Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Source
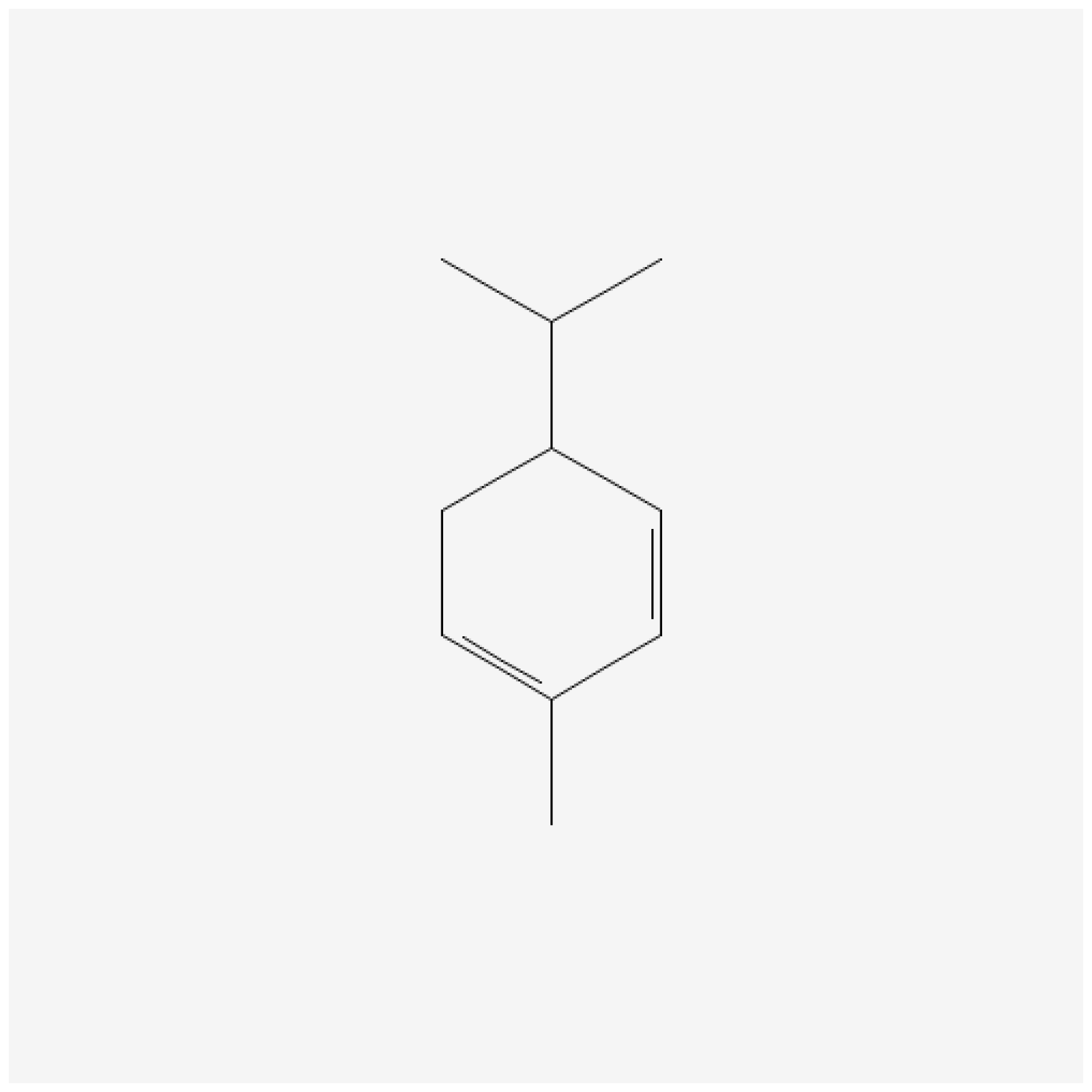
3.2. Chemistry and Biosynthesis
3.3. Alpha-Phellandrene Properties as Pure Molecule
3.3.1. Antitumoral Effects
3.3.2. Biopesticide and Repellent Activity
3.3.3. Food Preservative
3.3.4. Supplementary Data Concerning Specific Biological Activities
3.4. EOs Rich in Alpha-Phellandrene and Their Main Biological Activities
3.4.1. Antimicrobial Activity
3.4.2. Antitumoral Effects
3.4.3. Biopesticides and Repellent Activity
3.4.4. Food Preservative
3.4.5. Miscellaneous Data Concerning Specific Biological Activities
3.5. Toxicity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, Z.; Peng, J.; Chen, Y.; Tao, L.; Zhang, Y.; Fu, L.; Long, Q.; Shen, X. 1,8-Cineole: A Review of Source, Biological Activities, and Application. J. Asian Nat. Prod. Res. 2020, 23, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Reynoso, M.M.N.; Lucia, A.; Zerba, E.N.; Alzogaray, R.A. The Octopamine Receptor Is a Possible Target for Eugenol-Induced Hyperactivity in the Blood-Sucking Bug Triatoma Infestans (Hemiptera: Reduviidae). J. Med. Entomol. 2020, 57, 627–630. [Google Scholar] [CrossRef]
- Sell, C.S. The Chemistry of Fragrances: From Perfumer to Consumer; Royal Society of Chemistry: London, UK, 2006; ISBN 0854048243. [Google Scholar]
- Wojtunik-Kulesza, K.A.; Kasprzak, K.; Oniszczuk, T.; Oniszczuk, A. Natural Monoterpenes: Much More than Only a Scent. Chem. Biodivers. 2019, 16. [Google Scholar] [CrossRef]
- Tan, X.C.; Chua, K.H.; Ravishankar Ram, M.; Kuppusamy, U.R. Monoterpenes: Novel Insights into Their Biological Effects and Roles on Glucose Uptake and Lipid Metabolism in 3T3-L1 Adipocytes. Food Chem. 2016, 196, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Grassmann, J.; Elstner, E.F. Essential Oils | Properties and Uses. Encycl. Food Sci. Nutr. 2003, 75, 2177–2184. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals; Elsevier Health Sciences: Amsterdam, The Netherlands, 2013; ISBN 0702054348. [Google Scholar]
- Dhakad, A.K.; Pandey, V.V.; Beg, S.; Rawat, J.M. Biological, Medicinal and Toxicological Significance of Eucalyptus Leaf Essential Oil: A Review. J Sci Food Agri 2018, 98, 833–848. [Google Scholar] [CrossRef]
- LOTUS. Available online: https://lotus.naturalproducts.net/compound/lotus_id/LTS0234318 (accessed on 19 February 2022).
- Adams, T.B.; Gavin, C.L.; McGowen, M.M.; Waddell, W.J.; Cohen, S.M.; Feron, V.J.; Marnett, L.J.; Munro, I.C.; Portoghese, P.S.; Rietjens, I.M.C.M.; et al. The FEMA GRAS Assessment of Aliphatic and Aromatic Terpene Hydrocarbons Used as Flavor Ingredients. Food Chem. Toxicol. 2011, 49, 2471–2494. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.; Brennan, S.E.; et al. The Prisma 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Med. Flum. 2021, 57, 444–465. [Google Scholar] [CrossRef]
- Setzer, W.N.; Duong, L.; Poudel, A.; Mentreddy, S.R. Variation in the Chemical Composition of Five Varieties of Curcuma Longa Rhizome Essential Oils Cultivated in North Alabama. Foods 2021, 10, 212. [Google Scholar] [CrossRef]
- Vérité, P.; Nacer, A.; Kabouche, Z.; Seguin, E. Composition of Seeds and Stems Essential Oils of Pituranthos Scoparius (Coss. & Dur.) Schinz. Flavour Fragr. J. 2004, 19, 562–564. [Google Scholar] [CrossRef]
- Lucia, A.; Licastro, S.; Zerba, E.; Masuh, H. Yield, Chemical Composition, and Bioactivity of Essential Oils from 12 Species of Eucalyptus on Aedes Aegypti Larvae. Entomol. Exp. Appl. 2008, 129, 107–114. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.Ã.; Brophy, J.J. Volatile Leaf Oils of Some South-Western and Southern Australian Species of the Genus Eucalyptus ( Series I ). Part XVIII. A. Subgenus Monocalyptus. B. Subgenus Symphyomyrtus: ( I ) Section Guilfoyleanae; ( Ii ) Section Bisectaria, Series Ac. Flavour Fragr. J. 1997, 12, 423–432. [Google Scholar] [CrossRef]
- Bignell, C.M.; Dunlop, P.J.; Brophy, J.J. Volatile Leaf Oils of Some South-Western and Southern Australian Species of the Genus Eucalyptus ( Series 1 ). Part XIX. Flavour Fragr. J. 1998, 13, 131–139. [Google Scholar] [CrossRef]
- Amazonas, D.R.; Oliveira, C.; Barata, L.E.S.; Tepe, E.J.; Kato, M.J.; Mourão, R.H.V.; Yamaguchi, L.F. Chemical and Genotypic Variations in Aniba Rosiodora from the Brazilian Amazon Forest. Molecules 2020, 26, 69. [Google Scholar] [CrossRef] [PubMed]
- da Silva, H.M.; Andrade, E.H.A.; Grac, M.; Zoghbi, Ë.B.; Maia, Â.G.S.; Domingos, Ä. The Essential Oils of Lantana Camara L. Occurring in North Brazil. Flavour Fragr. J. 1999, 210, 208–210. [Google Scholar] [CrossRef]
- Cysne, J.B.; Canuto, K.M.; Pessoa, O.D.L.; Nunes, E.P.; Silveiraa, E.R. Leaf Essential Oils of Four Piper Species from the State of Ceará - Northeast of Brazil. J. Braz. Chem. Soc. 2005, 16, 1378–1381. [Google Scholar] [CrossRef]
- Teneva, D.; Denkova, Z.; Denkova-Kostova, R.; Goranov, B.; Kostov, G.; Slavchev, A.; Hristova-Ivanova, Y.; Uzunova, G.; Degraeve, P. Biological Preservation of Mayonnaise with Lactobacillus Plantarum LBRZ12, Dill, and Basil Essential Oils. Food Chem. 2021, 344, 128707. [Google Scholar] [CrossRef] [PubMed]
- Mercier, S.; Lorenzo, R.Y.; Pichette, A.; Côté, H.; Legault, J.; St-Gelais, A. Pili Tree (Canarium Ovatum) Resin’s Antibacterial Essential Oil and Hydrosol as Rich Sources of (S)-Phellandrenes Derivatives. Chem. Biodivers. 2020, 17, e2000561. [Google Scholar] [CrossRef]
- Quintero Ruiz, N.; Córdoba Campo, Y.; Stashenko, E.E.; Fuentes, J.L. Antigenotoxic Effect against Ultraviolet Radiation-Induced DNA Damage of the Essential Oils from Lippia Species. Photochem. Photobiol. 2017, 93, 1063–1072. [Google Scholar] [CrossRef]
- Malagón, O.; Cartche, P.; Montaño, A.; Cumbicus, N.; Gilardoni, G. A New Essential Oil from the Leaves of the Endemic Andean Species <i>Gynoxys Miniphylla Cuatrec. (Asteraceae): Chemical and Enantioselective Analyses. Plants 2022, 11, 398. [Google Scholar] [CrossRef]
- Andreani, S.; De Cian, M.C.; Paolini, J.; Desjobert, J.M.; Costa, J.; Muselli, A. Chemical Variability and Antioxidant Activity of Limbarda Crithmoides L. Essential Oil from Corsica. Chem. Biodivers. 2013, 10, 2061–2077. [Google Scholar] [CrossRef] [PubMed]
- Faber, B.; Bangert, K.; Mosandl, A. GC-IRMS and Enantioselective Analysis in Biochemical Studies in Dill (Anethum Graveolens L.). Flavour Fragr. J. 1997, 12, 305–314. [Google Scholar] [CrossRef]
- Kanjilal, P.B.; Kotoky, R.; Singh, R.S. Chemical Composition of the Leaf Oil of Altingia Excelsa Nornha. Flavour Fragr. J. 2003, 18, 449–450. [Google Scholar] [CrossRef]
- Agnihotri, V.K.; Thappa, R.K.; Meena, B.; Kapahi, B.K.; Saxena, R.K.; Qazi, G.N.; Agarwal, S.G. Essential Oil Composition of Aerial Parts of Angelica Glauca Growing Wild in North-West Himalaya (India). Phytochemistry 2004, 65, 2411–2413. [Google Scholar] [CrossRef] [PubMed]
- Madhava Naidu, M.; Vedashree, M.; Satapathy, P.; Khanum, H.; Ramsamy, R.; Hebbar, H.U. Effect of Drying Methods on the Quality Characteristics of Dill (Anethum Graveolens) Greens. Food Chem. 2016, 192, 849–856. [Google Scholar] [CrossRef]
- Sharma, R.K.; Kotoky, R.; Bhattacharyya, P.R. Volatile Oil from the Leaves of Callistemon Lanceolatus D.C. Grown in North-Eastern India. Flavour Fragr. J. 2006, 21, 239–240. [Google Scholar] [CrossRef]
- Joshi, R.K. Chemical Disparity in the Oil from Leaves of Cinnamomum Zeylanicum Blume. Flavour Fragr. J. 2019, 34, 443–449. [Google Scholar] [CrossRef]
- Sindhu, S.; Chempakam, B.; Leela, N.K.; Suseela Bhai, R. Chemoprevention by Essential Oil of Turmeric Leaves (Curcuma Longa L.) on the Growth of Aspergillus Flavus and Aflatoxin Production. Food Chem. Toxicol. 2011, 49, 1188–1192. [Google Scholar] [CrossRef]
- Debbarma, J.; Kishore, P.; Nayak, B.B.; Kannuchamy, N.; Gudipati, V. Antibacterial Activity of Ginger, Eucalyptus and Sweet Orange Peel Essential Oils on Fish-Borne Bacteria. J. Food Process. Preserv. 2013, 37, 1022–1030. [Google Scholar] [CrossRef]
- Sharma, A.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Chemical Profiling, Cytotoxicity and Phytotoxicity of Foliar Volatiles of Hyptis Suaveolens. Ecotoxicol. Environ. Saf. 2019, 171, 863–870. [Google Scholar] [CrossRef]
- Tavakkoli, E.; Bazargani-Gilani, B.; Pajohi-Alamoti, M. The Impacts of Tomato Residuum Extract with Arabic Gum and Dill Essential Oil on the Shelf Life Improvement of Trout Fillets Stored at Chilly Condition. J. Food Saf. 2020, 40, e12812. [Google Scholar] [CrossRef]
- Hajhashemi, V.; Abbasi, N. Hypolipidemic Activity of Anethum Graveolens in Rats. Phyther. Res. 2008, 22, 372–375. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, N.; Bruno, M.; Gagliano Candela, R.; Maggi, F. Chemical Composition of the Essential Oil of Elaeoselinum Asclepium (L.) Bertol Subsp. Meoides (Desf.) Fiori (Umbelliferae) Collected Wild in Central Sicily and Its Antimicrobial Activity. Nat. Prod. Res. 2022, 36, 789–797. [Google Scholar] [CrossRef] [PubMed]
- Piccaglia, R.; Marotti, M. Characterization of Some Italian Types of Wild Fennel (Foeniculum Vulgare Mill.). J. Agric. Food Chem. 2001, 49, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Ilardi, V.; Badalamenti, N.; Bruno, M. Chemical Composition of the Essential Oil from Different Vegetative Parts of Foeniculum Vulgare Subsp. Piperitum (Ucria) Coutinho (Umbelliferae) Growing Wild in Sicily. Nat. Prod. Res. 2020, 1–11. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Porcedda, S.; Tuveri, E.; Maxia, A. Comparative Analysis of the Oil and Supercritical CO2 Extract of Ridolfia Segetum (L.) Moris. Nat. Prod. Res. 2007, 21, 412–417. [Google Scholar] [CrossRef]
- Miyazawa, M.; Teranishi, A.; Ishikawa, Y. Components of the Essential Oil from Petasites Japonicus. Flavour Fragr. J. 2003, 18, 231–233. [Google Scholar] [CrossRef]
- Nait Irahal, I.; azzahra Lahlou, F.; Hmimid, F.; Errami, A.; Guenaou, I.; Diawara, I.; Kettani-Halabi, M.; Fahde, S.; Ouafik, L.; Bourhim, N. Identification of the Chemical Composition of Six Essential Oils with Mass Spectroscopy and Evaluation of Their Antibacterial and Antioxidant Potential. Flavour Fragr. J. 2021, 36, 465–476. [Google Scholar] [CrossRef]
- Onyenekwe, P.C.; Stahl, M.; Adejo, G. Post-Irradiation Changes of the Volatile Oil Constituents of Monodora Myristica (Gaertn) Dunal. Nat. Prod. Res. 2012, 26, 2030–2034. [Google Scholar] [CrossRef]
- Pereira, S.I.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D.; Silva, A.M.S. Chemical Composition of the Essential Oil Distilled from the Fruits of Eucalyptus Globulus Grown in Portugal. Flavour Fragr. J. 2005, 20, 407–409. [Google Scholar] [CrossRef]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Sevinate-pinto, I.; Antunes, T.; Fontinha, S.S. Composition of the Essential Oil of Lavandula Pinnata L. Fil. Var. Pinnata Grown on Madeira. Flavour Fragr. J. 1995, 10, 93–96. [Google Scholar] [CrossRef]
- El-Zaeddi, H.; Martínez-Tomé, J.; Calín-Sánchez, Á.; Burló, F.; Carbonell-Barrachina, Á.A. Volatile Composition of Essential Oils from Different Aromatic Herbs Grown in Mediterranean Regions of Spain. Foods 2016, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Palá-Paúl, J.; Pérez-Alonso, M.J.; Velasco-Negueruela, A.; Ballestros, M.T.; Sanz, J. Essential Oil Composition of Sideritis Hirsuta L. from Guadalajara Province, Spain. Flavour Fragr. J. 2006, 21, 410–415. [Google Scholar] [CrossRef]
- Clark, R.J.; Menary, R.C. The Effect of Harvest Date on the Yield and Composition of Tasmanian Dill Oil (Anethum Graveolens L.). J. Sci. Food Agric. 1984, 35, 1186–1190. [Google Scholar] [CrossRef]
- El Ayeb-Zakhamaa, A.; Sakka-Rouisa, L.; Flaminib, G.; Jannetc, H.B.; Harzallah-Skhiri, F. Chemical Composition and Allelopathic Potential of Essential Oils from Citharexylum Spinosum L. Grown in Tunisia. Chem. Biodivers. 2016, 14, e1600225. [Google Scholar] [CrossRef] [PubMed]
- Hayouni, E.A.; Chraief, I.; Abedrabba, M.; Bouix, M.; Leveau, J.Y.; Mohammed, H.; Hamdi, M. Tunisian Salvia Officinalis L. and Schinus Molle L. Essential Oils: Their Chemical Compositions and Their Preservative Effects against Salmonella Inoculated in Minced Beef Meat. Int. J. Food Microbiol. 2008, 125, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical Composition and Anticancer and Antioxidant Activities of Schinus Molle L. and Schinus Terebinthifolius Raddi Berries Essential Oils. J. Food Sci. 2010, 75, 466–472. [Google Scholar] [CrossRef]
- Elshafie, H.S.; Ghanney, N.; Mang, S.M.; Ferchichi, A.; Camele, I. An in Vitro Attempt for Controlling Severe Phytopathogens and Human Pathogens Using Essential Oils from Mediterranean Plants of Genus Schinus. J. Med. Food 2016, 19, 266–273. [Google Scholar] [CrossRef]
- Akgül, A.; Chialva, F. Constituents of the Essential Oil of Echinophora Tenuifolia L. Subsp. Sibthorpiana (Guss.) Tutin from Turkey. Flavour Fragr. J. 1989, 4, 67–68. [Google Scholar] [CrossRef]
- Özcan, M.M.; Chalchat, J.C.; Arslan, D.; Ateş, A.; Ünver, A. Comparative Essential Oil Composition and Antifungal Effect of Bitter Fennel (Foeniculum Vulgare Ssp. Piperitum) Fruit Oils Obtained during Different Vegetation. J. Med. Food 2006, 9, 552–561. [Google Scholar] [CrossRef]
- Mamadalieva, N.Z.; Abdullaeva, N.S.; Rosenau, T.; Fakhrutdinova, M.; Azimova, S.S.; Böhmdorfer, S. Composition of Essential Oils from Four Apiaceae and Asteraceae Species Growing in Uzbekistan. Nat. Prod. Res. 2018, 32, 1118–1122. [Google Scholar] [CrossRef] [PubMed]
- Ibañez, J.; Usubillaga, A. The Essential Oil of Espeletia Schultzii of Different Altitudinal Populations. Flavour Fragr. J. 2006, 21, 286–289. [Google Scholar] [CrossRef]
- Waser, M.; Rinner, U. From Biosynthesis to Total Synthesis: Strategies and Tactics for Natural Products. In Monoterpenes and Iridoids; Zografos, A.L., Ed.; Wiley: Hoboken, NJ, USA, 2016; pp. 196–235. ISBN 9781118754085. [Google Scholar]
- Lin, J.J.; Lin, J.U.H.; Hsu, S.C.; Weng, S.W.; Huang, Y.I.P.; Tang, N.Y.; Lin, J.G.; Chung, J.G. Alpha-Phellandrene Promotes Immune Responses in Normal Mice through Enhancing Macrophage Phagocytosis and Natural Killer Cell Activities. In Vivo 2013, 27, 809–814. [Google Scholar] [PubMed]
- Lin, J.J.; Lu, K.W.; Ma, Y.S.; Tang, N.Y.; Wu, P.P.; Wu, C.C.; Lu, H.F.; Lin, J.G.; Chung, J.G. Alpha-Phellandrene, a Natural Active Monoterpene, Influences a Murine WEHI-3 Leukemia Model in Vivo by Enhancing Macrophague Phagocytosis and Natural Killer Cell Activity. In Vivo 2014, 28, 583–588. [Google Scholar] [PubMed]
- Lin, J.-J.; Hsu, S.-C.; Lu, K.-W.; Ma, Y.-S.; Wu, C.-C.; Lu, H.-F.; Chen, J.-C.; Lin, J.-G.; Wu, P.-P.; Chung, J.-G. Alpha-Phellandrene-Induced Apoptosis in Mice Leukemia WEHI-3 Cells in Vitro. Wiley Online Libr. 2015. [Google Scholar] [CrossRef]
- Lin, J.J.; Yu, C.C.; Lu, K.W.; Chang, S.J.; Yu, F.S.; Liao, C.L.; Lin, J.G.; Chung, J.G. α-Phellandrene Alters Expression of Genes Associated with DNA Damage, Cell Cycle, and Apoptosis in Murine Leukemia WEHI-3 Cells. Anticancer Res. 2014, 34, 4161–4180. [Google Scholar] [PubMed]
- Lin, J.-J.; Wu, C.-C.; Hsu, S.-C.; Weng, S.-W.; Ma, Y.-S.; Huang, Y.-P.; Lin, J.-G.; Chung, J.-G. Alpha-Phellandrene-Induced DNA Damage and Affect DNA Repair Protein Expression in WEHI-3 Murine Leukemia Cells in Vitro. Wiley Online Libr. 2014. [Google Scholar] [CrossRef]
- Pinheiro-Neto, F.R.; Lopes, E.M.; Acha, B.T.; da Silva Gomes, L.; Dias, W.A.; dos Reis Filho, A.C.; de Sousa Leal, B.; do Nascimento Rodrigues, D.C.; do Nascimento Silva, J.; Dittz, D.; et al. α-Phellandrene Exhibits Antinociceptive and Tumor-Reducing Effects in a Mouse Model of Oncologic Pain. Toxicol. Appl. Pharmacol. 2021, 418, 115497. [Google Scholar] [CrossRef] [PubMed]
- Plata-Rueda, A.; Campos, J.M.; da Silva Rolim, G.; Martínez, L.C.; Dos Santos, M.H.; Fernandes, F.L.; Serrão, J.E.; Zanuncio, J.C. Terpenoid Constituents of Cinnamon and Clove Essential Oils Cause Toxic Effects and Behavior Repellency Response on Granary Weevil, Sitophilus Granarius. Ecotoxicol. Environ. Saf. 2018, 156, 263–270. [Google Scholar] [CrossRef]
- Jung, W.C.; Jang, Y.S.U.; Hieu, T.T.; Lee, C.K.; Ahn, Y.J. Toxicity of Myristica Fragrans Seed Compounds against Blattella Germanica (Dictyoptera: Blattellidae). J. Med. Entomol. 2007, 44, 524–529. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Tabanca, N.; Ozek, G.; Ozek, T.; Aytac, Z.; Bernier, U.R.; Agramonte, N.M.; Baser, K.H.C.; Khan, I.A. Essential Oils of Echinophora Lamondiana (Apiales: Umbelliferae): A Relationship between Chemical Profile and Biting Deterrence and Larvicidal Activity against Mosquitoes (Diptera: Culicidae). J. Med. Entomol. 2015, 52, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Bleeker, P.M.; Diergaarde, P.J.; Ament, K.; Guerra, J.; Weidner, M.; Schütz, S.; de Both, M.T.J.; Haring, M.A.; Schuurink, R.C. The Role of Specific Tomato Volatiles in Tomato-Whitefly Interaction. Plant Physiol. 2009, 151, 925–935. [Google Scholar] [CrossRef] [PubMed]
- Hieu, T.T.; Choi, W.S.; Kim, S.I.; Wang, M.; Ahn, Y.J. Enhanced Repellency of Binary Mixtures of Calophyllum Inophyllum Nut Oil Fatty Acids or Their Esters and Three Terpenoids to Stomoxys Calcitrans. Pest Manag. Sci. 2015, 71, 1213–1218. [Google Scholar] [CrossRef]
- Wu, C.C.; Lin, C.L.; Huang, C.Y.; Hsieh, S.; Liu, C.H.; Hsieh, S.L. α-Phellandrene Enhances the Immune Response and Resistance against Vibrio Alginolyticus in White Shrimp (Litopenaeus Vannamei). Fish Shellfish Immunol. 2019, 84, 1108–1114. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.F.; Brandã, M.S.; Moura, J.B.; Leitão, J.M.R.S.; Carvalho, F.A.A.; Miúra, L.M.C.V.; Leite, J.R.S.A.; Sousa, D.P.; Almeida, F.R.C. Antinociceptive Activity of the Monoterpene α-Phellandrene in Rodents: Possible Mechanisms of Action. J. Pharm. Pharmacol. 2012, 64, 283–292. [Google Scholar] [CrossRef]
- Marrelli, M.; Amodeo, V.; Viscardi, F.; De Luca, M.; Statti, G.; Conforti, F. Essential Oils of Foeniculum Vulgare Subsp. Piperitum and Their in Vitro Anti-Arthritic Potential. Chem. Biodivers. 2020, 17, e2000388. [Google Scholar] [CrossRef]
- González, S.; Guerra, P.E.; Bottaro, H.; Molares, S.; Demo, M.S.; Oliva, M.M.; Zunino, M.P.; Zygadlo, J.A. Aromatic Plants from Patagonia. Part I. Antimicrobial Activity and Chemical Composition of Schinus Polygamus (Cav.) Cabrera Essential Oil. Flavour Fragr. J. 2004, 19, 36–39. [Google Scholar] [CrossRef]
- Deka Bhuyan, P.; Chutia, M.; Pathak, M.G.; Baruah, P. Effect of Essential Oils from Lippia Geminata and Cymbopogon Jwarancusa on in Vitro Growth and Sporulation of Two Rice Pathogens. JAOCS J. Am. Oil Chem. Soc. 2010, 87, 1333–1340. [Google Scholar] [CrossRef]
- Moro, A.; Librán, C.M.; Berruga, M.I.; Zalacain, A.; Carmona, M. Mycotoxicogenic Fungal Inhibition by Innovative Cheese Cover with Aromatic Plants. J. Sci. Food Agric. 2013, 93, 1112–1118. [Google Scholar] [CrossRef] [PubMed]
- Martins, M.D.R.; Arantes, S.; Candeias, F.; Tinoco, M.T.; Cruz-Morais, J. Antioxidant, Antimicrobial and Toxicological Properties of Schinus Molle L. Essential Oils. J. Ethnopharmacol. 2014, 151, 485–492. [Google Scholar] [CrossRef]
- Ennigrou, A.; Casabianca, H.; Vulliet, E.; Hanchi, B.; Hosni, K. Assessing the Fatty Acid, Essential Oil Composition, Their Radical Scavenging and Antibacterial Activities of Schinus Terebinthifolius Raddi Leaves and Twigs. J. Food Sci. Technol. 2018, 55, 1582–1590. [Google Scholar] [CrossRef] [PubMed]
- Cole, E.R.; dos Santos, R.B.; Lacerda Júnior, V.; Martins, J.D.L.; Greco, S.J.; Cunha Neto, A. Chemical Composition of Essential Oil from Ripe Fruit of Schinus Terebinthifolius Raddi and Evaluation of Its Activity against Wild Strains of Hospital Origin. Brazilian J. Microbiol. 2014, 45, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Salem, M.Z.M.; El-Hefny, M.; Ali, H.M.; Elansary, H.O.; Nasser, R.A.; El-Settawy, A.A.A.; El Shanhorey, N.; Ashmawy, N.A.; Salem, A.Z.M. Antibacterial Activity of Extracted Bioactive Molecules of Schinus Terebinthifolius Ripened Fruits against Some Pathogenic Bacteria. Microb. Pathog. 2018, 120, 119–127. [Google Scholar] [CrossRef]
- Celaya, L.S.; Alabrudzińska, M.H.; Molina, A.C.; Viturro, C.I.; Moreno, S. The Inhibition of Methicillin-Resistant Staphylococcus Aureus by Essential Oils Isolated from Leaves and Fruits of Schinus Areira Depending on Their Chemical Compositions. Acta Biochim. Pol. 2014, 61, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Chaftar, N.; Girardot, M.; Quellard, N.; Labanowski, J.; Ghrairi, T.; Hani, K.; Frère, J.; Imbert, C. Activity of Six Essential Oils Extracted from Tunisian Plants against Legionella Pneumophila. Chem. Biodivers. 2015, 12, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- Essien, E.; Newby, J.; Walker, T.; Setzer, W.; Ekundayo, O. Characterization and Antimicrobial Activity of Volatile Constituents from Fresh Fruits of Alchornea Cordifolia and Canthium Subcordatum. Medicines 2016, 3, 1. [Google Scholar] [CrossRef]
- Boucher, M.A.; Côté, H.; Pichette, A.; Ripoll, L.; Legault, J. Chemical Composition and Antibacterial Activity of Tussilago Farfara (L.) Essential Oil from Quebec, Canada. Nat. Prod. Res. 2020, 34, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Dalli, M.; Azizi, S.E.; Benouda, H.; Azghar, A.; Tahri, M.; Bouammali, B.; Maleb, A.; Gseyra, N. Molecular Composition and Antibacterial Effect of Five Essential Oils Extracted from Nigella Sativa L. Seeds against Multidrug-Resistant Bacteria: A Comparative Study. Evidence-Based Complement. Altern. Med. 2021, 2021. [Google Scholar] [CrossRef] [PubMed]
- Lisboa, F.P.; Silvestre, W.P.; Castro, J.O.; Martins, G.V.; Segabinazzi, L.G.T.M.; Pauletti, G.F.; Dell’Aqua, J.A. In Vitro Antimicrobial Activity of Selected Essential Oils against Endometritis-Causing Microorganisms in Mares. J. Equine Vet. Sci. 2022, 110, 103840. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ahluwalia, V.; Singh, P.; Kumar, N.; Prakash Sati, O.; Sati, N. Antifungal and Phytotoxic Activity of Essential Oil from Root of Senecio Amplexicaulis Kunth. (Asteraceae) Growing Wild in High Altitude-Himalayan Region. Nat. Prod. Res. 2016, 30, 1875–1879. [Google Scholar] [CrossRef] [PubMed]
- Gakuubi, M.M.; Maina, A.W.; Wagacha, J.M. Antifungal Activity of Essential Oil of Eucalyptus Camaldulensis Dehnh. against Selected Fusarium spp. Int. J. Microbiol. 2017, 2017. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.; Miranda, M.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Assessment of Safe Bioactive Doses of Foeniculum Vulgare Mill. Essential Oil from Portugal. Nat. Prod. Res. 2017, 31, 2654–2659. [Google Scholar] [CrossRef] [PubMed]
- Bakarnga-Via, I.; Hzounda, J.B.; Fokou, P.V.T.; Tchokouaha, L.R.Y.; Gary-Bobo, M.; Gallud, A.; Garcia, M.; Walbadet, L.; Secka, Y.; Dongmo, P.M.J.; et al. Composition and Cytotoxic Activity of Essential Oils from Xylopia Aethiopica (Dunal) A. Rich, Xylopia Parviflora (A. Rich) Benth.) and Monodora Myristica (Gaertn) Growing in Chad and Cameroon. BMC Complement. Altern. Med. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Hsieh, S.L.; Li, Y.C.; Chang, W.C.; Chung, J.G.; Hsieh, L.C.; Wu, C.C. Induction of Necrosis in Human Liver Tumor Cells by α-Phellandrene. Nutr. Cancer 2014, 66, 970–979. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.C.; Hsieh, S.L.; Chen, C.T.; Chung, J.G.; Wang, J.J.; Wu, C.C. Induction of α-Phellandrene on Autophagy in Human Liver Tumor Cells. Am. J. Chin. Med. 2015, 43, 121–136. [Google Scholar] [CrossRef] [PubMed]
- de Lima, E.J.S.P.; Fontes, S.S.; Nogueira, M.L.; Silva, V.R.; Santos, L.D.S.; D’Elia, G.M.A.; Dias, R.B.; Sales, C.B.S.; Rocha, C.A.G.; Vannier-Santos, M.A.; et al. Essential Oil from Leaves of Conobea Scoparioides (Cham. & Schltdl.) Benth. (Plantaginaceae) Causes Cell Death in HepG2 Cells and Inhibits Tumor Development in a Xenograft Model. Biomed. Pharmacother. 2020, 129, 110402. [Google Scholar] [CrossRef]
- Aboalhaija, N.H.; Awwad, O.; Khalil, E.; Abbassi, R.; Abaza, I.F.; Afifi, F.U. Chemodiversity and Antiproliferative Activity of the Essential Oil of Schinus Molle Growing in Jordan. Chem. Biodivers. 2019, 16, e1900388. [Google Scholar] [CrossRef]
- Kiran, S.; Kujur, A.; Patel, L.; Ramalakshmi, K.; Prakash, B. Assessment of Toxicity and Biochemical Mechanisms Underlying the Insecticidal Activity of Chemically Characterized Boswellia Carterii Essential Oil against Insect Pest of Legume Seeds. Pestic. Biochem. Physiol. 2017, 139, 17–23. [Google Scholar] [CrossRef]
- Sánchez Chopa, C.; Descamps, L.R. Composition and Biological Activity of Essential Oils against Metopolophium Dirhodum (Hemiptera: Aphididae) Cereal Crop Pest. Pest Manag. Sci. 2012, 68, 1492–1500. [Google Scholar] [CrossRef]
- Kostić, I.; Lazarević, J.; Jovanović, D.Š.; Kostić, M.; Marković, T.; Milanović, S. Potential of Essential Oils from Anise, Dill and Fennel Seeds for the Gypsy Moth Control. Plants 2021, 10, 2194. [Google Scholar] [CrossRef] [PubMed]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Silva, M.A.N.; Deschamps, C.; Molento, M.B. Insecticide Activity of Curcuma Longa (Leaves) Essential Oil and Its Major Compound α-Phellandrene against Lucilia Cuprina Larvae (Diptera: Calliphoridae): Histological and Ultrastructural Biomarkers Assessment. Pestic. Biochem. Physiol. 2019, 153, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Sereshti, H.; Sedaghat, M.M.; Amani, A. Nanoemulsion of Dill Essential Oil as a Green and Potent Larvicide against Anopheles Stephensi. Environ. Sci. Pollut. Res. 2018, 25, 6466–6473. [Google Scholar] [CrossRef] [PubMed]
- Raj, G.A.; Chandrasekaran, M.; Krishnamoorthy, S.; Jayaraman, M.; Venkatesalu, V. Phytochemical Profile and Larvicidal Properties of Seed Essential Oil from Nigella Sativa L. (Ranunculaceae), against Aedes Aegypti, Anopheles Stephensi, and Culex Quinquefasciatus (Diptera: Culicidae). Parasitol. Res. 2015, 114, 3385–3391. [Google Scholar] [CrossRef]
- Seo, S.M.; Jung, C.S.; Kang, J.; Lee, H.R.; Kim, S.W.; Hyun, J.; Park, I.K. Larvicidal and Acetylcholinesterase Inhibitory Activities of Apiaceae Plant Essential Oils and Their Constituents against Aedes Albopictus and Formulation Development. J. Agric. Food Chem. 2015, 63, 9977–9986. [Google Scholar] [CrossRef]
- Santos Da Silva, R.C.; Milet-Pinheiro, P.; Bezerra Da Silva, P.C.; Gomes Da Silva, A.; Vanusa Da Silva, M.; Do Amaral Ferraz Navarro, D.M.; Da Silva, N.H. (E)-Caryophyllene and α-Humulene: Aedes Aegypti Oviposition Deterrents Elucidated by Gas Chromatography-Electrophysiological Assay of Commiphora Leptophloeos Leaf Oil. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef]
- Do Nascimento, J.C.; David, J.M.; Barbosa, L.C.; De Paula, V.F.; Demuner, A.J.; David, J.P.; Conserva, L.M.; Ferreira, J.C.; Guimarães, E.F. Larvicidal Activities and Chemical Composition of Essential Oils from Piper Klotzschianum (Kunth) C. DC. (Piperaceae). Pest Manag. Sci. 2013, 69, 1267–1271. [Google Scholar] [CrossRef] [PubMed]
- Jaenson, T.G.T.; Pålsson, K.; Borg-Karlson, A.K. Evaluation of Extracts and Oils of Mosquito (Diptera: Culicidae) Repellent Plants from Sweden and Guinea-Bissau. J. Med. Entomol. 2006, 43, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Ashitani, T.; Garboui, S.S.; Schubert, F.; Vongsombath, C.; Liblikas, I.; Pålsson, K.; Borg-Karlson, A.K. Activity Studies of Sesquiterpene Oxides and Sulfides from the Plant Hyptis Suaveolens (Lamiaceae) and Its Repellency on Ixodes Ricinus (Acari: Ixodidae). Exp. Appl. Acarol. 2015, 67, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, R.H.; Nepote, V.; Grosso, N.R. Aguaribay and Cedron Essential Oils as Natural Antioxidants in Oil-Roasted and Salted Peanuts. JAOCS J. Am. Oil Chem. Soc. 2012, 89, 2195–2205. [Google Scholar] [CrossRef]
- Benkhoud, H.; M’Rabet, Y.; Gara ali, M.; Mezni, M.; Hosni, K. Essential Oils as Flavoring and Preservative Agents: Impact on Volatile Profile, Sensory Attributes, and the Oxidative Stability of Flavored Extra Virgin Olive Oil. J. Food Process. Preserv. 2022, 46, 1–13. [Google Scholar] [CrossRef]
- Ayelo, P.M.; Yusuf, A.A.; Pirk, C.W.W.; Chailleux, A.; Mohamed, S.A.; Deletre, E. Terpenes from Herbivore-Induced Tomato Plant Volatiles Attract Nesidiocoris Tenuis (Hemiptera: Miridae), a Predator of Major Tomato Pests. Pest Manag. Sci. 2021, 77, 5255–5267. [Google Scholar] [CrossRef]
- Mademtzoglou, D.; Akmoutsou, P.; Kounatidis, I.; Franzios, G.; Drosopoulou, E.; Vokou, D.; Mavragani-Tsipidou, P. Applying the Drosophila Wing Spot Test to Assess the Genotoxic Impact of 10 Essential Oil Constituents Used as Flavouring Agents or Cosmetic Ingredients. Flavour Fragr. J. 2011, 26, 447–451. [Google Scholar] [CrossRef]
- de Groot, A.C.; Schmidt, E. Tea Tree Oil: Contact Allergy and Chemical Composition. Contact Dermatitis 2016, 75, 129–143. [Google Scholar] [CrossRef]
- ISO 9235:2021; Aromatic Natural Raw Materials—Vocabulary. International Organization for Standardization (ISO): Geneva, Switzerland, 2021. Available online: https://www.iso.org/standard/78908.html (accessed on 6 October 2022).
- Sadgrove, N.; Jones, G. A Contemporary Introduction to Essential Oils: Chemistry, Bioactivity and Prospects for Australian Agriculture. Agric. 2015, 5, 48–102. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Aljaafari, M.N.; Alali, A.O.; Baqais, L.; Alqubaisy, M.; Alali, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.S.; Lim, S.H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef]
- Wongkattiya, N.; Sanguansermsri, P.; Fraser, I.H.; Sanguansermsri, D. Antibacterial Activity of Cuminaldehyde on Food-Borne Pathogens, the Bioactive Component of Essential Oil from Cuminum Cyminum l. Collected in Thailand. J. Complement. Integr. Med. 2019, 16, 1–6. [Google Scholar] [CrossRef]
- Scalvenzi, L.; Grandini, A.; Spagnoletti, A.; Tacchini, M.; Neill, D.; Ballesteros, J.L.; Sacchetti, G.; Guerrini, A. Myrcia Splendens (Sw.) DC. (Syn. M. Fallax (Rich.) DC.) (Myrtaceae) Essential Oil from Amazonian Ecuador: A Chemical Characterization and Bioactivity Profile. Molecules 2017, 22, 1163. [Google Scholar] [CrossRef]
- Naveed, R.; Hussain, I.; Tawab, A.; Tariq, M.; Rahman, M.; Hameed, S.; Mahmood, M.S.; Siddique, A.B.; Iqbal, M. Antimicrobial Activity of the Bioactive Components of Essential Oils from Pakistani Spices against Salmonella and Other Multi-Drug Resistant Bacteria. BMC Complement. Altern. Med. 2013, 13, 265. [Google Scholar] [CrossRef]
- Lesgards, J.F.; Baldovini, N.; Vidal, N.; Pietri, S. Anticancer Activities of Essential Oils Constituents and Synergy with Conventional Therapies: A Review. Phyther. Res. 2014, 28, 1423–1446. [Google Scholar] [CrossRef]
- Spisni, E.; Petrocelli, G.; Imbesi, V.; Spigarelli, R.; Azzinnari, D.; Sarti, M.D.; Campieri, M.; Valerii, M.C. Antioxidant, Anti-Inflammatory, and Microbial-Modulating Activities of Essential Oils: Implications in Colonic Pathophysiology. Int. J. Mol. Sci. 2020, 21, 4152. [Google Scholar] [CrossRef]
- Di Martile, M.; Garzoli, S.; Ragno, R.; Del Bufalo, D. Essential Oils and Their Main Chemical Components: The Past 20 Years of Preclinical Studies in Melanoma. Cancers 2020, 12, 2650. [Google Scholar] [CrossRef]
- Lombrea, A.; Antal, D.; Ardelean, F.; Avram, S.; Pavel, I.Z.; Vlaia, L.; Mut, A.; Diaconeasa, Z.; Dehelean, C.A.; Soica, C.; et al. A Recent Insight Regarding the Phytochemistry and Bioactivity of Origanum Vulgare L. Essential Oil. Int. J. Mol. Sci. 2020, 21, 9653. [Google Scholar] [CrossRef]
- De Oliveira, J.R.; Esteves, S.; Camargo, A. Rosmarinus Officinalis L. ( Rosemary ) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 8, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Simes, E.; Santos, E.; Abreu, M.; Silva, J.; Nunes, N.; Costa, M.; Pessoa, O.; Pessoa, C.; Ferreira, P. Biomedical Properties and Potentiality of Lippia Microphylla Cham. and Its Essential Oils. J. Intercult. Ethnopharmacol. 2015, 4, 256. [Google Scholar] [CrossRef]
- Zhao, H.; Ren, S.; Yang, H.; Tang, S.; Guo, C.; Liu, M.; Tao, Q. Peppermint Essential Oil: Its Phytochemistry, Biological Activity, Pharmacological Effect and Application. Biomed. Pharmacother. 2022, 154, 113559. [Google Scholar] [CrossRef]
- El Yaagoubi, M.; Mechqoq, H.; El Hamdaoui, A.; Jrv Mukku, V.; El Mousadik, A.; Msanda, F.; El Aouad, N. A Review on Moroccan Thymus Species: Traditional Uses, Essential Oils Chemical Composition and Biological Effects. J. Ethnopharmacol. 2021, 278, 114205. [Google Scholar] [CrossRef]
- Kesraoui, S.; Andrés, M.F.; Berrocal-Lobo, M.; Soudani, S.; Gonzalez-Coloma, A. Direct and Indirect Effects of Essential Oils for Sustainable Crop Protection. Plants 2022, 11, 2144. [Google Scholar] [CrossRef]
- Alonso-Gato, M.; Astray, G.; Mejuto, J.C.; Simal-Gandara, J. Essential Oils as Antimicrobials in Crop Protection. Antibiotics 2021, 10, 34. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef]
- Durofil, A.; Radice, M.; Blanco-Salas, J.; Ruiz-Téllez, T. Piper Aduncum Essential Oil: A Promising Insecticide, Acaricide and Antiparasitic. A Review. Parasite 2021, 28, 42. [Google Scholar] [CrossRef] [PubMed]
- Osanloo, M.; Sedaghat, M.M.; Sanei-Dehkordi, A.; Amani, A. Plant-Derived Essential Oils; Their Larvicidal Properties and Potential Application for Control of Mosquito-Borne Diseases. Galen Med. J. 2019, 8, 1532. [Google Scholar] [CrossRef] [PubMed]
- Silvério, M.R.S.; Espindola, L.S.; Lopes, N.P.; Vieira, P.C. Plant Natural Products for the Control of Aedes Aegypti: The Main Vector of Important Arboviruses. Molecules 2020, 25, 3484. [Google Scholar] [CrossRef] [PubMed]
- Luna, E.C.; Luna, I.S.; Scotti, L.; Monteiro, A.F.M.; Scotti, M.T.; De Moura, R.O.; De Araújo, R.S.A.; Monteiro, K.L.C.; De Aquino, T.M.; Ribeiro, F.F.; et al. Active Essential Oils and Their Components in Use against Neglected Diseases and Arboviruses. Oxid. Med. Cell. Longev. 2019, 2019, 52. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y. Essential Oils as Repellents against Arthropods. Biomed Res. Int. 2018, 2018, 9. [Google Scholar] [CrossRef] [PubMed]


| Country | Species | Part | Extract | Alpha-Phellandrene (%) | Ref. |
|---|---|---|---|---|---|
| Alabama | Curcuma longa | Rhizomes | HD | 11.8 | [12] |
| Algeria | Pituranthos scoparius | Stems | HD | 7.1 | [13] |
| Argentina | Eucalyptus tereticornis | Seeds | HD | 9.4 | [14] |
| Australia | Eucalyptus calcicola | Leaves | VD | 11.0 | [15] |
| Australia | Eucalyptus incerata | Leaves | VD | 6.3 | [16] |
| Brazil | Aniba rosiodora | Aerial parts | HD | 22.8 | [17] |
| Brazil | Lantana camara | Aerial parts | HD | 16.4 | [18] |
| Brazil | Piper diltatum | Leaves | HD | 22.5 | [19] |
| Bulgaria | Anethumgraveolens | Aerial parts | HD | 22.7 | [20] |
| Canada | Canarium ovatum | Resin | HD | 64.9 | [21] |
| Colombia | Lippia origanoides | Aerial parts | HD | 13.0 | [22] |
| Ecuador | Gynoxys miniphylla | Leaves | SD | 16.6 | [23] |
| France | Limbarda crithmoides | Aerial parts | HD | 11.9 | [24] |
| Germany | Anethumgraveolens | Aerial parts | SD | 32.3 | [25] |
| India | Altingia excelsa | Leaves | HD | 15.9 | [26] |
| India | Angelica glauca | Aerial parts | HD | 13.5 | [27] |
| India | Anethumgraveolens | Aerial parts | HD | 19.1 | [28] |
| India | Callistemon lanceolatus | Leaves | HD | 5.8 | [29] |
| India | Cinnamomum zeylanicum | Leaves | HD | 6.2 | [30] |
| India | Curcuma longa | Leaves | HD | 24.4 | [31] |
| India | Eucalyptus camaldulensis | Aerial parts | HD | 27.5 | [32] |
| India | Hyptis suaveolens | Leaves | HD | 22.8 | [33] |
| Iran | Anethum graveolens | Aerial parts | HD | 30.2 | [34] |
| Iran | Anethum graveolens | Aerial parts | HD | 32.0 | [35] |
| Italy | Elaeoselinum ascelpium | Flowers | HD | 42.5 | [36] |
| Italy | Elaeoselinum ascelpium | Leaves | HD | 11.0 | [36] |
| Italy | Foeniculum vulgare | Aerial parts | SD | 82.1 | [37] |
| Italy | Foeniculum vulgare | Stems | HD | 36.9 | [38] |
| Italy | Ridolfia segetum | Flowers | HD | 19.4 | [39] |
| Italy | Ridolfia segetum | Stems | HD | 12.9 | [39] |
| Japan | Petasites japonicus | Flower stems | HD | 11.0 | [40] |
| Morocco | Schinus molle | Aerial parts | HD | 9.6 | [41] |
| Nigeria | Monodora myristica | Seeds | n.r. | 53.0 | [42] |
| Portugal | Eucalyptus globulus | Fruits | SD | 17.2 | [43] |
| Portugal | Lavandula pinnata | Flowers | SD | 15.9 | [44] |
| Spain | Anethum graveolens | Aerial parts | HD | 70.2 | [45] |
| Spain | Sideritis hirsute | Aerial parts | SD | 9.2 | [46] |
| Tasmania | Anethum graveolens | Aerial parts | n.r. | 49.1 | [47] |
| Tunisia | Citharexylum spinosum | Roots | HD | 30.8 | [48] |
| Tunisia | Schinus mole | Fruits | HD | 35.9 | [49] |
| Tunisia | Schinus molle | Fruits | SD | 46.5 | [50] |
| Tunisia | Schinus mole | Leaves | SD | 35.7 | [51] |
| Tunisia | Schinus terebinthifolius | Fruits | SD | 34.4 | [50] |
| Tunisia | Schinus terebintifolius | Fruits | SD | 44.3 | [51] |
| Turkey | Echinophora tenuifolia | Aerial parts | SD | 51.0 | [52] |
| Turkey | Foeniculum vulgare | Flowers | HD | 5.8 | [53] |
| Uzbekistan | Heracleum lehmannianum | Aerial parts | HD | 10.5 | [54] |
| Venezuela | Espeletia schultzii | Leaves | HD | 52.4 | [55] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radice, M.; Durofil, A.; Buzzi, R.; Baldini, E.; Martínez, A.P.; Scalvenzi, L.; Manfredini, S. Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications. Life 2022, 12, 1602. https://doi.org/10.3390/life12101602
Radice M, Durofil A, Buzzi R, Baldini E, Martínez AP, Scalvenzi L, Manfredini S. Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications. Life. 2022; 12(10):1602. https://doi.org/10.3390/life12101602
Chicago/Turabian StyleRadice, Matteo, Andrea Durofil, Raissa Buzzi, Erika Baldini, Amaury Pérez Martínez, Laura Scalvenzi, and Stefano Manfredini. 2022. "Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications" Life 12, no. 10: 1602. https://doi.org/10.3390/life12101602
APA StyleRadice, M., Durofil, A., Buzzi, R., Baldini, E., Martínez, A. P., Scalvenzi, L., & Manfredini, S. (2022). Alpha-Phellandrene and Alpha-Phellandrene-Rich Essential Oils: A Systematic Review of Biological Activities, Pharmaceutical and Food Applications. Life, 12(10), 1602. https://doi.org/10.3390/life12101602









