Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review
Abstract
:1. Introduction
2. Methods
2.1. Protocol Registration
2.2. Search Strategy
2.3. Inclusion and Exclusion Criteria
2.4. Article Selection and Data Extraction
2.5. Quality Assessment
2.6. Data Synthesis
3. Results
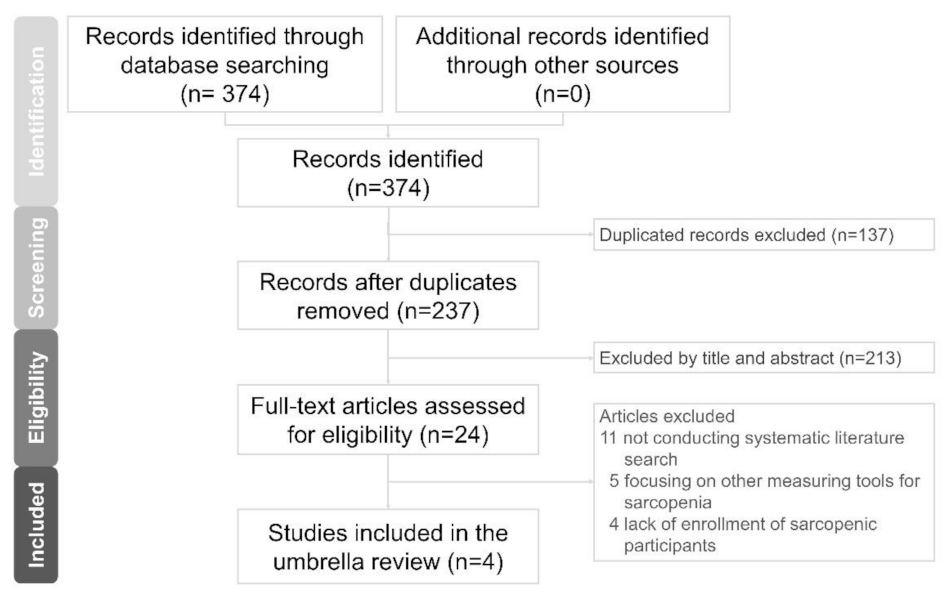
3.1. Literature Search
3.2. Study Characteristics
3.3. Methodological Quality of the Included Studies
3.4. Summary of the Outcome
3.4.1. Reliability and Validity of US Measurements
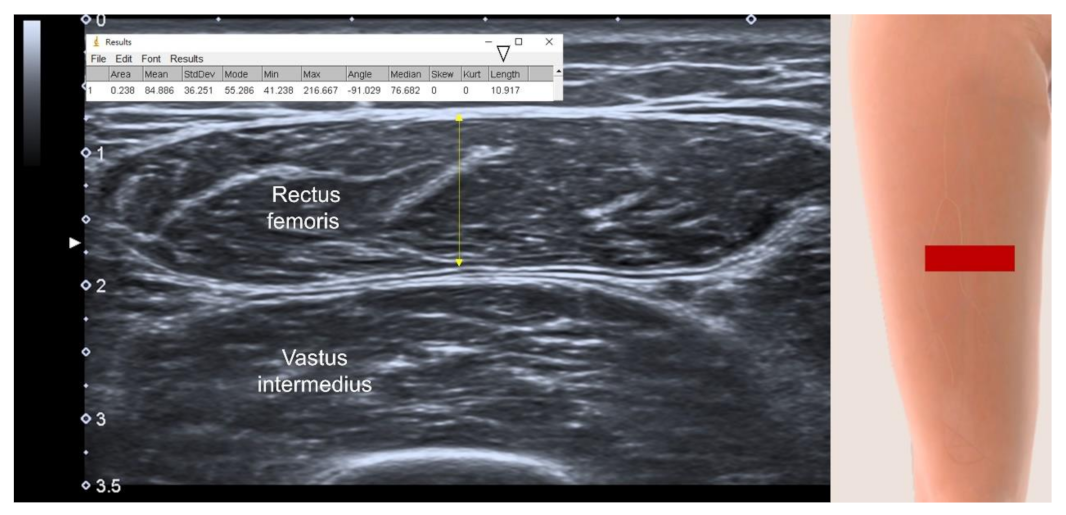
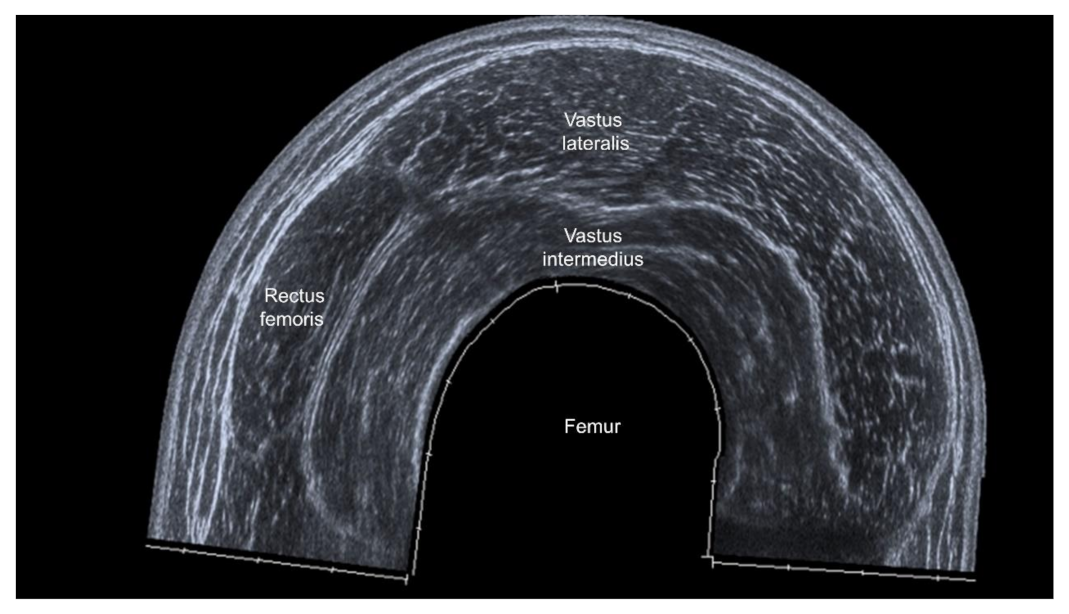
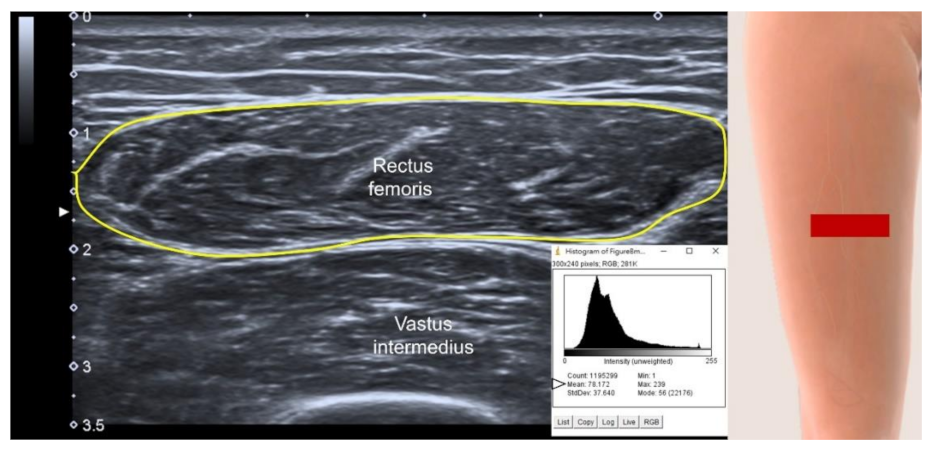
3.4.2. Sites of Muscle Measurement
3.4.3. US parameters in B-Mode
3.4.4. Muscle Stiffness Measurement by Sonoelastography
3.4.5. Contrast-Enhanced Assessment of Micro-Vascularity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shafiee, G.; Keshtkar, A.; Soltani, A.; Ahadi, Z.; Larijani, B.; Heshmat, R. Prevalence of sarcopenia in the world: A systematic review and meta- analysis of general population studies. J. Diabetes Metab. Disord. 2017, 16, 21. [Google Scholar] [CrossRef] [Green Version]
- Han, D.S.; Wu, W.T.; Hsu, P.C.; Chang, H.C.; Huang, K.C.; Chang, K.V. Sarcopenia Is Associated With Increased Risks of Rotator Cuff Tendon Diseases Among Community-Dwelling Elders: A Cross-Sectional Quantitative Ultrasound Study. Front. Med. 2021, 8, 630009. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.C.; Lee, T.M.; Wu, W.T.; Wang, T.G.; Han, D.S.; Chang, K.V. Assessment of Tongue Strength in Sarcopenia and Sarcopenic Dysphagia: A Systematic Review and Meta-Analysis. Front. Nutr. 2021, 8, 684840. [Google Scholar] [CrossRef]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Association Between Sarcopenia and Cognitive Impairment: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1164.e7–1164.e15. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Hsu, T.H.; Wu, W.T.; Huang, K.C.; Han, D.S. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age Ageing 2017, 46, 738–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dehghan, M.; Merchant, A.T. Is bioelectrical impedance accurate for use in large epidemiological studies? Nutr. J. 2008, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Hellmanns, K.; McBean, K.; Thoirs, K. Magnetic Resonance Imaging in the measurement of whole body muscle mass: A comparison of interval gap methods. Radiography 2015, 21, e35–e39. [Google Scholar] [CrossRef]
- Cao, Q.; Xiong, Y.; Zhong, Z.; Ye, Q. Computed Tomography-Assessed Sarcopenia Indexes Predict Major Complications following Surgery for Hepatopancreatobiliary Malignancy: A Meta-Analysis. Ann. Nutr. Metab. 2019, 74, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Buckinx, F.; Landi, F.; Cesari, M.; Fielding, R.A.; Visser, M.; Engelke, K.; Maggi, S.; Dennison, E.; Al-Daghri, N.M.; Allepaerts, S.; et al. Pitfalls in the measurement of muscle mass: A need for a reference standard. J. Cachexia Sarcopenia Muscle 2018, 9, 269–278. [Google Scholar] [CrossRef] [PubMed]
- Lam, F.M.H.; Su, Y.; Lu, Z.H.; Yu, R.; Leung, J.C.S.; Kwok, T.C.Y. Cumulative and Incremental Value of Sarcopenia Components on Predicting Adverse Outcomes. J. Am. Med. Dir. Assoc. 2020, 21, 1481–1489. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Wu, W.T.; Ozcakar, L. Ultrasound Imaging and Rehabilitation of Muscle Disorders: Part 1. Traumatic Injuries. Am. J. Phys. Med. Rehabil. 2019, 98, 1133–1141. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Ata, A.M.; Kaymak, B.; Özçakar, L. Ultrasound Imaging and Rehabilitation of Muscle Disorders: Part 2: Nontraumatic Conditions. Am. J. Phys. Med. Rehabil. 2020, 99, 636–644. [Google Scholar] [CrossRef]
- Creze, M.; Nordez, A.; Soubeyrand, M.; Rocher, L.; Maître, X.; Bellin, M.F. Shear wave sonoelastography of skeletal muscle: Basic principles, biomechanical concepts, clinical applications, and future perspectives. Skelet. Radiol. 2018, 47, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.V.; Lew, H.L.; Wang, T.G.; Chen, W.S. Use of contrast-enhanced ultrasonography in musculoskeletal medicine. Am. J. Phys. Med. Rehabil. 2012, 91, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Kaymak, B.; Frontera, W.; Ata, A.M.; Ricci, V.; Ekiz, T.; Chang, K.V.; Han, D.S.; Michail, X.; Quittan, M.; et al. Diagnosing sarcopenia: Functional perspectives and a new algorithm from the ISarcoPRM. J. Rehabil. Med. 2021, 53, jrm00209. [Google Scholar] [CrossRef] [PubMed]
- Abe, T.; Thiebaud, R.S.; Loenneke, J.P.; Loftin, M.; Fukunaga, T. Prevalence of site-specific thigh sarcopenia in Japanese men and women. Age 2014, 36, 417–426. [Google Scholar] [CrossRef] [Green Version]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Syst. Rev. 2021, 10, 89. [Google Scholar] [CrossRef] [PubMed]
- Shea, B.J.; Reeves, B.C.; Wells, G.; Thuku, M.; Hamel, C.; Moran, J.; Moher, D.; Tugwell, P.; Welch, V.; Kristjansson, E.; et al. AMSTAR 2: A critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 2017, 358, j4008. [Google Scholar] [CrossRef] [Green Version]
- Perkisas, S.; Bastijns, S.; Baudry, S.; Bauer, J.; Beaudart, C.; Beckwée, D.; Cruz-Jentoft, A.; Gasowski, J.; Hobbelen, H.; Jager-Wittenaar, H.; et al. Application of ultrasound for muscle assessment in sarcopenia: 2020 SARCUS update. Eur. Geriatr. Med. 2021, 12, 45–59. [Google Scholar] [CrossRef]
- Ticinesi, A.; Meschi, T.; Narici, M.V.; Lauretani, F.; Maggio, M. Muscle Ultrasound and Sarcopenia in Older Individuals: A Clinical Perspective. J. Am. Med. Dir. Assoc. 2017, 18, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Nijholt, W.; Scafoglieri, A.; Jager-Wittenaar, H.; Hobbelen, J.S.M.; van der Schans, C.P. The reliability and validity of ultrasound to quantify muscles in older adults: A systematic review. J. Cachexia Sarcopenia Muscle 2017, 8, 702–712. [Google Scholar] [CrossRef]
- Janczyk, E.M.; Champigny, N.; Michel, E.; Raffaelli, C.; Annweiler, C.; Zory, R.; Guérin, O.; Sacco, G. Sonoelastography to Assess Muscular Stiffness Among Older Adults and its Use for the Diagnosis of Sarcopenia: A Systematic Review. Ultraschall Med. 2021, 42, 634–642. [Google Scholar] [CrossRef]
- Merrigan, J.J.; White, J.B.; Hu, Y.E.; Stone, J.D.; Oliver, J.M.; Jones, M.T. Differences in elbow extensor muscle characteristics between resistance-trained men and women. Eur. J. Appl. Physiol. 2018, 118, 2359–2366. [Google Scholar] [CrossRef] [PubMed]
- Kruse, N.T.; Hughes, W.E.; Casey, D.P. Mechanistic insights into the modulatory role of the mechanoreflex on central hemodynamics using passive leg movement in humans. J. Appl. Physiol. 2018, 125, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Presland, J.D.; Timmins, R.G.; Bourne, M.N.; Williams, M.D.; Opar, D.A. The effect of Nordic hamstring exercise training volume on biceps femoris long head architectural adaptation. Scand. J. Med. Sci. Sports 2018, 28, 1775–1783. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xiao, Y.; Li, F.; Wang, C.; Wu, T.; Zhou, M.; Cui, L. Diagnosing Muscle Atrophy by Use of a Comprehensive Method of Assessing the Elastic Properties of Muscle During Passive Stretching. AJR. Am. J. Roentgenol. 2020, 214, 862–870. [Google Scholar] [CrossRef]
- Aromataris, E.; Fernandez, R.; Godfrey, C.M.; Holly, C.; Khalil, H.; Tungpunkom, P. Summarizing systematic reviews: Methodological development, conduct and reporting of an umbrella review approach. Int. J. Evid.-Based Healthc. 2015, 13, 132–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. Br. Med. J. 2015, 349, g7647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takai, Y.; Ohta, M.; Akagi, R.; Kato, E.; Wakahara, T.; Kawakami, Y.; Fukunaga, T.; Kanehisa, H. Applicability of ultrasound muscle thickness measurements for predicting fat-free mass in elderly population. J. Nutr. Health Aging 2014, 18, 579–585. [Google Scholar] [CrossRef]
- Takai, Y.; Ohta, M.; Akagi, R.; Kato, E.; Wakahara, T.; Kawakami, Y.; Fukunaga, T.; Kanehisa, H. Validity of ultrasound muscle thickness measurements for predicting leg skeletal muscle mass in healthy Japanese middle-aged and older individuals. J. Physiol. Anthr. 2013, 32, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.V.; Wu, W.T.; Huang, K.C.; Jan, W.H.; Han, D.S. Limb muscle quality and quantity in elderly adults with dynapenia but not sarcopenia: An ultrasound imaging study. Exp. Gerontol. 2018, 108, 54–61. [Google Scholar] [CrossRef]
- Narici, M.V.; Maganaris, C.N.; Reeves, N.D.; Capodaglio, P. Effect of aging on human muscle architecture. J. Appl. Physiol. 2003, 95, 2229–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narici, M.V.; Binzoni, T.; Hiltbrand, E.; Fasel, J.; Terrier, F.; Cerretelli, P. In vivo human gastrocnemius architecture with changing joint angle at rest and during graded isometric contraction. J. Physiol. 1996, 496 Pt 1, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Wijntjes, J.; van Alfen, N. Muscle ultrasound: Present state and future opportunities. Muscle Nerve 2021, 63, 455–466. [Google Scholar] [CrossRef] [PubMed]
- Bastijns, S.; De Cock, A.M.; Vandewoude, M.; Perkisas, S. Usability and Pitfalls of Shear-Wave Elastography for Evaluation of Muscle Quality and Its Potential in Assessing Sarcopenia: A Review. Ultrasound Med. Biol. 2020, 46, 2891–2907. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.A.; Chen, Y.J.; Chang, K.V.; Wu, W.T.; Ozcakar, L. Reliability of Sonoelastography Measurement of Tongue Muscles and Its Application on Obstructive Sleep Apnea. Front. Physiol. 2021, 12, 654667. [Google Scholar] [CrossRef]
- Mitchell, W.K.; Phillips, B.E.; Williams, J.P.; Rankin, D.; Smith, K.; Lund, J.N.; Atherton, P.J. Development of a new Sonovue™ contrast-enhanced ultrasound approach reveals temporal and age-related features of muscle microvascular responses to feeding. Physiol. Rep. 2013, 1, e00119. [Google Scholar] [CrossRef]
- Minetto, M.A.; Caresio, C.; Menapace, T.; Hajdarevic, A.; Marchini, A.; Molinari, F.; Maffiuletti, N.A. Ultrasound-Based Detection of Low Muscle Mass for Diagnosis of Sarcopenia in Older Adults. PM R J. Inj. Funct. Rehabil. 2016, 8, 453–462. [Google Scholar] [CrossRef]
- Abe, T.; Counts, B.R.; Barnett, B.E.; Dankel, S.J.; Lee, K.; Loenneke, J.P. Associations between Handgrip Strength and Ultrasound-Measured Muscle Thickness of the Hand and Forearm in Young Men and Women. Ultrasound Med. Biol. 2015, 41, 2125–2130. [Google Scholar] [CrossRef]
- Abe, T.; Thiebaud, R.S.; Loenneke, J.P.; Ogawa, M.; Mitsukawa, N. Association between forearm muscle thickness and age-related loss of skeletal muscle mass, handgrip and knee extension strength and walking performance in old men and women: A pilot study. Ultrasound Med. Biol. 2014, 40, 2069–2075. [Google Scholar] [CrossRef]
- Özkal, Ö.; Kara, M.; Topuz, S.; Kaymak, B.; Bakı, A.; Özçakar, L. Assessment of core and lower limb muscles for static/dynamic balance in the older people: An ultrasonographic study. Age Ageing 2019, 48, 881–887. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Frontera, W.R.; Özçakar, L. Measure What Matters Most in Sarcopenia: Regional vs. Appendicular Muscle Mass? J. Am. Med. Dir. Assoc. 2021, 22, 883–884. [Google Scholar] [CrossRef] [PubMed]
- Kara, M.; Kaymak, B.; Ata, A.M.; Özkal, Ö.; Kara, Ö.; Baki, A.; Şengül Ayçiçek, G.; Topuz, S.; Karahan, S.; Soylu, A.R.; et al. STAR-Sonographic Thigh Adjustment Ratio: A Golden Formula for the Diagnosis of Sarcopenia. Am. J. Phys. Med. Rehabil. 2020, 99, 902–908. [Google Scholar] [CrossRef]
- Tsukasaki, K.; Matsui, Y.; Arai, H.; Harada, A.; Tomida, M.; Takemura, M.; Otsuka, R.; Ando, F.; Shimokata, H. Association of Muscle Strength and Gait Speed with Cross-Sectional Muscle Area Determined by Mid-Thigh Computed Tomography—A Comparison with Skeletal Muscle Mass Measured by Dual-Energy X-Ray Absorptiometry. J. Frailty Aging 2020, 9, 82–89. [Google Scholar] [CrossRef] [PubMed]

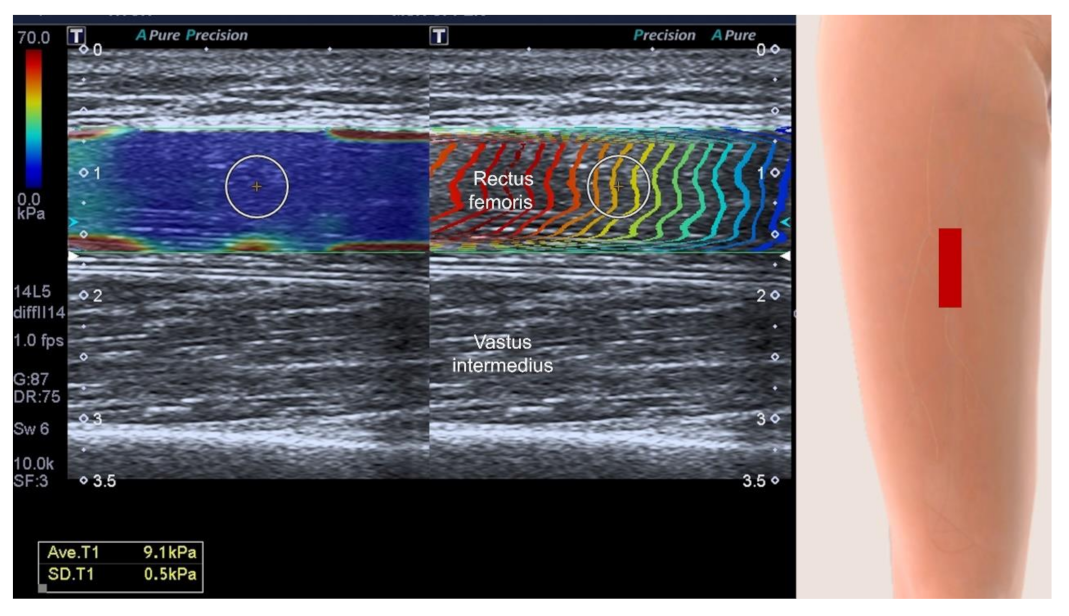
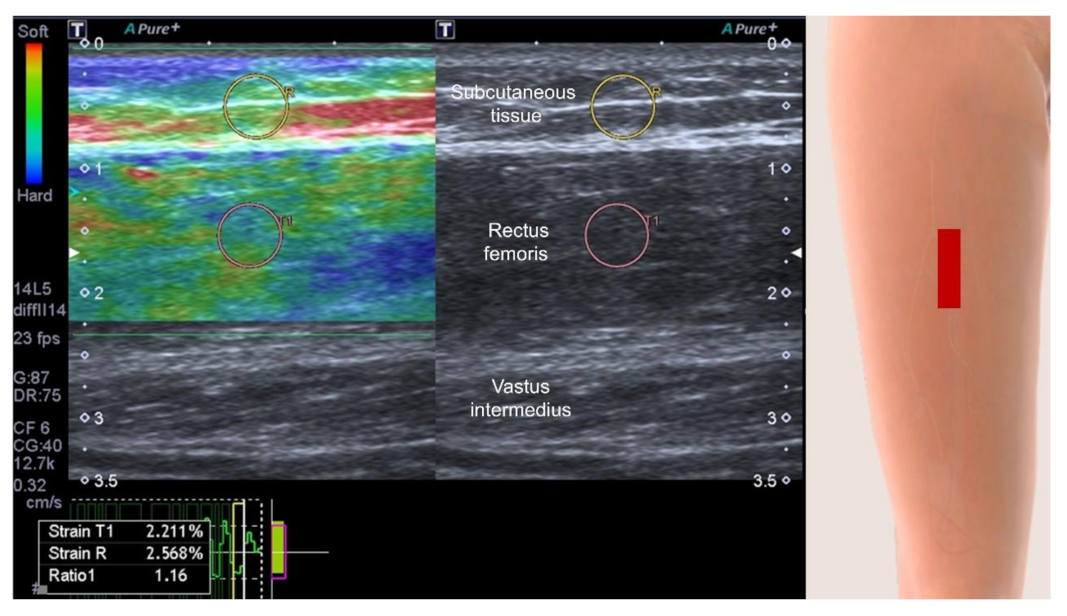
| Author, Year | Country | Protocol Registration | Included Studies (n) | Searched Database | Research Question | Ultrasound Modes | Main Conclusion |
|---|---|---|---|---|---|---|---|
| Ticinesi et al., 2017 [20] | Italy, UK | No | 44 | PubMed, Scopus | To review the role of muscle US for detecting muscle mass loss in older individuals | B-mode, Contrast-enhanced US | US parameters may be theoretically useful for detecting muscle mass loss and functionality in geriatric patients |
| Nijholt et al., 2017 [21] | Netherlands | No | 17 | PubMed, Cochrane, Cumulative index to Nursing and Allied Health Literature | To evaluate the reliability and validity of US for assessing muscle size in older adults | B-mode | US is a reliable and valid tool for the assessment of muscle size in older adults |
| Janczyk et al., 2020 [22] | France | PROSPERO (CRD42020165653) | 10 | Medline, Google Scholar, Scopus, SpringerLink, Science Direct | To examine whether sonoelastograpy can be a reliable method to evaluate sarcopenia in older patients | Sonoelastography (shear wave and strain modes) | No conclusion could be made about the usefulness of sonoelastograpy to assess sarcopenia due to substantial heterogenicity of actual data |
| Perkisas et al., 2021 [19] | Belgium, Germany, Netherlands, Spain, Poland | PROSPERO (CRD42019126106) | 65 | PubMed, Scopus, Web of Science | To provide standardization for the assessment of muscles of specific limbs | B-mode, Sonoelastography, Contrast-enhanced US | Different approaches for US assessment are found to likely impact the values measured |
| AMSTAR-2 Item Number | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author, Year | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 |
| Ticinesi et al., 2017 [20] | N | N | Y | Y | N | N | N | N | N | N | N/A | N/A | N | N | N/A | N |
| Nijholt et al., 2017 [21] | Y | N | Y | Y | Y | Y | N | Y | N | N | N/A | N/A | N | N | N/A | N |
| Janczyk et al., 2020 [22] | Y | Y | Y | Y | Y | Y | N | Y | Y | N | N/A | N/A | Y | N | N/A | N |
| Perkisas et al., 2021 [19] | Y | Y | Y | Y | Y | Y | N | PY | N | N | N/A | N/A | N | N | N/A | N |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.-C.; Wu, W.-T.; Chang, K.-V.; Chen, L.-R.; Chi, S.-Y.; Kara, M.; Özçakar, L. Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review. Life 2022, 12, 9. https://doi.org/10.3390/life12010009
Wang J-C, Wu W-T, Chang K-V, Chen L-R, Chi S-Y, Kara M, Özçakar L. Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review. Life. 2022; 12(1):9. https://doi.org/10.3390/life12010009
Chicago/Turabian StyleWang, Jia-Chi, Wei-Ting Wu, Ke-Vin Chang, Lan-Rong Chen, Shao-Yu Chi, Murat Kara, and Levent Özçakar. 2022. "Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review" Life 12, no. 1: 9. https://doi.org/10.3390/life12010009
APA StyleWang, J.-C., Wu, W.-T., Chang, K.-V., Chen, L.-R., Chi, S.-Y., Kara, M., & Özçakar, L. (2022). Ultrasound Imaging for the Diagnosis and Evaluation of Sarcopenia: An Umbrella Review. Life, 12(1), 9. https://doi.org/10.3390/life12010009








