A Severe Acute Pancreatitis Mouse Model Transited from Mild Symptoms Induced by a “Two-Hit” Strategy with L-Arginine
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals and Treatment
2.3. Methods
2.3.1. H&E Staining
2.3.2. Measurement of Serum Lipase and α-Amylase Activity
2.3.3. ELISA of IL-6 and TNF-α
2.3.4. The Organ Index Measurement
2.3.5. Permeability of the Intestinal Tissue
2.3.6. Statistical Analysis
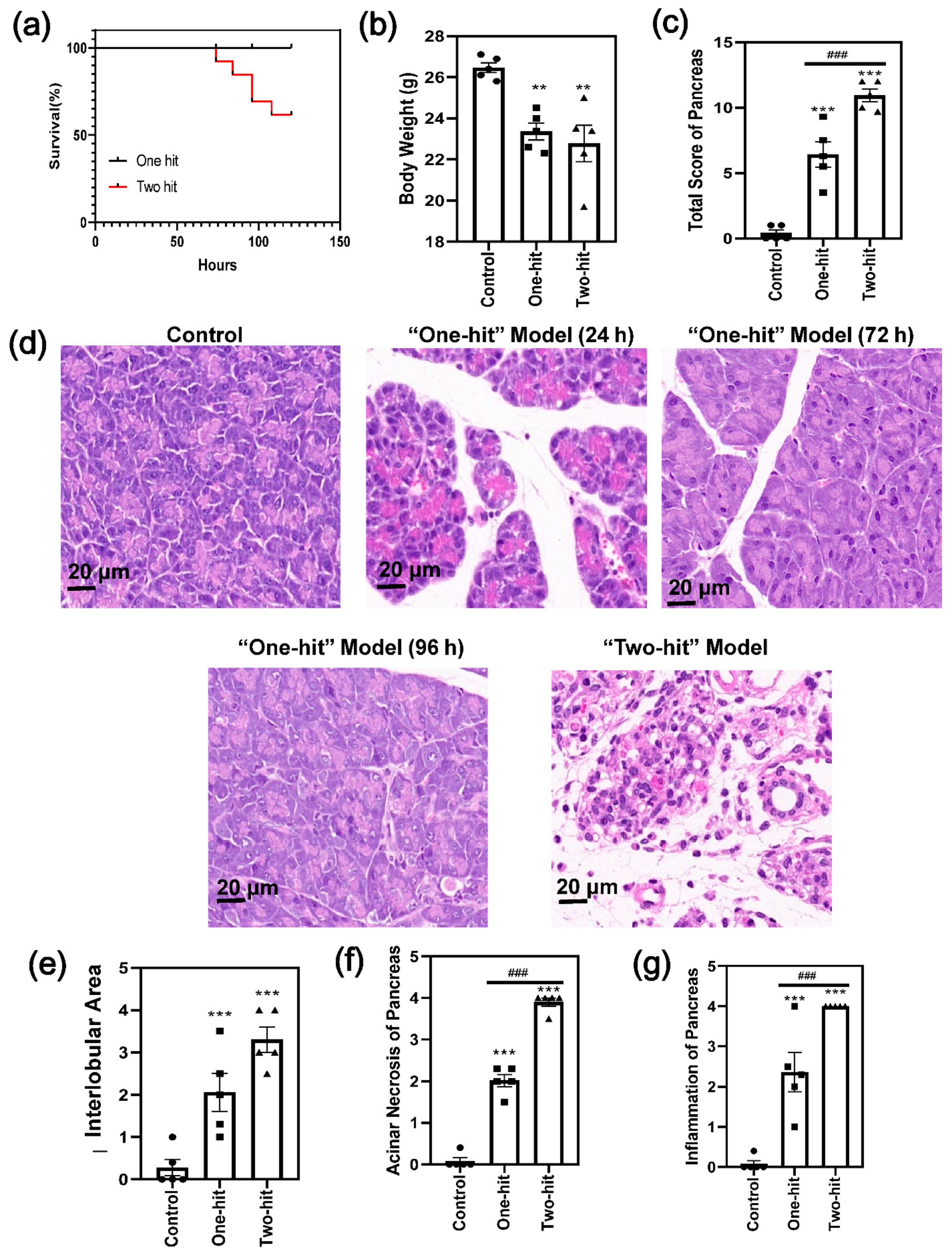
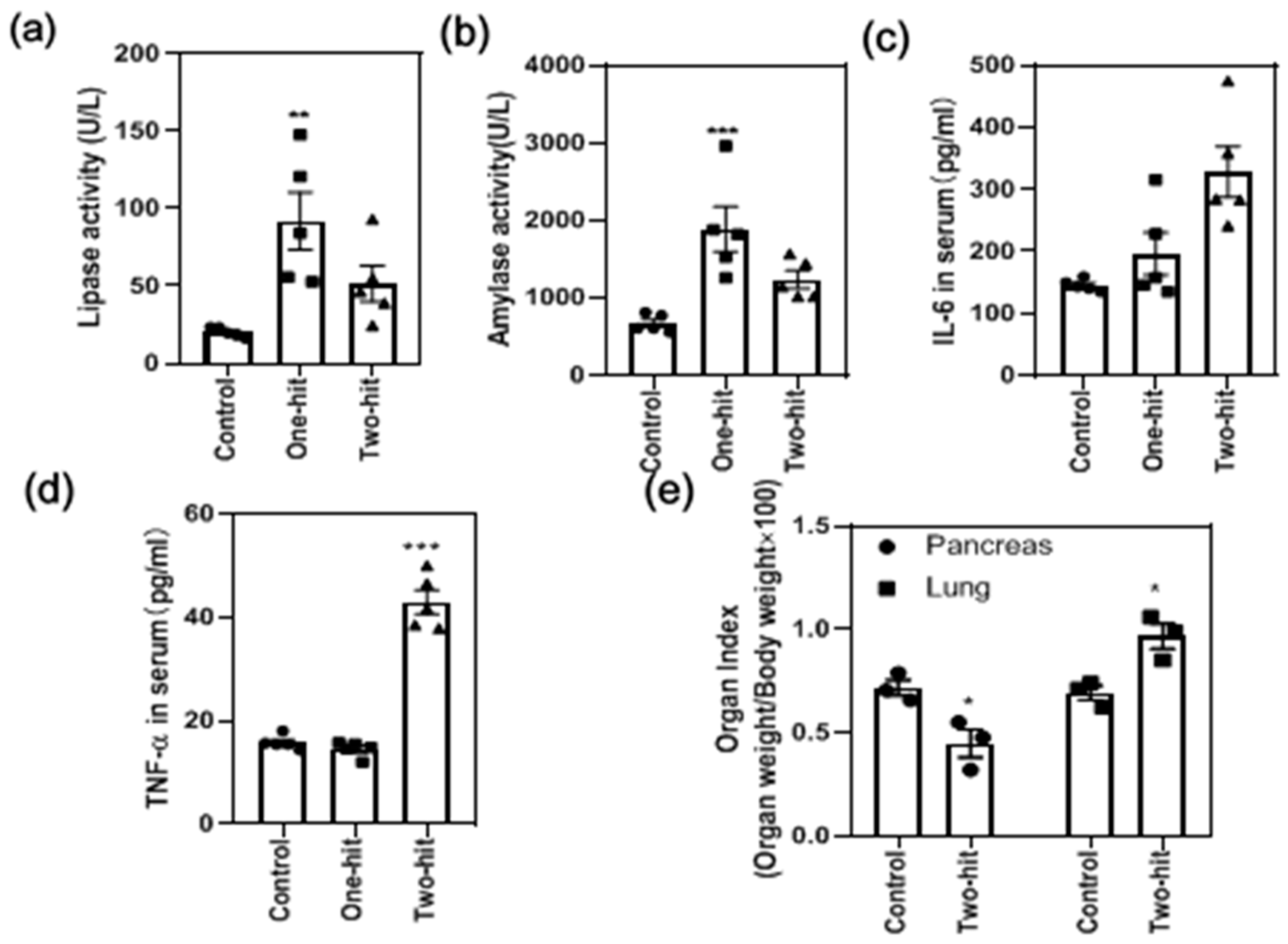
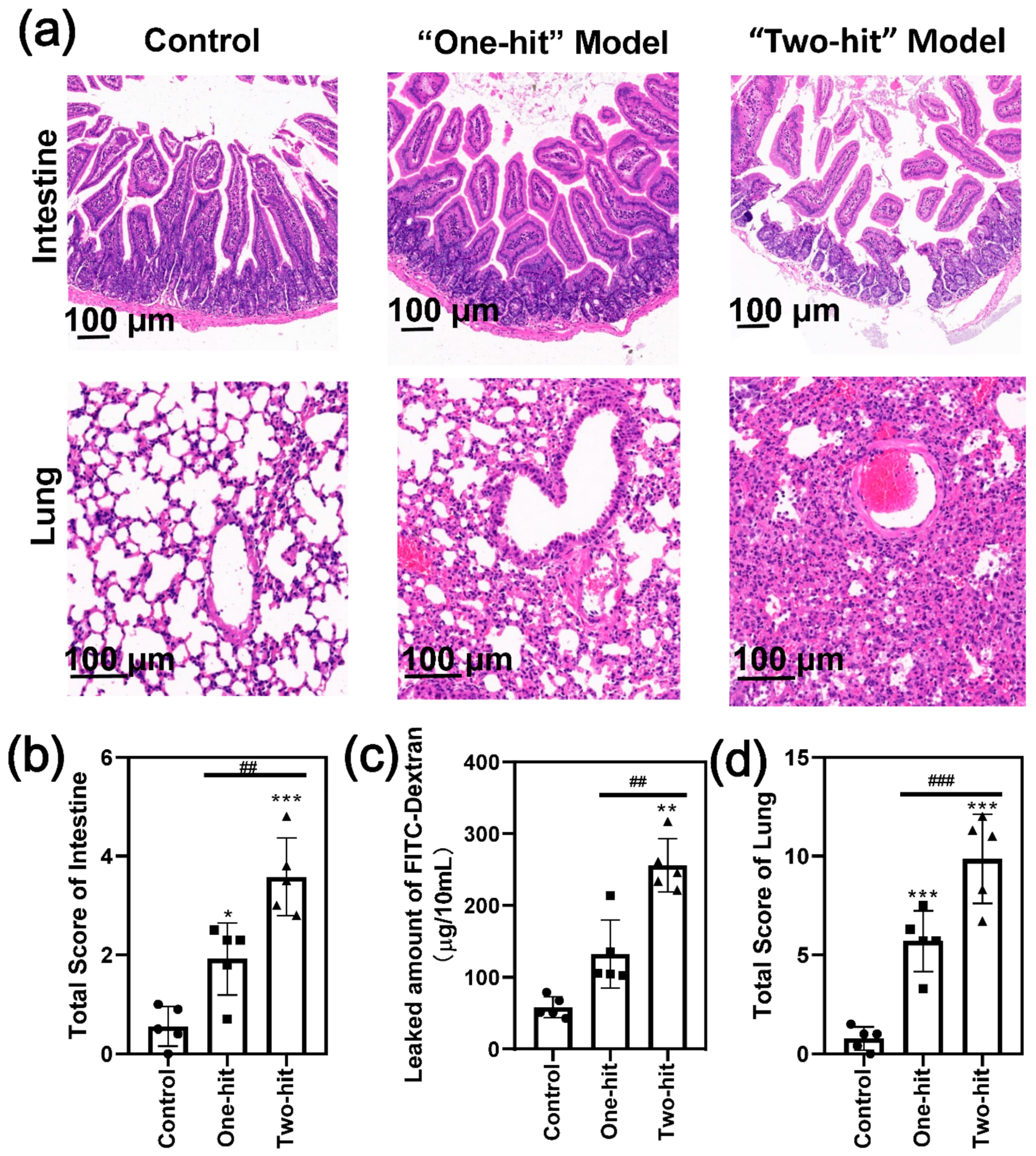
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mayerle, J.; Hlouschek, V.; Lerch, M.M. Current management of acute pancreatitis. Nat. Clin. Pract. Gastroenterol. Hepatol. 2005, 2, 473–483. [Google Scholar] [CrossRef]
- Lee, P.J.; Papachristou, G.I. New insights into acute pancreatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 479–496. [Google Scholar] [CrossRef]
- Thomson, J.E.; Brand, M.; Fonteh, P. The immune imbalance in the second hit of pancreatitis is independent of IL-17A. Pancreatology 2018, 18, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.K.; Singh, V.P. Organ Failure Due to Systemic Injury in Acute Pancreatitis. Gastroenterology 2019, 156, 2008–2023. [Google Scholar] [CrossRef]
- Hegyi, P.; Szakács, Z.; Sahin-Tóth, M. Lipotoxicity and Cytokine Storm in Severe Acute Pancreatitis and COVID-19. Gastroenterology 2020, 159, 824–827. [Google Scholar] [CrossRef] [PubMed]
- Shamoon, M.; Deng, Y.; Chen, Y.Q.; Bhatia, M.; Sun, J. Therapeutic implications of innate immune system in acute pancreatitis. Expert Opin. Ther. Targets 2016, 20, 73–87. [Google Scholar] [CrossRef]
- Niederau, C.; Ferrell, L.D.; Grendell, J.H. Caerulein-Induced Acute Necrotizing Pancreatitis in Mice; Protective Effects of Proglumide Benzotript, and Secretin. Gastroenterology 1985, 88, 1192–1204. [Google Scholar] [CrossRef]
- Le, T.; Eisses, J.F.; Lemon, K.L.; Ozolek, J.A.; Pociask, D.A.; Orabi, A.I.; Husain, S.Z. Intraductal Infusion of Taurocholate Followed by Distal Common Bile Duct Ligation Leads to a Severe Necrotic Model of Pancreatitis in Mice. Pancreas 2015, 44, 493–499. [Google Scholar] [CrossRef]
- Laukkarinen, J.M.; Van Acker, G.J.D.; Weiss, E.R.; Steer, M.L.; Perides, G. A mouse model of acute biliary pancreatitis induced by retrograde pancreatic duct infusion of Na-taurocholate. Gut 2007, 56, 1590–1598. [Google Scholar] [CrossRef]
- Dawra, R.; Sharif, R.; Phillips, P.; Dudeja, V.; Dhaulakhandi, D.; Saluja, A.K. Development of a new mouse model of acute pancreatitis induced by administration of l-arginine. Am. J. Physiol. Liver Physiol. 2007, 292, G1009–G1018. [Google Scholar] [CrossRef]
- Yasuda, H.; Kataoka, K.; Ichimura, H.; Mitsuyoshi, M.; Iida, T.; Kita, M.; Imanishi, J. Cytokine Expression and Induction of Acinar Cell Apoptosis after Pancreatic Duct Ligation in Mice. J. Interf. Cytokine Res. 1999, 19, 637–644. [Google Scholar] [CrossRef]
- Dembiński, A.; Warzecha, Z.; Ceranowicz, P.; Stachura, J.; Tomaszewska, R.; Konturek, S.J.; Sendur, R.; Dembiński, M.; Pawlik, W.W. Pancreatic damage and regeneration in the course of ischemia-reperfusion induced pancreatitis in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2001, 52, 221–235. [Google Scholar]
- Jamdar, S.; Siriwardena, A.K. Contemporary management of infected necrosis complicating severe acute pancreatitis. Crit. Care 2005, 10, 101. [Google Scholar] [CrossRef][Green Version]
- Fusco, R.; Cordaro, M.; Siracusa, R.; D’Amico, R.; Genovese, T.; Gugliandolo, E.; Peritore, A.F.; Crupi, R.; Impellizzeri, D.; Cuzzocrea, S.; et al. Biochemical Evaluation of the Antioxidant Effects of Hydroxytyrosol on Pancreatitis-Associated Gut Injury. Antioxidants 2020, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Cordaro, M.; Fusco, R.; D’Amico, R.; Siracusa, R.; Peritore, A.; Gugliandolo, E.; Genovese, T.; Crupi, R.; Mandalari, G.; Cuzzocrea, S.; et al. Cashew (Anacardium occidentale L.) Nuts Modulate the Nrf2 and NLRP3 Pathways in Pancreas and Lung after Induction of Acute Pancreatitis by Cerulein. Antioxidants 2020, 9, 992. [Google Scholar] [CrossRef] [PubMed]
- Hegyi, P.; Rakonczay, Z., Jr.; Sári, R.; Góg, C.; Lonovics, J.; Takács, T.; Czakó, L. L-arginine-induced experimental pancreatitis. World J. Gastroenterol. 2004, 10, 2003–2009. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Vati, S.; Rana, S.; Bhasin, D.K.; Malhotra, S. Severe Chronic pancreatitis due to recurrent acute injury: Non-Invasive Chronic pancreatitis model of Rat. JOP J. Pancreas 2017, 18, 107–120. [Google Scholar]
- Elder, A.S.F.; Saccone, G.T.P.; Bersten, A.D.; Dixon, D.-L. L-Arginine–induced acute pancreatitis results in mild lung inflammation without altered respiratory mechanics. Exp. Lung Res. 2011, 37, 1–9. [Google Scholar] [CrossRef]
- Tani, S.; Itoh, H.; Okabayashi, Y.; Nakamura, T.; Fujii, M.; Fujisawa, T.; Koide, M.; Otsuki, M. New model of acute necrotizing pancreatitis induced by excessive doses of arginine in rats. Dig. Dis. Sci. 1990, 35, 367–374. [Google Scholar] [CrossRef]
- Ruiz, S.; Vardon-Bounes, F.; Merlet-Dupuy, V.; Conil, J.-M.; Buléon, M.; Fourcade, O.; Tack, I.; Minville, V. Sepsis modeling in mice: Ligation length is a major severity factor in cecal ligation and puncture. Intensiv. Care Med. Exp. 2016, 4, 1–13. [Google Scholar] [CrossRef]
- Shrum, B.; Anantha, R.V.; Xu, S.X.; Donnelly, M.; Haeryfar, S.M.; McCormick, J.K.; Mele, T. A robust scoring system to evaluate sepsis severity in an animal model. BMC Res. Notes 2014, 7, 233. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Li, Y.; Gao, L.; Tong, Z.; Ye, B.; Liu, S.; Li, B.; Chen, Y.; Yang, Q.; Meng, L.; et al. Development of a novel model of hypertriglyceridemic acute pancreatitis in mice. Sci. Rep. 2017, 7, 40799. [Google Scholar] [CrossRef] [PubMed]
- Chiu, C.-J.; McArdle, A.H.; Brown, R.; Scott, H.J.; Gurd, F.N. Intestinal Mucosal Lesion in Low-Flow States. Arch. Surg. 1970, 101, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, T.; Takeyama, Y.; Ueda, T.; Shinzeki, M.; Kishi, S.; Sawa, H.; Nakajima, T.; Kuroda, Y. Protective Effect of Caspase Inhibitor on Intestinal Integrity in Experimental Severe Acute Pancreatitis. J. Surg. Res. 2007, 138, 300–307. [Google Scholar] [CrossRef]
- Takeyama, Y. Long-Term Prognosis of Acute Pancreatitis in Japan. Clin. Gastroenterol. Hepatol. 2009, 7, S15–S17. [Google Scholar] [CrossRef]
- Dembinski, A.; Warzecha, Z.; Konturek, P.C.; Ceranowicz, P.; Konturek, S.J.; Tomaszewska, R.; Stachura, J. Adaptation of pancreas to repeated caerulein-induced pancreatitis in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 1996, 47, 455–467. [Google Scholar]
- Warzecha, Z.; Dembiński, A.; Ceranowicz, P.; Konturek, P.C.; Niemiec, J.; Stachura, J.; Tomaszewska, R.; Konturek, S.J. The influence of sensory nerves and CGRP on the pancreatic regeneration after repeated episodes of acute pancreatitis in rats. J. Physiol. Pharmacol. Off. J. Pol. Physiol. Soc. 2000, 51, 449–461. [Google Scholar]
- Schneider, L.; Hackert, T.; Heck, M.; Hartwig, W.; Fritz, S.; Strobel, O.; Gebhard, M.-M.; Werner, J. Capsaicin Reduces Tissue Damage in Experimental Acute Pancreatitis. Pancreas 2009, 38, 676–680. [Google Scholar] [CrossRef]
- Ismail, O.Z.; Bhayana, V. Lipase or amylase for the diagnosis of acute pancreatitis? Clin. Biochem. 2017, 50, 1275–1280. [Google Scholar] [CrossRef]
- Jm, A.; Xma, B.; Yi, L.A.; Cheng, Y.A.; Tong, Z.A.; Pw, A.; Ykpa, C.; Hwja, C. Effects of a rhizome aqueous extract of Dioscorea batatas and its bioactive compound, allantoin in high fat diet and streptozotocin-induced diabetic mice and the regulation of liver, pancreas and skeletal muscle dysfunction. J. Ethnopharmacol. 2020, 259, 112926. [Google Scholar]
- Staubli, S.M.; Oertli, D.; Nebiker, C.A. Laboratory markers predicting severity of acute pancreatitis. Crit. Rev. Clin. Lab. Sci. 2015, 52, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Mei, Q.-X.; Hu, J.-H.; Huang, Z.-H.; Fan, J.-J.; Huang, C.-L.; Lu, Y.-Y.; Wang, X.-P.; Zeng, Y. Pretreatment with chitosan oligosaccharides attenuate experimental severe acute pancreatitis via inhibiting oxidative stress and modulating intestinal homeostasis. Acta Pharmacol. Sin. 2021, 42, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Neuhöfer, P.; Song, L.; Rabe, B.; Lesina, M.; Kurkowski, M.U.; Treiber, M.; Wartmann, T.; Regnér, S.; Thorlacius, H.; et al. IL-6 trans-signaling promotes pancreatitis-associated lung injury and lethality. J. Clin. Investig. 2013, 123, 1019–1031. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, G.; Wang, X.; Wang, L.; Liu, X.; Jin, L.; Xu, D. Studies on the conformational transformations of l-arginine molecule in aqueous solution with temperature changing by circular dichroism spectroscopy and optical rotations. J. Mol. Struct. 2012, 1026, 71–77. [Google Scholar] [CrossRef]
- Schwieger, C.; Blume, A. Interaction of Poly(l-arginine) with Negatively Charged DPPG Membranes: Calorimetric and Monolayer Studies. Biomacromolecules 2009, 10, 2152–2161. [Google Scholar] [CrossRef] [PubMed]
| Score | Edema | Acinar Necrosis | Inflammation |
|---|---|---|---|
| 0 | Absent | Absent | 0–5 leukocytes/HPF |
| 1 | Diffuse expansion of the interlobar septae | 1–4 necrotic cells/(High power field, HPF) | 6–15 leukocytes/HPF |
| 2 | Diffuse expansion of the interlobubar septae | 5–10 necrotic cells/HPF | 16–25 leukocytes/HPF |
| 3 | Diffuse expansion of the interacinar septae | 11–16 necrotic cells/HPF (foci of confluent necrosis) | 26–35 leukocytes/HPF |
| 4 | Diffuse expansion of the intercellular spaces | >16 necrotic cells/HPF (extensive confluent necrosis) | >36 leukocytes/HPF |
| Score | |
|---|---|
| 0 | Normal mucosal villi. |
| 1 | Development of subepithelial Gruenhagen’s space, usually at the apex of the villus; often with capillary congestion. |
| 2 | Extension of the subepithelial space with moderate lifting of the epithelial layer from the lamina propria. |
| 3 | Massive epithelial lifting down the sides of villi. A few tips may be denuded. |
| 4 | Denuded villi with lamina propria and dilated capillaries exposed. Increased cellularity of the lamina propria may be noted. |
| 5 | Digestion and disintegration of the lamina propria; hemorrhage and ulceration. |
| Score | Thickness of the Alveolar | Infiltration of the Neutrophils | Alveolar Congestion |
|---|---|---|---|
| 0 | Absent | Absent | Absent |
| 1 | Discrete | Discrete | Small foci |
| 2 | Moderate | Moderate | Large foci |
| 3 | Severe | Severe | Diffuse |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Tang, X.; Wu, Q.; Ren, P.; Yan, Y. A Severe Acute Pancreatitis Mouse Model Transited from Mild Symptoms Induced by a “Two-Hit” Strategy with L-Arginine. Life 2022, 12, 126. https://doi.org/10.3390/life12010126
Yang J, Tang X, Wu Q, Ren P, Yan Y. A Severe Acute Pancreatitis Mouse Model Transited from Mild Symptoms Induced by a “Two-Hit” Strategy with L-Arginine. Life. 2022; 12(1):126. https://doi.org/10.3390/life12010126
Chicago/Turabian StyleYang, Jing, Xujiao Tang, Qingqing Wu, Panpan Ren, and Yishu Yan. 2022. "A Severe Acute Pancreatitis Mouse Model Transited from Mild Symptoms Induced by a “Two-Hit” Strategy with L-Arginine" Life 12, no. 1: 126. https://doi.org/10.3390/life12010126
APA StyleYang, J., Tang, X., Wu, Q., Ren, P., & Yan, Y. (2022). A Severe Acute Pancreatitis Mouse Model Transited from Mild Symptoms Induced by a “Two-Hit” Strategy with L-Arginine. Life, 12(1), 126. https://doi.org/10.3390/life12010126






