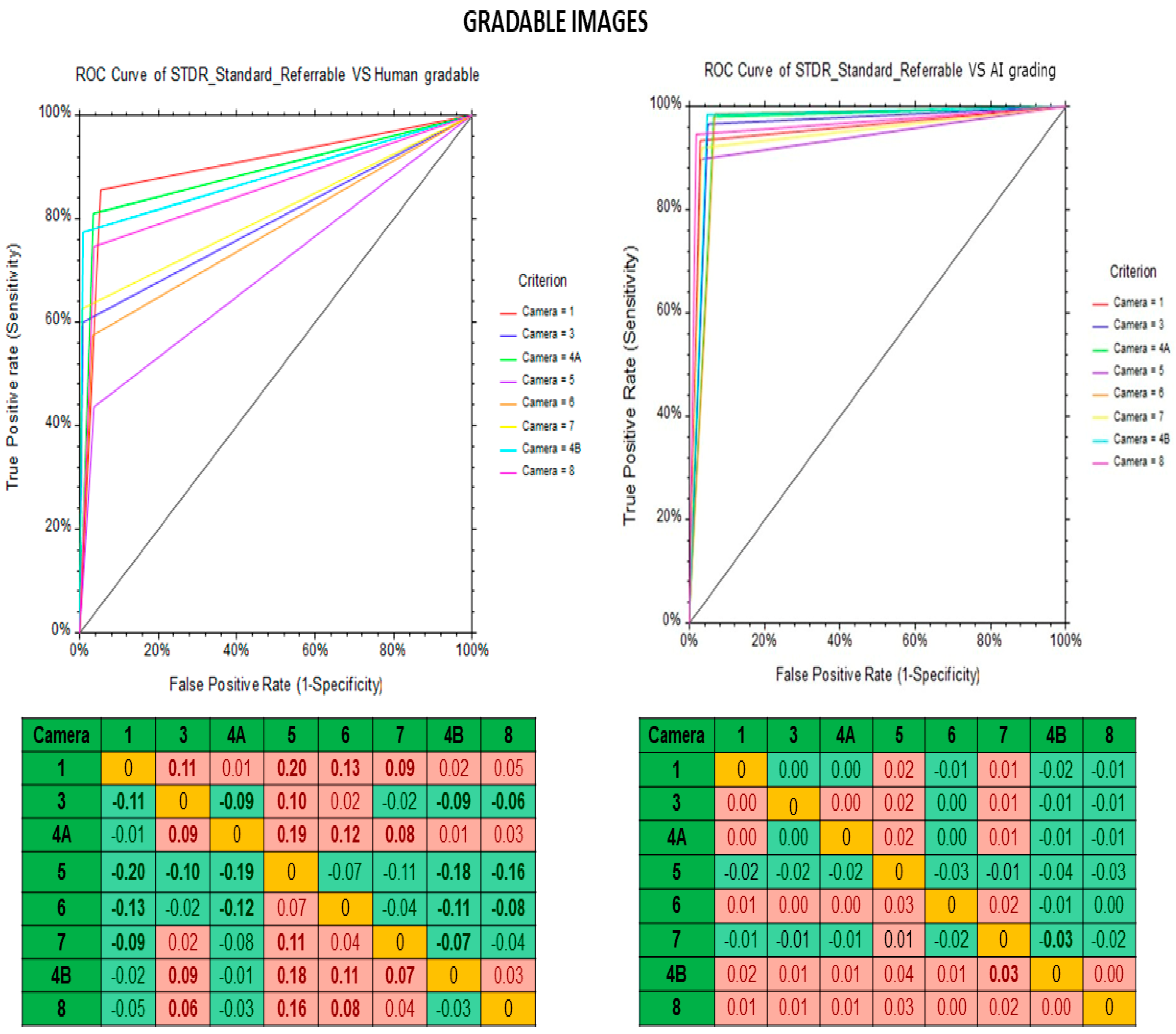
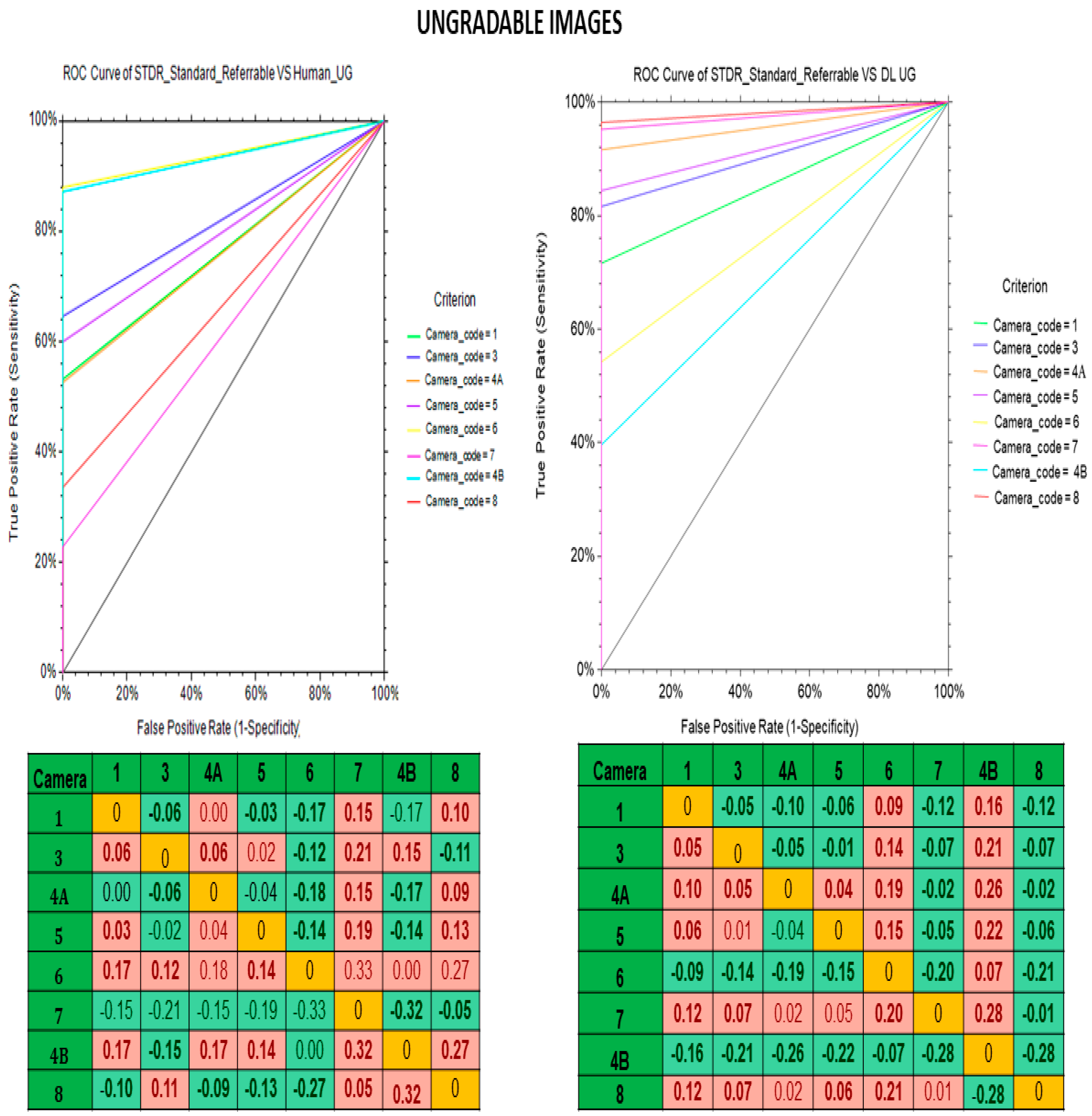
| Fundus Camera | Gradable Images | Ungradable Images | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Human Graders vs. Reference Standard | Deep Learning Algorithm vs. Standard Reference | Human Graders vs. Reference Standard | Deep Learning Algorithm vs. Standard Reference | |||||||||
| Sensitivity | Specificity | Kappa | Sensitivity | Specificity | Kappa | Sensitivity | Specificity | Kappa | Sensitivity | Specificity | Kappa | |
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||||
| Kowa Nonmyd | 85.6 | 94.6 | 0.72 | 93.5 | 97.2 | 0.85 | 53.2 | 98.7 | 0.37 | 71.5 | 99.7 | 0.97 |
| (79.0–90.8) | (93.2–95.9) | (88.3–96.8) | (96.1–98.1) | (47.8–58.6) | (97.8–1.0) | (66.4–76.2) | (99.8–1.0) | |||||
| Kowa Nonmyd α-DⅢ | 59.9 | 99.1 | 0.68 | 96.6 | 95.1 | 0.72 | 64.5 | 98.9 | 0.75 | 81.6 | 99.7 | 0.88 |
| (53.3–66.3) | (98.7–99.4) | (93.3–98.5) | (94.3–95.9) | (61.0–67.9) | (97.8–1.0) | (78.6–84.2) | (99.1–1.0) | |||||
| Kowa Nonmyd 7 | 81 | 96.7 | 0.8 | 97.9 | 93.7 | 0.86 | 52.5 | 99.5 | 0.66 | 91.5 | 99 | 0.81 |
| (76.9–84.6) | (95.6–97.5) | (96.1–99.0) | (92.3- 94.8) | (43.2–61.8) | (99.1–1.0) | (85.0–95.9) | (98.9–1.0) | |||||
| Kowa Nonmyd WX | 43.6 | 96.4 | 0.39 | 89.7 | 97 | 0.72 | 60 | 99.7 | 0.74 | 84.4 | 98.7 | 0.91 |
| (27.8–60.4) | (94.7–97.7) | (75.8–97.1) | (95.4–98.1) | (44.3–74.3) | (99.5–1.0) | (70.5–93.5) | (98.4–1.0) | |||||
| Kowa VX 10 α | 57.4 | 96.7 | 0.61 | 98.7 | 93.3 | 0.84 | 88 | 99.3 | 0.66 | 54.3 | 99.7 | 0.95 |
| (49.1–65.5) | (94.9–98.0) | (95.2–99.8) | (90.9–95.2) | (84.5–91.0) | (99.1–1.0) | (49.3–59.2) | (99.1–1.0) | |||||
| Kowa VX 20 | 62.6 | 99.5 | 0.72 | 91.9 | 96.9 | 0.75 | 22.7 | 99.9 | 0.93 | 95.1 | 99.1 | 0.67 |
| (55.5–69.4) | (99.2–99.7) | (87.2–95.3) | (96.2–97.5) | (19.1–26.7) | (99.7–1.0) | (92.8–96.9) | (97.4–1.0) | |||||
| Kowa Nonmyd 7 | 74.6 | 96.5 | 0.68 | 94.6 | 98.2 | 0.88 | 30.7 | 98.2 | 0.3 | 96.4 | 98.9 | 0.97 |
| (65.4–82.4) | (95.3–97.5) | (88.5–98.0) | (97.3–98.9) | (65.4–36.6) | (98.0–1.0) | (95.1–97.5) | (98.6–1.0) | |||||
| Nidek AFC 210 | 77.4 | 99.2 | 0.82 | 98.4 | 95.4 | 0.8 | 84.8 | 99.4 | 0.91 | 39.6 | 99.7 | 0.51 |
| (72.3–82.0) | (98.8–99.5) | (96.2–99.5) | (94.6–96.2) | (72.3–89.5) | (99.2–1.0) | (36.2–43.0) | (99.2–1.0) | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srinivasan, R.; Surya, J.; Ruamviboonsuk, P.; Chotcomwongse, P.; Raman, R. Influence of Different Types of Retinal Cameras on the Performance of Deep Learning Algorithms in Diabetic Retinopathy Screening. Life 2022, 12, 1610. https://doi.org/10.3390/life12101610
Srinivasan R, Surya J, Ruamviboonsuk P, Chotcomwongse P, Raman R. Influence of Different Types of Retinal Cameras on the Performance of Deep Learning Algorithms in Diabetic Retinopathy Screening. Life. 2022; 12(10):1610. https://doi.org/10.3390/life12101610
Chicago/Turabian StyleSrinivasan, Ramyaa, Janani Surya, Paisan Ruamviboonsuk, Peranut Chotcomwongse, and Rajiv Raman. 2022. "Influence of Different Types of Retinal Cameras on the Performance of Deep Learning Algorithms in Diabetic Retinopathy Screening" Life 12, no. 10: 1610. https://doi.org/10.3390/life12101610
APA StyleSrinivasan, R., Surya, J., Ruamviboonsuk, P., Chotcomwongse, P., & Raman, R. (2022). Influence of Different Types of Retinal Cameras on the Performance of Deep Learning Algorithms in Diabetic Retinopathy Screening. Life, 12(10), 1610. https://doi.org/10.3390/life12101610







