RETRACTED: Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Maintenance and Embryo Collection
2.2. Viability, Morphology, Hatching, and Heart Rate after PEAOXA Exposure
2.3. Application of LPS to Zebrafish Embryos
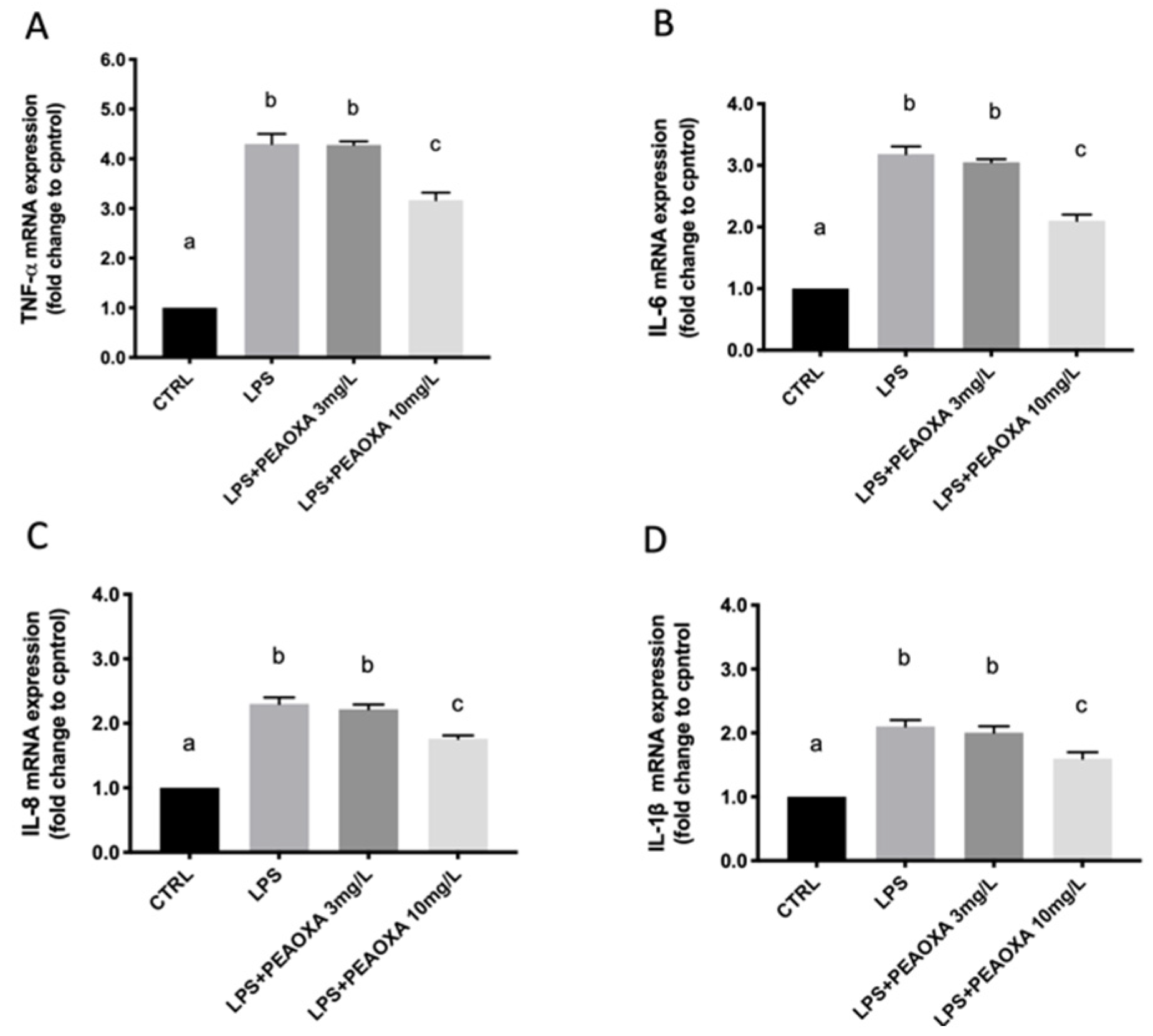
2.4. Gene Expression Analysis
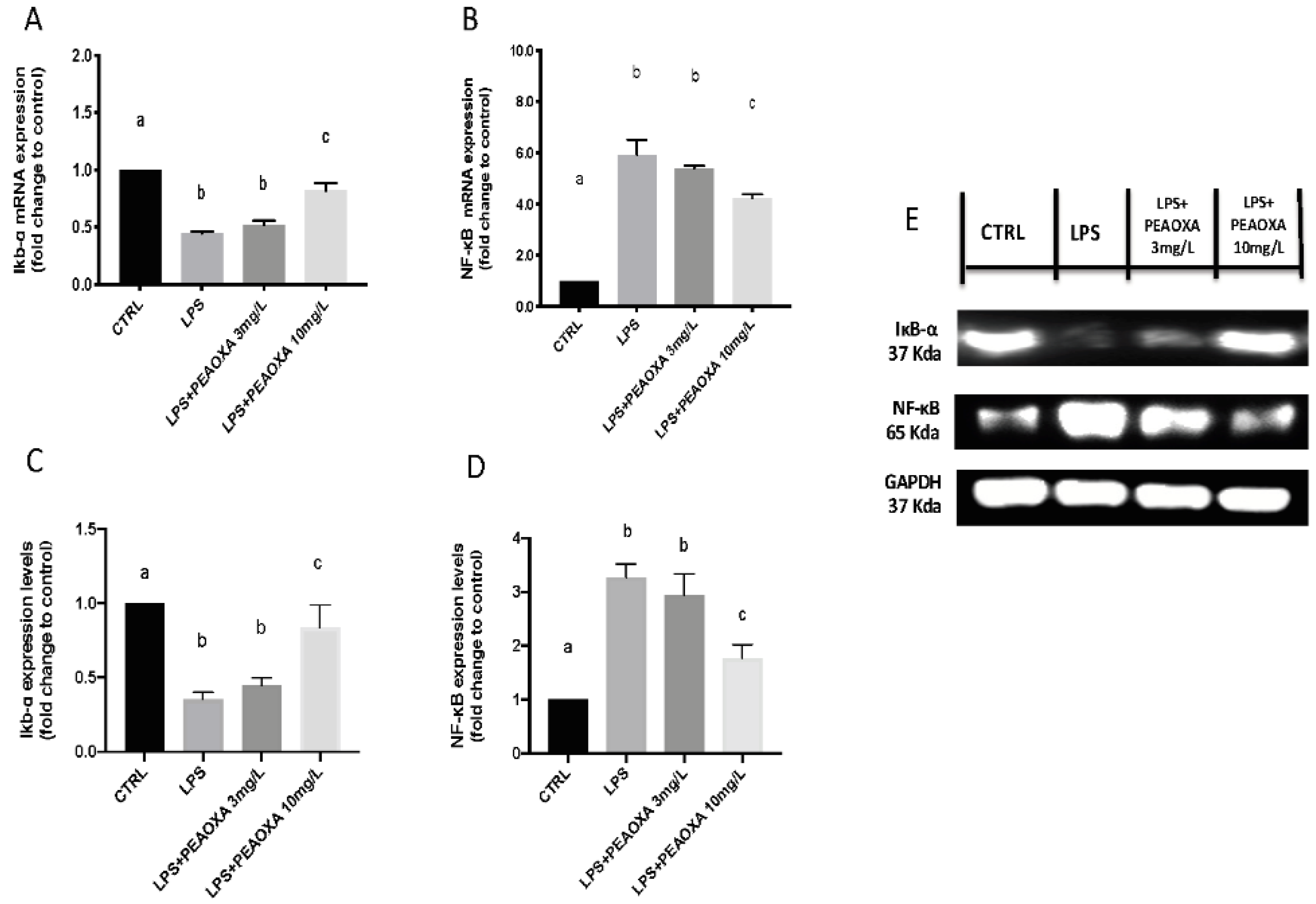
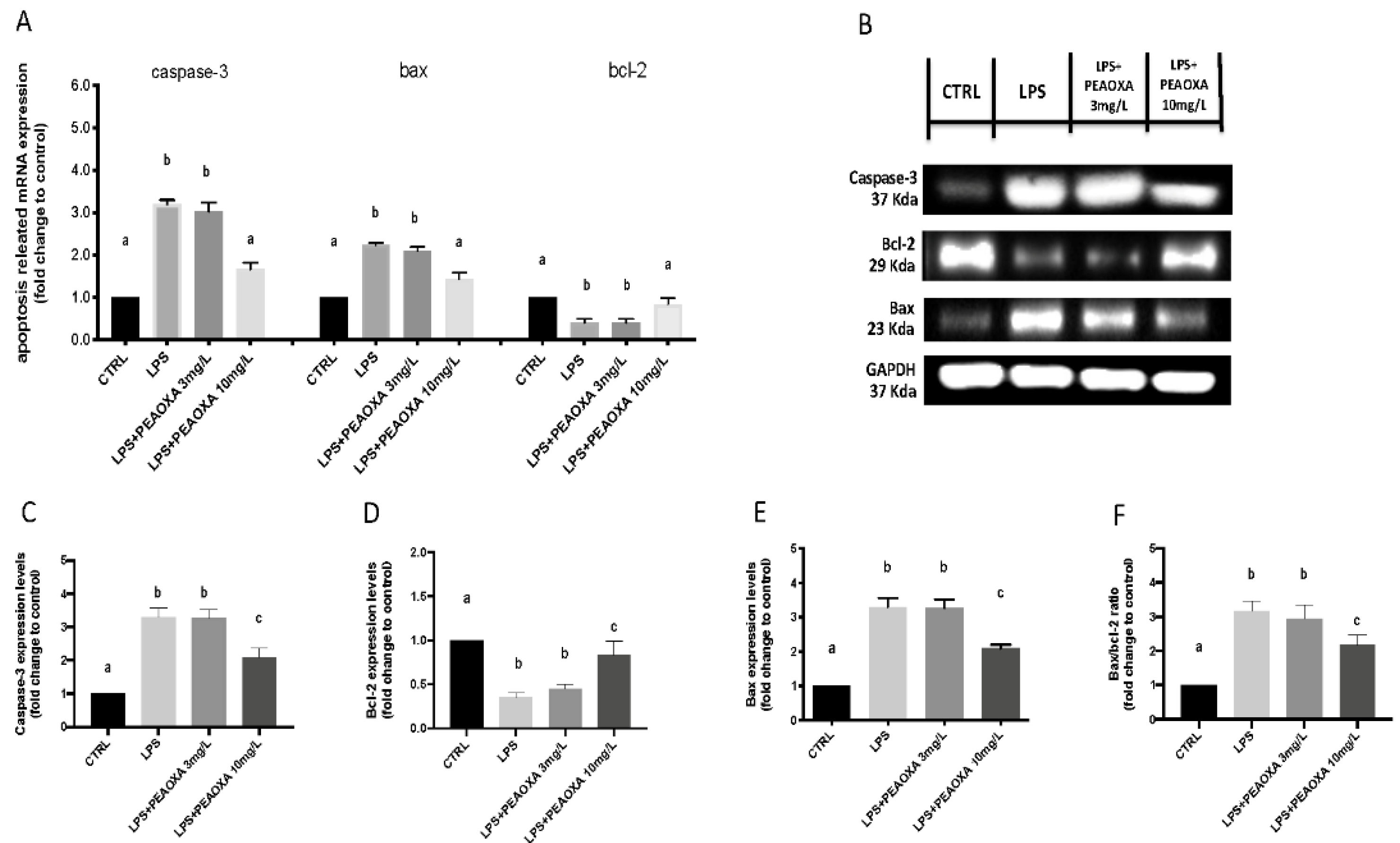
2.5. Western Blot
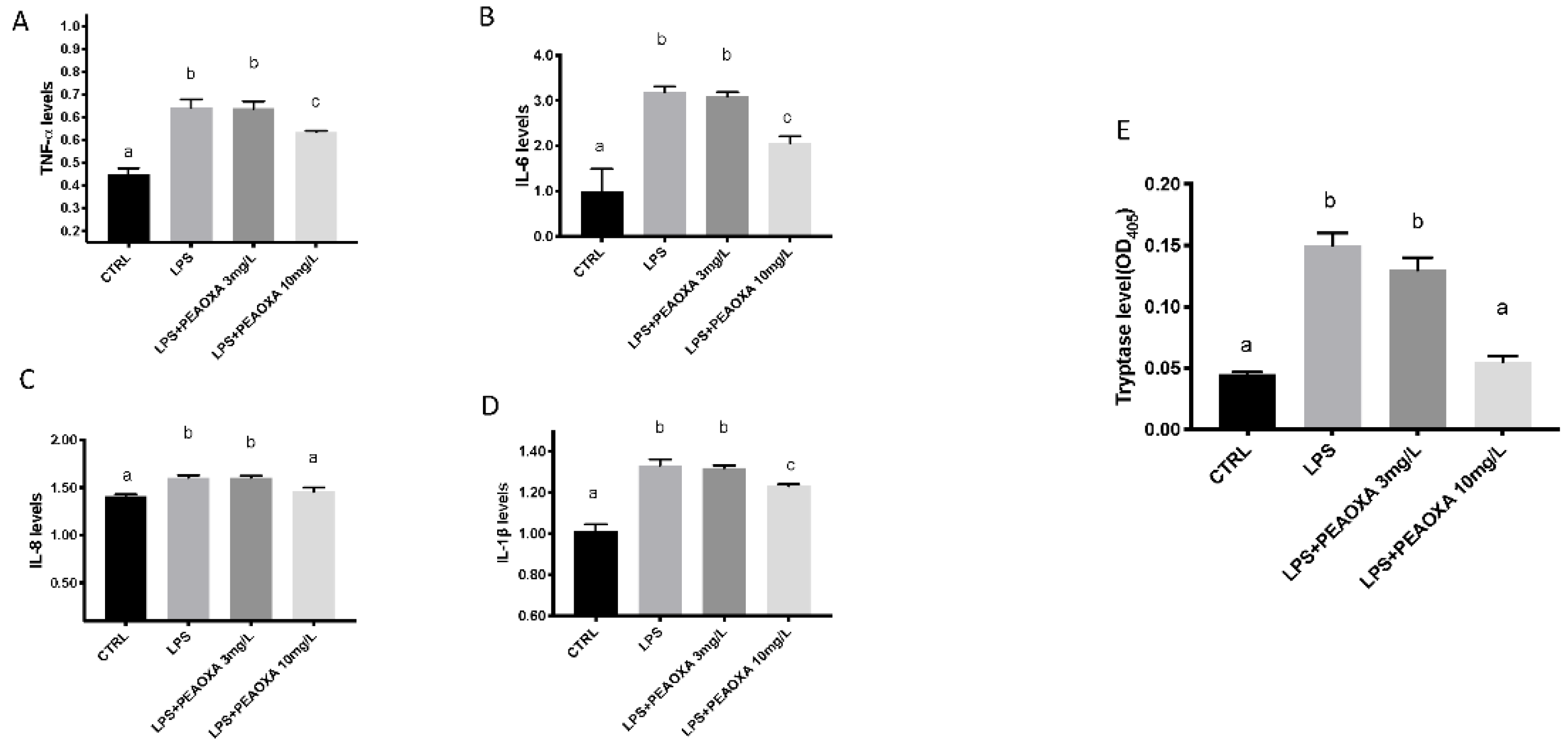
2.6. Determination of the Levels of Cytokines and Tryptase in Larval Zebrafish
2.7. Yolk Sac Areas and Body Axis Curvature Percentage
2.8. Statistical Evaluation
3. Results
3.1. Morphology
3.2. Survival, Heart, and Hatching Rate
3.3. Effects of PEAOXA on the Levels of Cytokines and Tryptase Release on LPS-Induced Inflammation
3.4. Effect of PEAOXA on the mRNA Expression of LPS-Induced Cytokines
3.5. Effect of PEAOXA on the mRNA Expression and Protein of LPS-Induced NF-κB Pathway
3.6. Effects of PEAOXA on LPS-Induced Apoptotic Pathway
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Phull, A.R.; Kim, S.J. Fucoidan as bio-functional molecule: Insights into the anti-inflammatory potential and associated molecular mechanisms. J. Funct. Foods 2017, 38, 415–426. [Google Scholar] [CrossRef]
- Wang, Y.; Xing, M.; Cao, Q.; Ji, A.; Liang, H.; Song, S. Biological activities of fucoidan and the factors mediating its therapeutic effects: A review of recent studies. Mar. Drugs 2019, 17, 183. [Google Scholar] [CrossRef] [Green Version]
- Haddad, J.J. Cytokines and related receptor-mediated signaling pathways. Biochem. Biophys. Res. Commun. 2002, 297, 700–713. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tomkovich, S.; Jobin, C. Could a swimming creature inform us on intestinal diseases? Lessons from zebrafish. Inflamm. Bowel Dis. 2014, 20, 956–966. [Google Scholar] [CrossRef] [Green Version]
- Dong, D.; Zhou, H.; Na, S.-Y.; Niedra, R.; Peng, Y.; Wang, H.; Seed, B.; Zhou, G.L. GPR108, an NF-κB activator suppressed by TIRAP, negatively regulates TLR-triggered immune responses. PLoS ONE 2018, 13, e0205303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tu, Q.; Yan, W.; Xiao, D.; Zeng, Z.; Ouyang, Y.; Huang, L.; Cai, J.; Zeng, X.; Chen, Y.-J. CXC195 suppresses proliferation and inflammatory response in LPS-induced human hepatocellular carcinoma cells via regulating TLR4-MyD88-TAK1-mediated NF-κB and MAPK pathway. Biochem. Biophys. Res. Commun. 2015, 456, 373–379. [Google Scholar] [CrossRef]
- Barton, G.M.; Medzhitov, R. Toll-like receptor signaling pathways. Science 2003, 300, 1524–1525. [Google Scholar] [CrossRef]
- Ryu, S.-J.; Choi, H.-S.; Yoon, K.-Y.; Lee, O.-H.; Kim, K.-J.; Lee, B.-Y. Oleuropein suppresses LPS-induced inflammatory responses in RAW 264.7 cell and zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef]
- Peritore, A.F.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Fusco, R.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Di Paola, R.; Cuzzocrea, S.; et al. Management of Acute Lung Injury: Palmitoylethanolamide as a New Approach. Int. J. Mol. Sci. 2021, 22, 5533. [Google Scholar] [CrossRef]
- Gugliandolo, E.; Fusco, R.; Licata, P.; Peritore, A.F.; D’Amico, R.; Cordaro, M.; Siracusa, R.; Cuzzocrea, S.; Crupi, R. Protective Effect of Hydroxytyrosol on LPS-Induced Inflammation and Oxidative Stress in Bovine Endometrial Epithelial Cell Line. Vet. Sci. 2020, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Yang, X.; Xia, B.; Yang, Z.; Wang, Z.; Wang, J.; Li, T.; Lin, P.; Song, X.; Guo, S. The fucoidan from sea cucumber Apostichopus japonicus attenuates lipopolysaccharide-challenged liver injury in C57BL/6J mice. J. Funct. Foods 2019, 61, 103493. [Google Scholar] [CrossRef]
- Wang, Y.-M.; Xu, M.; Wang, D.; Yang, C.-R.; Zeng, Y.; Zhang, Y.-J. Anti-inflammatory compounds of “Qin-Jiao”, the roots of Gentiana dahurica (Gentianaceae). J. Ethnopharmacol. 2013, 147, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Zhu, J.; Cao, F.; Chen, F. Anti-inflammatory properties of extracts from Chimonanthus nitens Oliv. leaf. PLoS ONE 2017, 12, e0181094. [Google Scholar] [CrossRef]
- Gasparrini, M.; Forbes-Hernandez, T.Y.; Giampieri, F.; Afrin, S.; Alvarez-Suarez, J.M.; Mazzoni, L.; Mezzetti, B.; Quiles, J.L.; Battino, M. Anti-inflammatory effect of strawberry extract against LPS-induced stress in RAW 264.7 macrophages. Food Chem. Toxicol. 2017, 102, 1–10. [Google Scholar] [CrossRef]
- Toranzo, A.E.; Magariños, B.; Romalde, J.L. A review of the main bacterial fish diseases in mariculture systems. Aquaculture 2005, 246, 37–61. [Google Scholar] [CrossRef]
- Swain, P.; Nayak, S.; Nanda, P.; Dash, S. Biological effects of bacterial lipopolysaccharide (endotoxin) in fish: A review. Fish Shellfish Immunol. 2008, 25, 191–201. [Google Scholar] [CrossRef]
- Lesley, R.; Ramakrishnan, L. Insights into early mycobacterial pathogenesis from the zebrafish. Curr. Opin. Microbiol. 2008, 11, 277–283. [Google Scholar] [CrossRef] [Green Version]
- Traver, D.; Herbomel, P.; Patton, E.E.; Murphey, R.D.; Yoder, J.A.; Litman, G.W.; Catic, A.; Amemiya, C.T.; Zon, L.I.; Trede, N.S. The zebrafish as a model organism to study development of the immune system. Adv. Immunol. 2003, 81, 253–330. [Google Scholar] [PubMed]
- Trede, N.S.; Langenau, D.M.; Traver, D.; Look, A.T.; Zon, L.I. The use of zebrafish to understand immunity. Immunity 2004, 20, 367–379. [Google Scholar] [CrossRef] [Green Version]
- H Meijer, A.; P Spaink, H. Host-pathogen interactions made transparent with the zebrafish model. Curr. Drug Targets 2011, 12, 1000–1017. [Google Scholar] [CrossRef]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Crupi, R.; Pascali, J.; Alfonsi, D.; Marcolongo, G.; Cuzzocrea, S. 2-pentadecyl-2-oxazoline: Identification in coffee, synthesis and activity in a rat model of carrageenan-induced hindpaw inflammation. Pharm. Res. 2016, 108, 23–30. [Google Scholar] [CrossRef]
- Petrosino, S.; Campolo, M.; Impellizzeri, D.; Paterniti, I.; Allara, M.; Gugliandolo, E.; D’Amico, R.; Siracusa, R.; Cordaro, M.; Esposito, E.; et al. 2-Pentadecyl-2-Oxazoline, the Oxazoline of Pea, Modulates Carrageenan-Induced Acute Inflammation. Front Pharm. 2017, 8, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impellizzeri, D.; Cordaro, M.; Bruschetta, G.; Siracusa, R.; Crupi, R.; Esposito, E.; Cuzzocrea, S. N-Palmitoylethanolamine-Oxazoline as a New Therapeutic Strategy to Control Neuroinflammation: Neuroprotective Effects in Experimental Models of Spinal Cord and Brain Injury. J. Neurotrauma 2017, 34, 2609–2623. [Google Scholar] [CrossRef] [PubMed]
- Impellizzeri, D.; Siracusa, R.; Cordaro, M.; Crupi, R.; Peritore, A.F.; Gugliandolo, E.; D’Amico, R.; Petrosino, S.; Evangelista, M.; Di Paola, R.; et al. N-Palmitoylethanolamine-oxazoline (PEA-OXA): A new therapeutic strategy to reduce neuroinflammation, oxidative stress associated to vascular dementia in an experimental model of repeated bilateral common carotid arteries occlusion. Neurobiol. Dis. 2019, 125, 77–91. [Google Scholar] [CrossRef]
- Buschmann, J. The OECD guidelines for the testing of chemicals and pesticides. Methods Mol. Biol. 2013, 947, 37–56. [Google Scholar] [CrossRef]
- Manzo, E.; Schiano Moriello, A.; Tinto, F.; Verde, R.; Allarà, M.; De Petrocellis, L.; Pagano, E.; Izzo, A.A.; Di Marzo, V.; Petrosino, S. A Glucuronic Acid-Palmitoylethanolamide Conjugate (GLUPEA) Is an Innovative Drug Delivery System and a Potential Bioregulator. Cells 2021, 10, 450. [Google Scholar] [CrossRef]
- Kuder, R.S.; Gundala, H.P. Developmental toxicity of deltamethrin and 3-phenoxybenzoic acid in embryo-larval stages of zebrafish (Danio rerio). Toxicol. Mech. Methods 2018, 28, 415–422. [Google Scholar] [CrossRef]
- Wang, S.; Ni, L.; Fu, X.; Duan, D.; Xu, J.; Gao, X. A Sulfated Polysaccharide from Saccharina japonica Suppresses LPS-Induced Inflammation Both in a Macrophage Cell Model via Blocking MAPK/NF-κB Signal Pathways In Vitro and a Zebrafish Model of Embryos and Larvae In Vivo. Mar. Drugs 2020, 18, 593. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Chen, R.; Liu, W.; Fu, Z. Effect of endocrine disrupting chemicals on the transcription of genes related to the innate immune system in the early developmental stage of zebrafish (Danio rerio). Fish Shellfish Immun. 2010, 28, 854–861. [Google Scholar] [CrossRef]
- Varela, M.; Dios, S.; Novoa, B.; Figueras, A. Characterisation, expression and ontogeny of interleukin-6 and its receptors in zebrafish (Danio rerio). Dev. Comp. Immunol. 2012, 37, 97–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Takagi, N.; Yuan, B.; Zhou, Y.; Si, N.; Wang, H.; Yang, J.; Wei, X.; Zhao, H.; Bian, B. The protection of indolealkylamines from LPS-induced inflammation in zebrafish. J. Ethnopharmacol. 2019, 243, 112122. [Google Scholar] [CrossRef] [PubMed]
- Hunt, R.F.; Hortopan, G.A.; Gillespie, A.; Baraban, S.C. A novel zebrafish model of hyperthermia-induced seizures reveals a role for TRPV4 channels and NMDA-type glutamate receptors. Exp. Neurol. 2012, 237, 199–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steenbergen, P.J.; Bardine, N. Antinociceptive effects of buprenorphine in zebrafish larvae: An alternative for rodent models to study pain and nociception? Appl. Anim. Behav. Sci. 2014, 152, 92–99. [Google Scholar] [CrossRef]
- Jin, Y.; Zhang, S.; Tao, R.; Huang, J.; He, X.; Qu, L.; Fu, Z. Oral exposure of mice to cadmium (II), chromium (VI) and their mixture induce oxidative-and endoplasmic reticulum-stress mediated apoptosis in the livers. Environ. Toxicol. 2016, 31, 693–705. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zeng, C.; Sun, H.; Xie, P.; Wang, J.; Zhang, G.; Chen, N.; Yan, W.; Li, G. The role of apoptosis in MCLR-induced developmental toxicity in zebrafish embryos. Aquat. Toxicol. 2014, 149, 25–32. [Google Scholar] [CrossRef]
- D’Amico, R.; Fusco, R.; Cordaro, M.; Siracusa, R.; Peritore, A.F.; Gugliandolo, E.; Crupi, R.; Scuto, M.; Cuzzocrea, S.; Di Paola, R.; et al. Modulation of NLRP3 Inflammasome through Formyl Peptide Receptor 1 (Fpr-1) Pathway as a New Therapeutic Target in Bronchiolitis Obliterans Syndrome. Int. J. Mol. Sci. 2020, 21, 2144. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef]
- Liu, Z.; Fu, Z.; Jin, Y. Immunotoxic effects of atrazine and its main metabolites at environmental relevant concentrations on larval zebrafish (Danio rerio). Chemosphere 2017, 166, 212–220. [Google Scholar] [CrossRef]
- Fusco, R.; Gugliandolo, E.; Siracusa, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Evangelista, M.; Peli, A.; Peritore, A.F.; Impellizzeri, D.; et al. Formyl Peptide Receptor 1 Signaling in Acute Inflammation and Neural Differentiation Induced by Traumatic Brain Injury. Biology 2020, 9, 238. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Lao, Q.C.; Yu, H.P.; Zhang, Y.; Liu, H.C.; Luan, L.; Sun, H.M.; Li, C.Q. Tween-80 and impurity induce anaphylactoid reaction in zebrafish. J. Appl. Toxicol. 2015, 35, 295–301. [Google Scholar] [CrossRef]
- Langheinrich, U. Zebrafish: A new model on the pharmaceutical catwalk. Bioessays 2003, 25, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Sanjeewa, K.A.; Jayawardena, T.U.; Kim, S.-Y.; Kim, H.-S.; Ahn, G.; Kim, J.; Jeon, Y.-J. Fucoidan isolated from invasive Sargassum horneri inhibit LPS-induced inflammation via blocking NF-κB and MAPK pathways. Algal Res. 2019, 41, 101561. [Google Scholar] [CrossRef]
- Zou, Y.; Fu, X.; Liu, N.; Duan, D.; Wang, X.; Xu, J.; Gao, X. The synergistic anti-inflammatory activities of agaro-oligosaccharides with different degrees of polymerization. J. Appl. Phycol. 2019, 31, 2547–2558. [Google Scholar] [CrossRef]
- Dobson, J.T.; Seibert, J.; Teh, E.M.; Da’as, S.; Fraser, R.B.; Paw, B.H.; Lin, T.-J.; Berman, J.N. Carboxypeptidase A5 identifies a novel mast cell lineage in the zebrafish providing new insight into mast cell fate determination. Blood J. Am. Soc. Hematol. 2008, 112, 2969–2972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- da Silva, E.Z.M.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef]
- Abraham, S.N.; John, A.L.S. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [Green Version]
- Da’as, S.I.; Coombs, A.J.; Balci, T.B.; Grondin, C.A.; Ferrando, A.A.; Berman, J.N. The zebrafish reveals dependence of the mast cell lineage on Notch signaling in vivo. Blood J. Am. Soc. Hematol. 2012, 119, 3585–3594. [Google Scholar] [CrossRef]
- Gugliandolo, E.; D’amico, R.; Cordaro, M.; Fusco, R.; Siracusa, R.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; Di Paola, R. Effect of PEA-OXA on neuropathic pain and functional recovery after sciatic nerve crush. J. Neuroinflamm. 2018, 15, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Fusco, R.; Scuto, M.; Cordaro, M.; D’Amico, R.; Gugliandolo, E.; Siracusa, R.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S. N-palmitoylethanolamide-oxazoline protects against middle cerebral artery occlusion injury in diabetic rats by regulating the SIRT1 pathway. Int. J. Mol. Sci. 2019, 20, 4845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watzke, J.; Schirmer, K.; Scholz, S. Bacterial lipopolysaccharides induce genes involved in the innate immune response in embryos of the zebrafish (Danio rerio). Fish Shellfish Immunol. 2007, 23, 901–905. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, L.A.; Bowie, A.G. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 2007, 7, 353–364. [Google Scholar] [CrossRef]
- Ghosh, S.; Hayden, M.S. New regulators of NF-κB in inflammation. Nat. Rev. Immunol. 2008, 8, 837–848. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Kim, E.-A.; Kang, M.-C.; Lee, J.-H.; Yang, H.-W.; Lee, J.-S.; Lim, T.I.; Jeon, Y.-J. Polyphenol-rich fraction from Ecklonia cava (a brown alga) processing by-product reduces LPS-induced inflammation in vitro and in vivo in a zebrafish model. Algae 2014, 29, 165–174. [Google Scholar] [CrossRef] [Green Version]
- Wolf, B.B.; Green, D.R. Suicidal tendencies: Apoptotic cell death by caspase family proteinases. J. Biol. Chem. 1999, 274, 20049–20052. [Google Scholar] [CrossRef] [Green Version]


| Gene | Primer Orientation | Nucleotide Sequence |
|---|---|---|
| b-actin | forward | 5′-AGAGCTATGAGCTGCCTGACG-3′ |
| reverse | 5′-CCGCAAGATTCCATACCCA-3′ | |
| Inflammatory pathway genes | ||
| TNF-α | forward | 5′-GCTGGATCTTCAAAGTCGGGTGTA-3′ |
| reverse | 5′-TGTGAGTCTCAGCACACTTCCATC- -3′ | |
| Il-1β | forward | 5′-TGGACTTCGCAGCACAAAATG-3′ |
| reverse | 5′-GTTCACTTCACGCTCTTGGATG-3′ | |
| Il-6 | forward | 5′- -AGACCGCTGCCTGTCTAAAA-3′ |
| reverse | 5′-TTTGATGTCGTTCACCAGGA-3′ | |
| Il-8 | forward | 5′-GTCGCTGCATTGAAACAGAA- -3′ |
| reverse | 5′-CTTAACCCATGGAGCAGAGG-3′ | |
| NF-κB | forward | 5′-GAGCCCTTTGTGCAAGAGAC-3′ |
| reverse | 5′-TGGGATACGTCCTCCTGTTC-3′ | |
| IκBα | forward | 5′-TTTCGGAGGAGATGGAGAGA-3′ |
| reverse | 5′-CTGTTCAGGTACGGGTCGTT-3′ | |
| Apoptosis pathway genes | ||
| cas-3 | forward | 5′-CCGCTGCCCATCACTA-3′ |
| reverse | 5′-ATCCTTTCACGACCATCT-3′ | |
| Bax | forward | 5′-GGCTATTTCAACCAGGGTTCC-3′ |
| reverse | 5′-TGCGAATCACCAATGCTGT-3′ | |
| bcl-2 | forward | 5′-TCACTCGTTCAGACCCTCAT-3′ |
| reverse | 5′-ACGCTTTCCACGCACAT-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Paola, D.; Natale, S.; Gugliandolo, E.; Cordaro, M.; Crupi, R.; Siracusa, R.; D’Amico, R.; Fusco, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. RETRACTED: Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio). Life 2022, 12, 128. https://doi.org/10.3390/life12010128
Di Paola D, Natale S, Gugliandolo E, Cordaro M, Crupi R, Siracusa R, D’Amico R, Fusco R, Impellizzeri D, Cuzzocrea S, et al. RETRACTED: Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio). Life. 2022; 12(1):128. https://doi.org/10.3390/life12010128
Chicago/Turabian StyleDi Paola, Davide, Sabrina Natale, Enrico Gugliandolo, Marika Cordaro, Rosalia Crupi, Rosalba Siracusa, Ramona D’Amico, Roberta Fusco, Daniela Impellizzeri, Salvatore Cuzzocrea, and et al. 2022. "RETRACTED: Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio)" Life 12, no. 1: 128. https://doi.org/10.3390/life12010128
APA StyleDi Paola, D., Natale, S., Gugliandolo, E., Cordaro, M., Crupi, R., Siracusa, R., D’Amico, R., Fusco, R., Impellizzeri, D., Cuzzocrea, S., Spanò, N., Marino, F., & Peritore, A. F. (2022). RETRACTED: Assessment of 2-Pentadecyl-2-oxazoline Role on Lipopolysaccharide-Induced Inflammation on Early Stage Development of Zebrafish (Danio rerio). Life, 12(1), 128. https://doi.org/10.3390/life12010128












