Adulthood Psychosocial Disadvantages and Risk of Hypertension in U.S. Workers: Effect Modification by Adverse Childhood Experiences
Abstract
1. Introduction
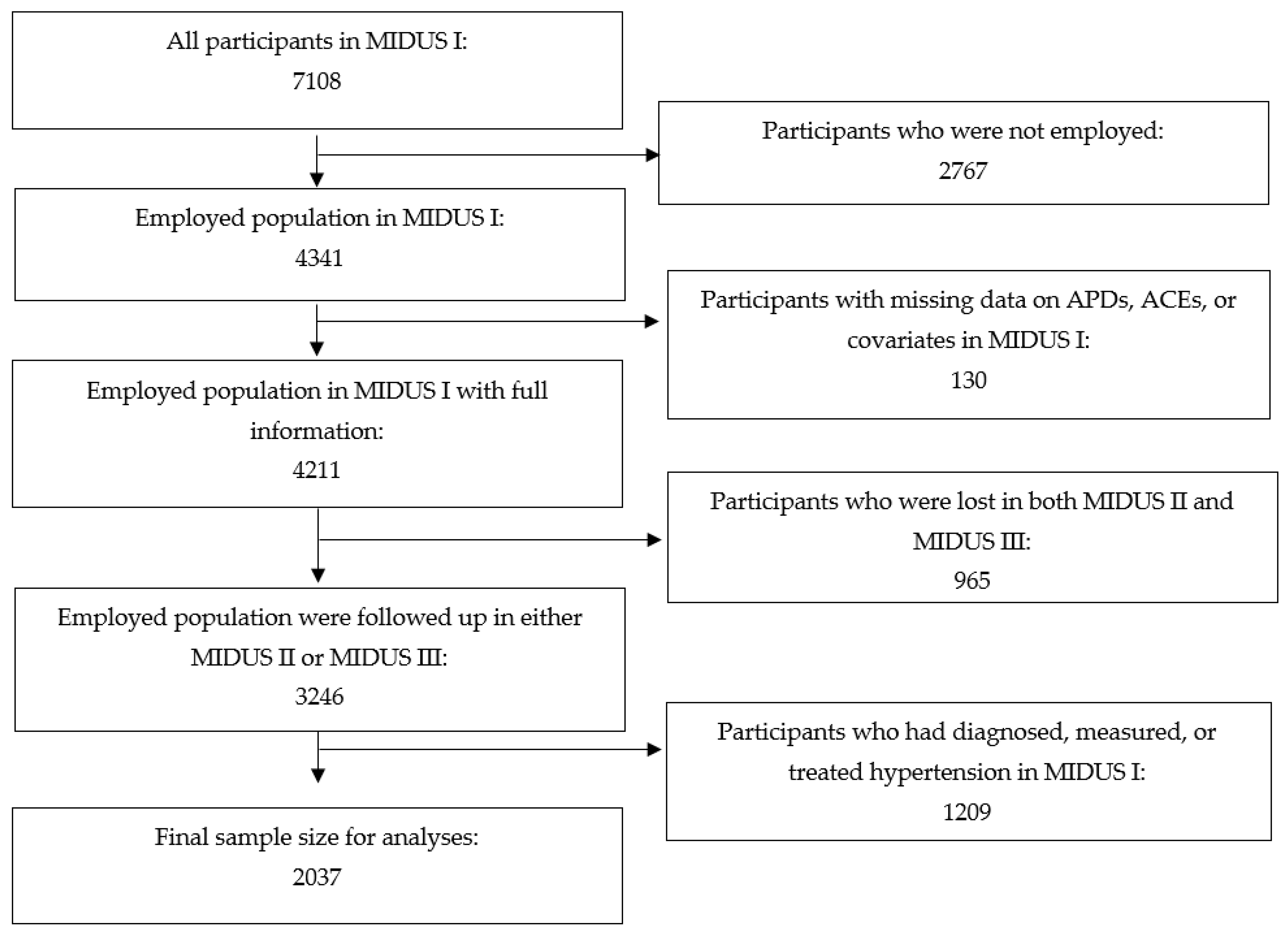
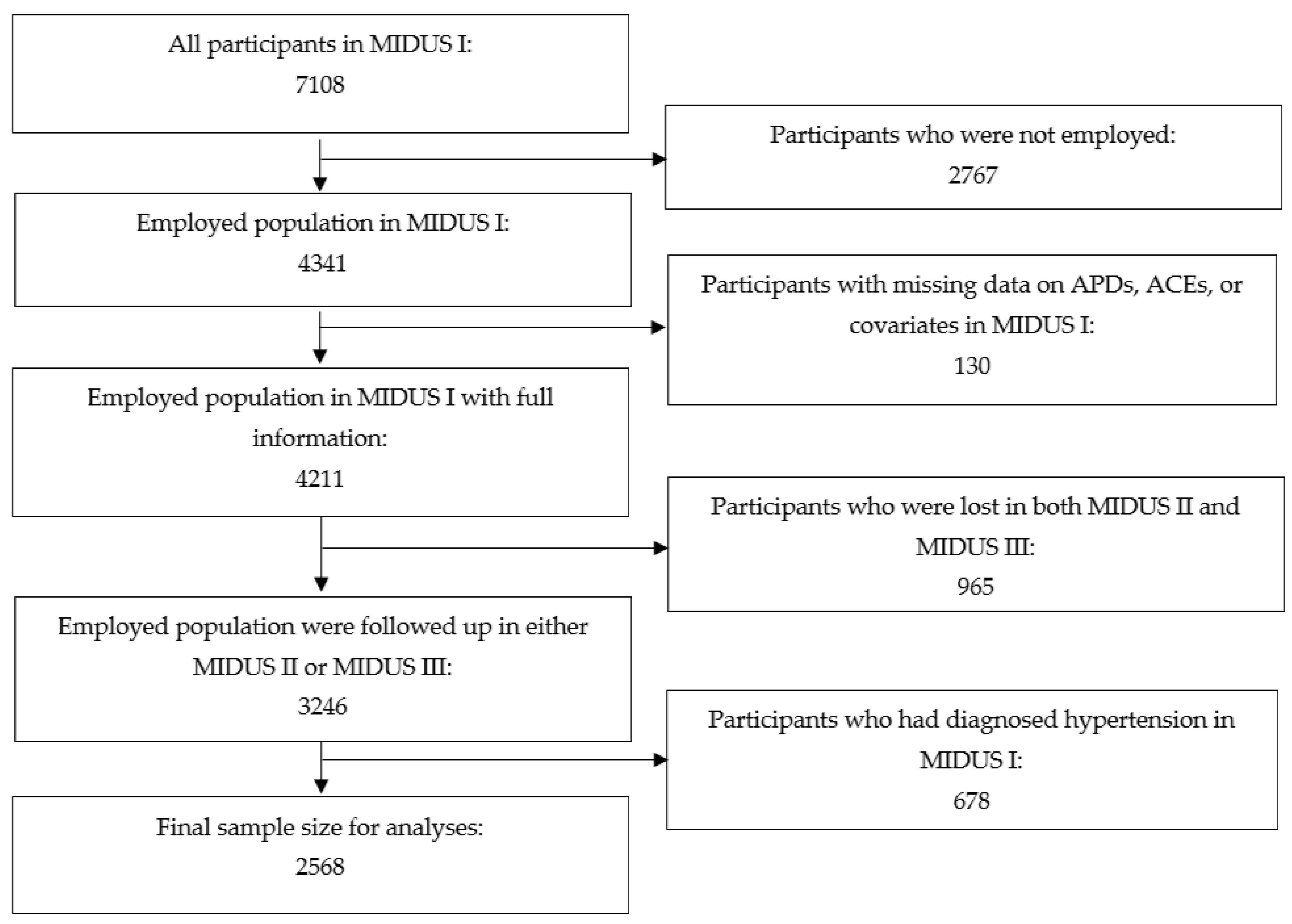
2. Materials and Methods
2.1. Sample Population
2.2. Materials and Measures
2.3. Outcome
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Participant Characteristics
3.2. Associations of Adverse Childhood Experiences and Adulthood Psychosocial Disadvantages at Baseline with Risk of Hypertension
3.3. Effect Modification of Adverse Childhood Experiences
3.4. Sensitivity Analyses
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Variables (n, %) | |
|---|---|
| Mean age (SD) | 42.12 (10.35) |
| Sex | |
| Male | 980 (48.11) |
| Female | 1057 (51.89) |
| Race | |
| White | 1888 (92.69) |
| Black | 70 (3.44) |
| Non-white | 79 (3.88) |
| Educational attainment | |
| University or more | 817 (40.11) |
| Some college | 608 (29.85) |
| High school or less | 612 (30.04) |
| Household income (annual USD) | |
| <45,000 | 683 (33.53) |
| 45,000–89,999 | 736 (36.13) |
| ≥90,000 | 618 (30.34) |
| Smoking status | |
| No | 1610 (79.04) |
| Yes | 427 (20.96) |
| Alcohol consumption | |
| Low to moderate drinking | 1943 (95.39) |
| Heavy drinking | 94 (4.61) |
| Physical activity | |
| High | 1482 (72.75) |
| Moderate | 371 (18.21) |
| Low | 184 (9.03) |
| Major depressive episode | |
| No | 1815 (89.10) |
| Yes | 222 (10.90) |
| Adverse childhood experiences | |
| Low | 1249 (61.32) |
| High | 788 (38.68) |
| Adulthood psychosocial disadvantages | |
| Low | 1356 (66.57) |
| Moderate | 604 (29.65) |
| High | 77 (3.78) |
| Incident hypertension | |
| No | 1351 (66.32) |
| Yes | 686 (33.68) |
| Number of Exposed Participants (Number of Incident Hypertension Cases) | Incidence Rate of Hypertension (Per 1000 Person Years) | Model 0 | Model I | Model II | Model III | Model IV | |
|---|---|---|---|---|---|---|---|
| ACEs | |||||||
| Low | 1249 (400) | 23.01 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| High | 788 (286) | 26.66 | 1.16 (0.99, 1.35) | 1.13 (0.97, 1.31) | 1.07 (0.92, 1.25) | 1.04 (0.89, 1.22) | 1.04 (0.89, 1.22) |
| APDs | |||||||
| Low | 1356 (431) | 22.88 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate | 604 (216) | 26.21 | 1.14 (0.97, 1.35) | 1.20 (1.02, 1.42) * | 1.18 (1.00, 1.40) * | 1.16 (0.98, 1.37) | 1.15 (0.98, 1.36) |
| High | 77 (39) | 37.97 | 1.67 (1.20, 2.32) ** | 1.80 (1.30, 2.50) ** | 1.71 (1.22, 2.39) ** | 1.62 (1.15, 2.27) ** | 1.61 (1.15, 2.26) ** |
| Number of Exposed Participants (Number of Incident Hypertension Cases) | Incidence Rate of Hypertension (Per 1000 Person Years) | Model 0 | Model I | Model II | Model III | Model IV | |
|---|---|---|---|---|---|---|---|
| ACEs (low) (n = 1249) | |||||||
| APDs | |||||||
| Low | 871 (274) | 25.16 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate | 336 (108) | 27.98 | 1.03 (0.83, 1.29) | 1.11 (0.89, 1.39) | 1.09 (0.87, 1.36) | 1.06 (0.84, 1.33) | 1.06 (0.84, 1.32) |
| High | 42 (18) | 36.57 | 1.39 (0.86, 2.24) | 1.54 (0.95, 2.48) | 1.49 (0.92, 2.41) | 1.38 (0.85, 2.24) | 1.36 (0.83, 2.22) |
| ACEs (high) (n = 788) | |||||||
| APDs | |||||||
| Low | 485 (157) | 23.51 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate | 268 (108) | 30.03 | 1.30 (1.01, 1.66) * | 1.34 (1.05, 1.72) * | 1.36 (1.06, 1.76) * | 1.35 (1.04, 1.74) * | 1.35 (1.04, 1.75) * |
| High | 35 (21) | 46.37 | 2.07 (1.31, 3.63) ** | 2.14 (1.35, 3.37) ** | 2.04 (1.27, 3.27) ** | 2.11 (1.31, 3.39) ** | 2.11 (1.31, 3.40) ** |
References
- Tsao, C.W.; Aday, A.W.; Almarzooq, Z.I.; Alonso, A.; Beaton, A.Z.; Bittencourt, M.S.; Boehme, A.K.; Buxton, A.E.; Carson, A.P.; Commodore-Mensah, Y.; et al. Heart Disease and Stroke Statistics—2022 Update: A Report From the American Heart Association. Circulation 2022, 145, e153–e639. [Google Scholar] [CrossRef] [PubMed]
- Andersson, C.; Vasan, R.S. Epidemiology of Cardiovascular Disease in Young Individuals. Nat. Rev. Cardiol. 2018, 15, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Harris, K.M.; Woolf, S.H.; Gaskin, D.J. High and Rising Working-Age Mortality in the US: A Report From the National Academies of Sciences, Engineering, and Medicine. JAMA 2021, 325, 2045–2046. [Google Scholar] [CrossRef] [PubMed]
- GBD 2017 Risk Factor Collaborators. Global, Regional, and National Comparative Risk Assessment of 84 Behavioural, Environmental and Occupational, and Metabolic Risks or Clusters of Risks for 195 Countries and Territories, 1990-2017: A Systematic Analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1923–1994. [Google Scholar] [CrossRef]
- Liu, M.-Y.; Li, N.; Li, W.A.; Khan, H. Association between Psychosocial Stress and Hypertension: A Systematic Review and Meta-Analysis. Neurol. Res. 2017, 39, 573–580. [Google Scholar] [CrossRef]
- Babu, G.R.; Jotheeswaran, A.T.; Mahapatra, T.; Mahapatra, S.; Kumar, A.; Detels, R.; Pearce, N. Is Hypertension Associated with Job Strain? A Meta-Analysis of Observational Studies. Occup. Environ. Med. 2014, 71, 220–227. [Google Scholar] [CrossRef]
- Gilbert-Ouimet, M.; Trudel, X.; Brisson, C.; Milot, A.; Vézina, M. Adverse Effects of Psychosocial Work Factors on Blood Pressure: Systematic Review of Studies on Demand-Control-Support and Effort-Reward Imbalance Models. Scand. J. Work. Environ. Health 2014, 40, 109–132. [Google Scholar] [CrossRef]
- Xia, N.; Li, H. Loneliness, Social Isolation, and Cardiovascular Health. Antioxid. Redox Signal. 2018, 28, 837–851. [Google Scholar] [CrossRef]
- Naito, R.; Leong, D.P.; Bangdiwala, S.I.; McKee, M.; Subramanian, S.V.; Rangarajan, S.; Islam, S.; Avezum, A.; Yeates, K.E.; Lear, S.A.; et al. Impact of Social Isolation on Mortality and Morbidity in 20 High-Income, Middle-Income and Low-Income Countries in Five Continents. BMJ Glob. Health 2021, 6, e004124. [Google Scholar] [CrossRef]
- Holt-Lunstad, J.; Smith, T.B. Loneliness and Social Isolation as Risk Factors for CVD: Implications for Evidence-Based Patient Care and Scientific Inquiry. Heart 2016, 102, 987–989. [Google Scholar] [CrossRef]
- Nakagomi, A.; Yasufuku, Y.; Ueno, T.; Kondo, K. Social Determinants of Hypertension in High-Income Countries: A Narrative Literature Review and Future Directions. Hypertens. Res. 2022, 45, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Suglia, S.F.; Koenen, K.C.; Boynton-Jarrett, R.; Chan, P.S.; Clark, C.J.; Danese, A.; Faith, M.S.; Goldstein, B.I.; Hayman, L.L.; Isasi, C.R.; et al. Childhood and Adolescent Adversity and Cardiometabolic Outcomes: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e15–e28. [Google Scholar] [CrossRef] [PubMed]
- Obi, I.E.; McPherson, K.C.; Pollock, J.S. Childhood Adversity and Mechanistic Links to Hypertension Risk in Adulthood. Br. J. Pharmacol. 2019, 176, 1932–1950. [Google Scholar] [CrossRef]
- Yang, Y.C.; Boen, C.; Gerken, K.; Li, T.; Schorpp, K.; Harris, K.M. Social Relationships and Physiological Determinants of Longevity across the Human Life Span. Proc. Natl. Acad. Sci. USA 2016, 113, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Pulkki-Råback, L.; Elovainio, M.; Virtanen, M.; Kivimäki, M.; Hintsanen, M.; Hintsa, T.; Jokela, M.; Puttonen, S.; Joensuu, M.; Lipsanen, J.; et al. Job Demands and Job Control as Predictors of Depressive Symptoms: Moderating Effects of Negative Childhood Socioemotional Experiences. Stress Health J. Int. Soc. Investig. Stress 2016, 32, 383–394. [Google Scholar] [CrossRef]
- Westerlund, H.; Gustafsson, P.E.; Theorell, T.; Janlert, U.; Hammarström, A. Social Adversity in Adolescence Increases the Physiological Vulnerability to Job Strain in Adulthood: A Prospective Population-Based Study. PLoS ONE 2012, 7, e35967. [Google Scholar] [CrossRef]
- Hemmingsson, T.; Lundberg, I. Is the Association between Low Job Control and Coronary Heart Disease Confounded by Risk Factors Measured in Childhood and Adolescence among Swedish Males 40–53 Years of Age? Int. J. Epidemiol. 2006, 35, 616–622. [Google Scholar] [CrossRef]
- Thomas, C.; Power, C. Do Early Life Exposures Explain Associations in Mid-Adulthood between Workplace Factors and Risk Factors for Cardiovascular Disease? Int. J. Epidemiol. 2010, 39, 812–824. [Google Scholar] [CrossRef]
- Kivimäki, M.; Hintsanen, M.; Keltikangas-Järvinen, L.; Elovainio, M.; Pulkki-Råback, L.; Vahtera, J.; Viikari, J.S.A.; Raitakari, O.T. Early Risk Factors, Job Strain, and Atherosclerosis Among Men in Their 30s: The Cardiovascular Risk in Young Finns Study. Am. J. Public Health 2007, 97, 450–452. [Google Scholar] [CrossRef]
- Hintsa, T.; Shipley, M.J.; Gimeno, D.; Elovainio, M.; Chandola, T.; Jokela, M.; Keltikangas-Järvinen, L.; Vahtera, J.; Marmot, M.G.; Kivimäki, M. Do Pre-Employment Influences Explain the Association between Psychosocial Factors at Work and Coronary Heart Disease? The Whitehall II Study. Occup. Environ. Med. 2010, 67, 330–334. [Google Scholar] [CrossRef]
- Gilbert-Ouimet, M.; Brisson, C.; Milot, A.; Vézina, M. Double Exposure to Adverse Psychosocial Work Factors and High Family Responsibilities as Related to Ambulatory Blood Pressure at Work: A 5-Year Prospective Study in Women With White-Collar Jobs. Psychosom. Med. 2017, 79, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Brim, O.G.; Baltes, P.B.; Bumpass, L.L.; Cleary, P.D.; Featherman, D.L.; Hazzard, W.R.; Kessler, R.C.; Lachman, M.E.; Markus, H.R.; Marmot, M.G.; et al. Midlife in the United States (MIDUS 1), 1995–1996 1996. Available online: https://www.icpsr.umich.edu/web/ICPSR/studies/2760 (accessed on 3 March 2022).
- Ryff, C.; Almeida, D.; Ayanian, J.; Binkley, N.; Carr, D.S.; Coe, C.; Davidson, R.; Grzywacz, J.; Karlamangla, A.; Krueger, R.; et al. Midlife in the United States (MIDUS 3), 2013–2014 2019. Available online: https://www.icpsr.umich.edu/web/ICPSR/studies/4652 (accessed on 3 March 2022).
- Ryff, C.; Almeida, D.M.; Ayanian, J.; Carr, D.S.; Cleary, P.D.; Coe, C.; Davidson, R.; Krueger, R.F.; Lachman, M.E.; Marks, N.F.; et al. Midlife in the United States (MIDUS 2), 2004–2006 2017. Available online: https://www.icpsr.umich.edu/web/ICPSR/studies/36346 (accessed on 3 March 2022).
- Friedman, E.M.; Karlamangla, A.S.; Gruenewald, T.L.; Koretz, B.; Seeman, T.E. Early Life Adversity and Adult Biological Risk Profiles. Psychosom. Med. 2015, 77, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Alhowaymel, F.; Kalmakis, K.; Jacelon, C. Developing the Concept of Adverse Childhood Experiences: A Global Perspective. J. Pediatr. Nurs. 2021, 56, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.C.; Merrick, M.T.; Parks, S.E.; Breiding, M.J.; Gilbert, L.K.; Edwards, V.J.; Dhingra, S.S.; Barile, J.P.; Thompson, W.W. Examination of the Factorial Structure of Adverse Childhood Experiences and Recommendations for Three Subscale Scores. Psychol. Violence 2014, 4, 432–444. [Google Scholar] [CrossRef] [PubMed]
- Karasek, R.A. Job Demands, Job Decision Latitude, and Mental Strain: Implications for Job Redesign. Adm. Sci. Q. 1979, 24, 285. [Google Scholar] [CrossRef]
- Karasek, R.; Brisson, C.; Kawakami, N.; Houtman, I.; Bongers, P.; Amick, B. The Job Content Questionnaire (JCQ): An Instrument for Internationally Comparative Assessments of Psychosocial Job Characteristics. J. Occup. Health Psychol. 1998, 3, 322–355. [Google Scholar] [CrossRef]
- Matthews, T.A.; Robbins, W.; Preisig, M.; von Känel, R.; Li, J. Associations of Job Strain and Family Strain with Risk of Major Depressive Episode: A Prospective Cohort Study in U.S. Working Men and Women. J. Psychosom. Res. 2021, 147, 110541. [Google Scholar] [CrossRef]
- Matthews, T.A.; Chen, L.; Li, J. Increased Job Strain and Cardiovascular Disease Mortality: A Prospective Cohort Study in U.S. Workers. Ind. Health 2022, 2021-0233. [Google Scholar] [CrossRef]
- Berkman, L.F.; Syme, S.L. Social Networks, Host Resistance, and Mortality: A Nine-Year Follow-up Study of Alameda County Residents. Am. J. Epidemiol. 1979, 109, 186–204. [Google Scholar] [CrossRef]
- Liu, X.; Matthews, T.A.; Chen, L.; Li, J. Job Strain and Leisure-Time Physical Activity on Risk of Hypertension: The Population-Based Midlife in the United States Cohort Study. Epidemiol. Health 2022, e2022073. [Google Scholar] [CrossRef]
- Capistrant, B.D.; Moon, J.R.; Glymour, M.M. Spousal Caregiving and Incident Hypertension. Am. J. Hypertens. 2012, 25, 437–443. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020–2025; U.S. Department of Agriculture and U.S. Department of Health and Human Services: Washington, DC, USA, 2020.
- Li, J.; Matthews, T.A.; Chen, L.; Seamans, M.; Leineweber, C.; Siegrist, J. Effort–Reward Imbalance at Work and Drug Misuse: Evidence from a National Survey in the U.S. Int. J. Environ. Res. Public. Health 2021, 18, 13334. [Google Scholar] [CrossRef] [PubMed]
- US Preventive Services Task Force Screening for Hypertension in Adults: US Preventive Services Task Force Reaffirmation Recommendation Statement. JAMA 2021, 325, 1650–1656. [CrossRef]
- Meng, L.; Chen, D.; Yang, Y.; Zheng, Y.; Hui, R. Depression Increases the Risk of Hypertension Incidence: A Meta-Analysis of Prospective Cohort Studies. J. Hypertens. 2012, 30, 842–851. [Google Scholar] [CrossRef] [PubMed]
- Muntner, P.; Carey, R.M.; Gidding, S.; Jones, D.W.; Taler, S.J.; Wright, J.T.; Whelton, P.K. Potential US Population Impact of the 2017 ACC/AHA High Blood Pressure Guideline. Circulation 2018, 137, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Cuffee, Y.; Ogedegbe, C.; Williams, N.J.; Ogedegbe, G.; Schoenthaler, A. Psychosocial Risk Factors for Hypertension: An Update of the Literature. Curr. Hypertens. Rep. 2014, 16, 483. [Google Scholar] [CrossRef]
- Ben-Shlomo, Y.; Kuh, D. A Life Course Approach to Chronic Disease Epidemiology: Conceptual Models, Empirical Challenges and Interdisciplinary Perspectives. Int. J. Epidemiol. 2002, 31, 285–293. [Google Scholar] [CrossRef]
- Kuh, D.; Ben-Shlomo, Y.; Lynch, J.; Hallqvist, J.; Power, C. Life Course Epidemiology. J. Epidemiol. Community Health 2003, 57, 778–783. [Google Scholar] [CrossRef]
- Baron, R.M.; Kenny, D.A. The Moderator-Mediator Variable Distinction in Social Psychological Research: Conceptual, Strategic, and Statistical Considerations. J. Pers. Soc. Psychol. 1986, 51, 1173–1182. [Google Scholar] [CrossRef]
- Belsky, J.; Jonassaint, C.; Pluess, M.; Stanton, M.; Brummett, B.; Williams, R. Vulnerability Genes or Plasticity Genes? Mol. Psychiatry 2009, 14, 746–754. [Google Scholar] [CrossRef]
- Gunnar, M.R.; Frenn, K.; Wewerka, S.S.; Ryzin, M.J.V. Moderate versus Severe Early Life Stress: Associations with Stress Reactivity and Regulation in 10–12-Year-Old Children. Psychoneuroendocrinology 2009, 34, 62. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.; Matthews, K.A. Cognitive Appraisal Biases: An Approach to Understanding the Relation between Socioeconomic Status and Cardiovascular Reactivity in Children. Ann. Behav. Med. 2001, 23, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hintsanen, M.; Kivimäki, M.; Hintsa, T.; Theorell, T.; Elovainio, M.; Raitakari, O.T.; Viikari, J.S.A.; Keltikangas-Järvinen, L. A Prospective Cohort Study of Deficient Maternal Nurturing Attitudes Predicting Adulthood Work Stress Independent of Adulthood Hostility and Depressive Symptoms. Stress 2010, 13, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Loman, M.M.; Gunnar, M.R. Early Experience and the Development of Stress Reactivity and Regulation in Children. Neurosci. Biobehav. Rev. 2010, 34, 867–876. [Google Scholar] [CrossRef]
- Wiley, J.F.; Bei, B.; Bower, J.E.; Stanton, A.L. Relationship of Psychosocial Resources with Allostatic Load: A Systematic Review. Psychosom. Med. 2017, 79, 283–292. [Google Scholar] [CrossRef]
- Kivimäki, M.; Steptoe, A. Effects of Stress on the Development and Progression of Cardiovascular Disease. Nat. Rev. Cardiol. 2018, 15, 215–229. [Google Scholar] [CrossRef]
- McEwen, B.S. Stress, Adaptation, and Disease: Allostasis and Allostatic Load. Ann. N. Y. Acad. Sci. 1998, 840, 33–44. [Google Scholar] [CrossRef]
- Dube, S.R.; Williamson, D.F.; Thompson, T.; Felitti, V.J.; Anda, R.F. Assessing the Reliability of Retrospective Reports of Adverse Childhood Experiences among Adult HMO Members Attending a Primary Care Clinic. Child Abus. Negl. 2004, 28, 729–737. [Google Scholar] [CrossRef]
- Hardt, J.; Rutter, M. Validity of Adult Retrospective Reports of Adverse Childhood Experiences: Review of the Evidence. J. Child Psychol. Psychiatry 2004, 45, 260–273. [Google Scholar] [CrossRef]
- Gonçalves, V.S.S.; Andrade, K.R.C.; Carvalho, K.M.B.; Silva, M.T.; Pereira, M.G.; Galvao, T.F. Accuracy of Self-Reported Hypertension: A Systematic Review and Meta-Analysis. J. Hypertens. 2018, 36, 970–978. [Google Scholar] [CrossRef]
| Variables (n, %) | |
|---|---|
| Mean age (SD) | 42.89 (10.48) |
| Sex | |
| Male | 1301 (50.66) |
| Female | 1267 (49.34) |
| Race | |
| White | 2388 (92.99) |
| Black | 82 (3.19) |
| Other | 98 (3.82) |
| Educational attainment | |
| University or more | 1033 (40.23) |
| Some college | 784 (30.53) |
| High school or less | 751 (29.24) |
| Household income (annual USD) | |
| <45,000 | 883 (34.38) |
| 45,000–89,999 | 928 (36.14) |
| ≥90,000 | 757 (29.48) |
| Smoking status | |
| No | 2034 (79.21) |
| Yes | 534 (20.79) |
| Alcohol consumption | |
| Low to moderate drinking | 2437 (94.90) |
| Heavy drinking | 131 (5.10) |
| Physical activity | |
| High | 1842 (71.73) |
| Moderate | 491 (19.12) |
| Low | 235 (9.15) |
| Major depressive episode | |
| No | 2283 (88.90) |
| Yes | 285 (11.10) |
| Adverse childhood experiences | |
| Low | 1576 (61.37) |
| High | 992 (38.63) |
| Adulthood psychosocial disadvantages | |
| Low | 1733 (67.48) |
| Moderate | 742 (28.89) |
| High | 93 (3.62) |
| Incident hypertension | |
| No | 1634 (63.63) |
| Yes | 934 (36.37) |
| Number of Exposed Participants (Number of Incident Hypertension Cases) | Incidence Rate of Hypertension (Per 1000 Person Years) | Model 0 | Model I | Model II | Model III | Model IV | |
|---|---|---|---|---|---|---|---|
| ACEs | |||||||
| Low | 1576 (562) | 26.05 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| High | 992 (372) | 27.73 | 1.06 (0.93, 1.21) | 1.03 (0.90, 1.17) | 0.99 (0.86, 1.13) | 0.96 (0.84, 1.11) | 0.96 (0.84, 1.10) |
| APDs | |||||||
| Low | 1733 (607) | 25.53 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate | 742 (281) | 28.14 | 1.11 (0.96, 1.28) | 1.20 (1.04, 1.39) * | 1.17 (1.02, 1.35) * | 1.15 (0.99, 1.32) | 1.14 (0.99, 1.32) |
| High | 93 (46) | 37.40 | 1.48 (1.10, 2.00) * | 1.66 (1.23, 2.24) ** | 1.55 (1.15, 2.11) ** | 1.48 (1.09, 2.01) * | 1.48 (1.09, 2.01) * |
| Number of Exposed Participants (Number of Incident Hypertension Cases) | Incidence Rate of Hypertension (Per 1000 Person Years) | Model 0 | Model I | Model II | Model III | Model IV | |
|---|---|---|---|---|---|---|---|
| ACEs (low) (n = 1576) | |||||||
| APDs | |||||||
| Low | 1103 (386) | 25.52 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate | 422 (153) | 26.58 | 1.05 (0.87, 1.26) | 1.14 (0.94, 1.37) | 1.10 (0.91, 1.33) | 1.08 (0.89, 1.30) | 1.08 (0.89, 1.30) |
| High | 51 (23) | 33.09 | 1.29 (0.85, 1.96) | 1.49 (0.98, 2.28) | 1.43 (0.93, 2.19) | 1.35 (0.88, 2.08) | 1.34 (0.87, 2.06) |
| ACEs (high) (n = 992) | |||||||
| APDs | |||||||
| Low | 630 (221) | 25.95 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Moderate | 320 (128) | 30.27 | 1.21 (0.97, 1.50) | 1.30 (1.04, 1.61) * | 1.29 (1.03, 1.61) * | 1.27 (1.01, 1.59) * | 1.27 (1.01, 1.60) * |
| High | 42 (23) | 43.00 | 1.76 (1.14, 2.70) * | 1.88 (1.22, 2.90) ** | 1.81 (1.16, 2.81) ** | 1.83 (1.17, 2.85) ** | 1.83 (1.17, 2.86) ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matthews, T.A.; Zhu, Y.; Robbins, W.; Rezk-Hanna, M.; Macey, P.M.; Song, Y.; Li, J. Adulthood Psychosocial Disadvantages and Risk of Hypertension in U.S. Workers: Effect Modification by Adverse Childhood Experiences. Life 2022, 12, 1507. https://doi.org/10.3390/life12101507
Matthews TA, Zhu Y, Robbins W, Rezk-Hanna M, Macey PM, Song Y, Li J. Adulthood Psychosocial Disadvantages and Risk of Hypertension in U.S. Workers: Effect Modification by Adverse Childhood Experiences. Life. 2022; 12(10):1507. https://doi.org/10.3390/life12101507
Chicago/Turabian StyleMatthews, Timothy A., Yifang Zhu, Wendie Robbins, Mary Rezk-Hanna, Paul M. Macey, Yeonsu Song, and Jian Li. 2022. "Adulthood Psychosocial Disadvantages and Risk of Hypertension in U.S. Workers: Effect Modification by Adverse Childhood Experiences" Life 12, no. 10: 1507. https://doi.org/10.3390/life12101507
APA StyleMatthews, T. A., Zhu, Y., Robbins, W., Rezk-Hanna, M., Macey, P. M., Song, Y., & Li, J. (2022). Adulthood Psychosocial Disadvantages and Risk of Hypertension in U.S. Workers: Effect Modification by Adverse Childhood Experiences. Life, 12(10), 1507. https://doi.org/10.3390/life12101507






