Metabolomic Profiles of the Creeping Wood Sorrel Oxalis corniculata in Radioactively Contaminated Fields in Fukushima: Dose-Dependent Changes in Key Metabolites
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Measurements of Ground Radiation Dose Rates and Radioactivity Concentrations
2.3. LC–MS: Analysis, Peak Detection, Alignment, and Annotation
2.4. Statistical Analysis of the Peak Area Data
3. Results
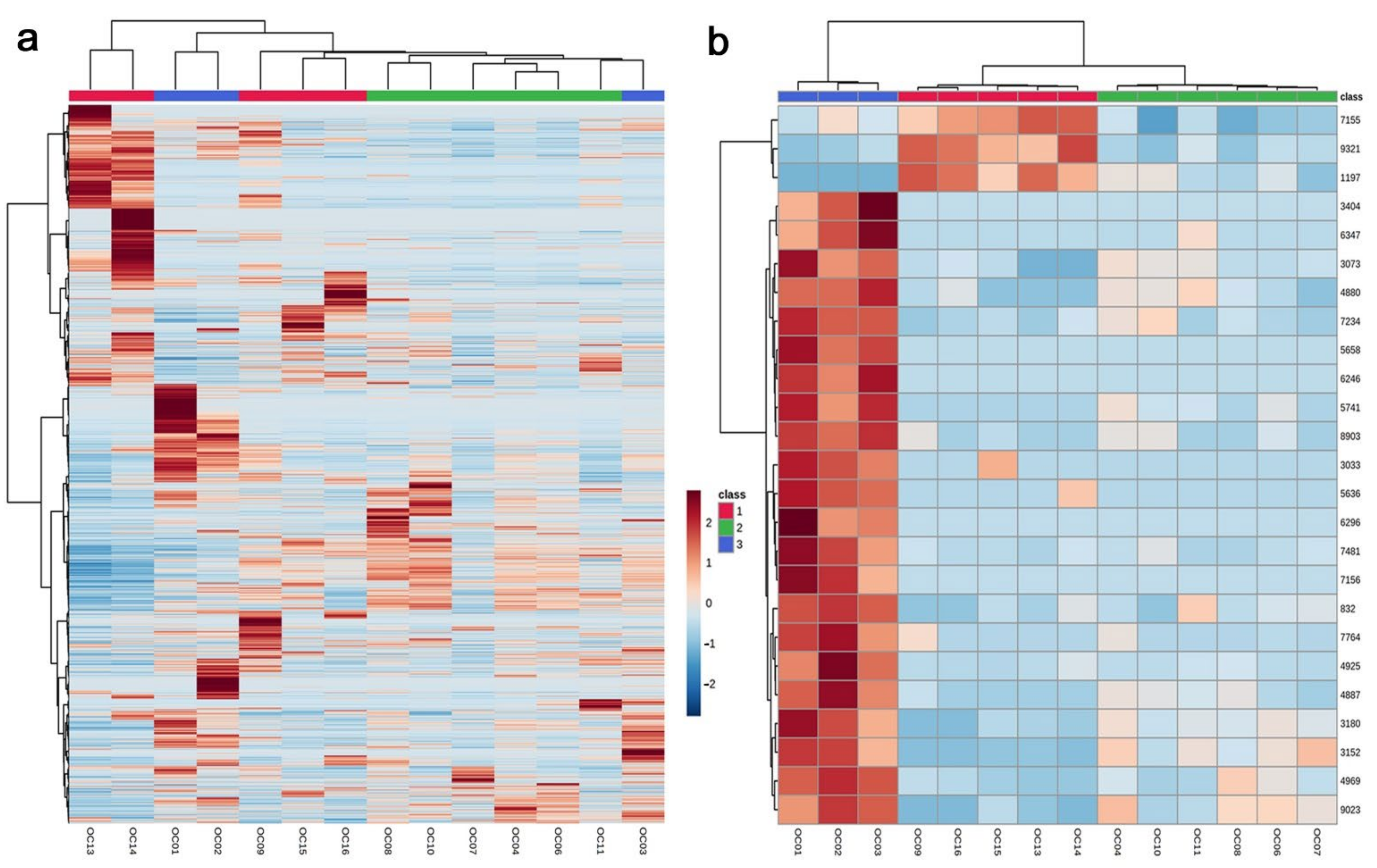
3.1. Clustering Analyses: PCA and Heatmaps
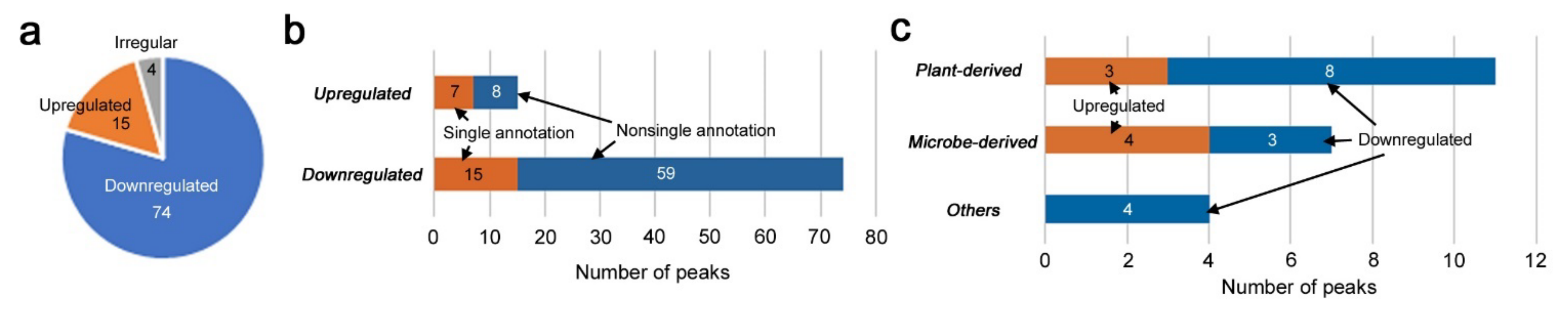
3.2. Identification of Upregulated and Downregulated Peaks
3.3. Candidate Compounds for Upregulated and Downregulated Peaks
3.4. Correlation Analyses of Upregulated Peaks
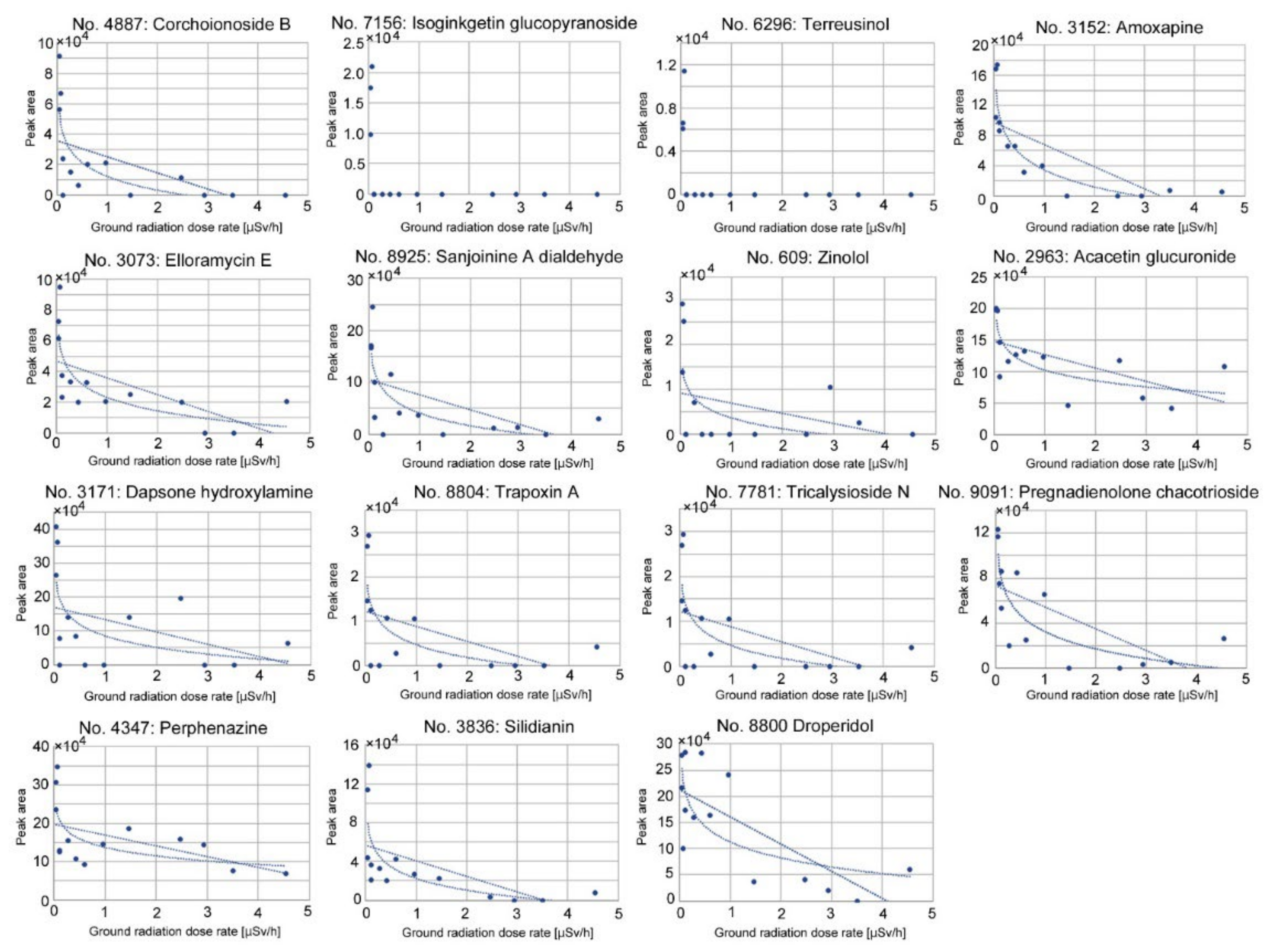
3.5. Correlation Analyses of Downregulated Peaks
3.6. Peaks Upregulated or Downregulated at the “Low Level”
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Mello, J.P.F. Preface. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2020; pp. xxv–xxxvi. [Google Scholar]
- Arapis, G.D.; Karandinos, M.G. Migration of 137Cs in the soil of sloping semi-natural ecosystems in Northern Greece. J. Environ. Radioact. 2004, 77, 133–142. [Google Scholar] [CrossRef]
- Tahir, S.N.A.; Jamil, K.; Zaidi, J.H.; Arif, M.; Ahmed, N. Activity concentration of 137Cs in soil samples from Punjab province (Pakistan) and estimation of gamma-ray dose rate for external exposure. Radiat. Prot. Dosim. 2006, 118, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, F.; Stellato, L.; Sabbarese, C. A case study on possible radiological contamination in the Lo Uttara landfill site (Caserta, Italy). J. Phys. Conf. Ser. 2020, 1548, 012001. [Google Scholar] [CrossRef]
- Endo, S.; Kimura, S.; Takatsuji, T.; Nanasawa, K.; Imanaka, T.; Shizuma, K. Measurement of soil contamination by radionuclides due to the Fukushima Dai-ichi Nuclear Power Plant accident and associated estimated cumulative external dose estimation. J. Environ. Radioact. 2021, 111, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Hinton, T.G.; Alexakhin, R.; Balonov, M.; Gentner, N.; Hendry, J.; Prister, B.; Strand, P.; Woodhead, D. Radiation-induced effects on plants and animals: Findings of the United Nations Chernobyl Forum. Health Phys. 2007, 93, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Geras’kin, S.A.; Fesenko, S.V.; Alexakhin, R.M. Effects of non-human species irradiation after the Chernobyl NPP accident. Environ. Int. 2008, 34, 880–897. [Google Scholar] [CrossRef] [PubMed]
- Beresford, N.A.; Scott, E.M.; Copplestone, D. Field effects studies in the Chernobyl Exclusion Zone: Lessons to be learnt. J. Environ. Radioact. 2020, 211, 105893. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.P.; Mousseau, T.A. Biological consequences of Chernobyl: 20 years on. Trends Ecol. Evol. 2006, 21, 200–207. [Google Scholar] [CrossRef] [PubMed]
- Mousseau, T.A. The biology of Chernobyl. Annu. Rev. Ecol. Evol. Syst. 2021, 52, 87–109. [Google Scholar] [CrossRef]
- Møller, A.P.; Hagiwara, A.; Matsui, S.; Kasahara, S.; Kawatsu, K.; Nishiumi, I.; Suzuki, H.; Mousseau, T.A. Abundance of birds in Fukushima as judges from Chernobyl. Environ. Pollut. 2012, 164, 36–39. [Google Scholar] [CrossRef] [PubMed]
- Bonisoli-Alquati, A.; Koyama, K.; Tedeschi, D.J.; Kitamura, W.; Sukuzi, H.; Ostermiller, S.; Arai, E.; Møller, A.P.; Mousseau, T.A. Abundance and genetic damage of barn swallows from Fukushima. Sci. Rep. 2015, 5, 9432. [Google Scholar] [CrossRef]
- Murase, K.; Murase, J.; Horie, R.; Endo, K. Effects of the Fukushima Daiichi nuclear accident on goshawk reproduction. Sci. Rep. 2015, 5, 9405. [Google Scholar] [CrossRef]
- Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 570. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Nohara, C.; Taira, W.; Kinjo, S.; Iwata, M.; Otaki, J.M. The Fukushima nuclear accident and the pale grass blue butterfly: Evaluating biological effects of long-term low-dose exposures. BMC Evol. Biol. 2013, 13, 168. [Google Scholar] [CrossRef]
- Nohara, C.; Hiyama, A.; Taira, W.; Tanahara, A.; Otaki, J.M. The biological impacts of ingested radioactive materials on the pale grass blue butterfly. Sci. Rep. 2014, 4, 4946. [Google Scholar] [CrossRef] [PubMed]
- Hiyama, A.; Taira, W.; Nohara, C.; Iwasaki, M.; Kinjo, S.; Iwata, M.; Otaki, J.M. Spatiotemporal abnormality dynamics of the pale grass blue butterfly: Three years of monitoring (2011–2013) after the Fukushima nuclear accident. BMC Evol. Biol. 2015, 15, 15. [Google Scholar] [CrossRef]
- Akimoto, S. Morphological abnormalities in gall-forming aphids in a radiation-contaminated area near Fukushima Daiichi: Selective impact of fallout? Ecol. Evol. 2014, 4, 355–369. [Google Scholar] [CrossRef] [PubMed]
- Akimoto, S.I.; Li, Y.; Imanaka, T.; Sato, H.; Ishida, K. Effects of radiation from contaminated soil and moss in Fukushima on embryogenesis and egg hatching of the aphid Prociphilus oriens. J. Hered. 2018, 109, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Hayama, S.; Tsuchiya, M.; Ochiai, K.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Kato, T.; Tanaka, A.; Konno, F.; Kawamoto, Y.; et al. Small head size and delayed body weight growth in wild Japanese monkey fetuses after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2017, 7, 3528. [Google Scholar] [CrossRef] [PubMed]
- Ochiai, K.; Hayama, S.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Uno, T.; Kato, T.; Konno, F.; Kawamoto, Y.; Tsuchida, S.; et al. Low blood cell counts in wild Japanese monkeys after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2014, 4, 5793. [Google Scholar] [CrossRef] [PubMed]
- Urushihara, Y.; Suzuki, T.; Shimizu, Y.; Ohtaki, M.; Kuwahara, Y.; Suzuki, M.; Uno, T.; Fujita, S.; Saito, A.; Yamashiro, H.; et al. Haematological analysis of Japanese macaques (Macaca fuscata) in the area affected by the Fukushima Daiichi Nuclear Power Plant accident. Sci. Rep. 2018, 8, 16748. [Google Scholar] [CrossRef]
- Horiguchi, T.; Yoshii, H.; Mizuno, S.; Shiraishi, H. Decline in intertidal biota after the 2011 Great East Japan Earthquake and Tsunami and the Fukushima nuclear disaster: Field observations. Sci. Rep. 2016, 6, 20416. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, G.; Shibato, J.; Imanaka, T.; Cho, K.; Kubo, A.; Kikuchi, S.; Satoh, K.; Kimura, S.; Ozawa, S.; Fukutani, S.; et al. Unraveling low-level gamma radiation-responsive changes in expression of early and late genes in leaves of rice seedlings at Iitate Village, Fukushima. J. Hered. 2014, 105, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ichikawa, S.; Kubota, M.; Hoshino, J.; Kubota, Y.; Maruyama, K.; Fuma, S.; Kawaguchi, I.; Yoschenko, V.I.; Yoshida, S. Morphological defects in native Japanese fir trees around the Fukushima Daiichi Nuclear Power Plant. Sci. Rep. 2015, 5, 13232. [Google Scholar] [CrossRef] [PubMed]
- Yoschenko, V.; Nanba, K.; Yoshida, S.; Watanabe, Y.; Takase, T.; Sato, N.; Keitoku, K. Morphological abnormalities in Japanese red pine (Pinus densiflora) at the territories contaminated as a result of the accident at Fukushima Dai-ichi Nuclear Power Plant. J. Environ. Radioact. 2016, 165, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Rakwal, R.; Hayashi, G.; Shibato, J.; Deepak, S.A.; Gundimeda, S.; Simha, U.; Padmanaban, A.; Gupta, R.; Han, S.; Kim, S.T.; et al. Progress toward rice seed OMICS in low-level gamma radiation environment in Iitate Village, Fukushima. J. Hered. 2018, 109, 2089–2211. [Google Scholar] [CrossRef] [PubMed]
- Sakauchi, K.; Taira, W.; Toki, M.; Tsuhako, M.; Umetsu, K.; Otaki, J.M. Nutrient imbalance of the host plant for larvae of the pale grass blue butterfly may mediate the field effect of low-dose radiation exposure in Fukushima: Dose-dependent changes in the sodium content. Insects 2021, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Sakauchi, K.; Taira, W.; Otaki, J.M. Metabolomic response of the creeping wood sorrel Oxalis corniculata to low-dose radiation exposure from Fukushima’s contaminated soil. Life 2021, 11, 990. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.; Vo, N.T.K.; Omar-Nazir, L.; Batlle, J.V.I.; Otaki, J.M.; Hiyama, A.; Byun, S.H.; Seymour, C.B.; Mothersill, C. Transgenerational effects of historic radiation dose in pale grass blue butterflies around Fukushima following the Fukushima Dai-ichi Nuclear Power Plant meltdown accident. Environ. Res. 2019, 168, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Sakauchi, K.; Taira, W.; Hiyama, A.; Imanaka, T.; Otaki, J.M. The pale grass blue butterfly in ex-evacuation zones 5.5 years after the Fukushima nuclear accident: Contributions of initial high-dose exposure to transgenerational effects. J. Asia-Pac. Entomol. 2020, 23, 242–252. [Google Scholar] [CrossRef]
- Nohara, C.; Taira, W.; Hiyama, A.; Tanahara, A.; Takatsuji, T.; Otaki, J.M. Ingestion of radioactively contaminated diets for two generations in the pale grass blue butterfly. BMC Evol. Biol. 2014, 14, 193. [Google Scholar] [CrossRef] [PubMed]
- Taira, W.; Hiyama, A.; Nohara, C.; Sakauchi, K.; Otaki, J.M. Ingestional and transgenerational effects of the Fukushima nuclear accident on the pale grass blue butterfly. J. Radiat. Res. 2015, 56, i2–i18. [Google Scholar] [CrossRef]
- Taira, W.; Nohara, C.; Hiyama, A.; Otaki, J.M. Fukushima’s biological impacts: The case of the pale grass blue butterfly. J. Hered. 2014, 105, 710–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taira, W.; Toki, M.; Kakinohana, K.; Sakauchi, K.; Otaki, J.M. Developmental and hemocytological effects of ingesting Fukushima’s radiocesium on the cabbage white butterfly Pieris rapae. Sci. Rep. 2019, 9, 2625. [Google Scholar] [CrossRef] [PubMed]
- Nohara, C.; Hiyama, A.; Taira, W.; Otaki, J.M. Robustness and radiation resistance of the pale grass blue butterfly from radioactively contaminated areas: A possible case of adaptive evolution. J. Hered. 2018, 109, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Gurung, R.D.; Taira, W.; Sakauchi, K.; Iwata, M.; Hiyama, A.; Otaki, J.M. Tolerance of high oral doses of nonradioactive and radioactive caesium chloride in the pale grass blue butterfly Zizeeria maha. Insects 2019, 10, 290. [Google Scholar] [CrossRef]
- Otaki, J.M. Understanding low-dose exposure and field effects to resolve the field-laboratory paradox: Multifaceted biological effects from the Fukushima nuclear accident. In New Trends in Nuclear Science; Awwad, N.S., AlFaify, S.A., Eds.; IntechOpen: London, UK, 2018; pp. 49–71. [Google Scholar] [CrossRef]
- D’Mello, J.P.F. Phytotoxins. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2020; pp. 3–18. [Google Scholar]
- Zagrobelny, M.; de Castro, É.C.P.; Møller, B.L.; Bak, S. Cyanogenesis in arthropods: From chemical warfare to nuptial gifts. Insects 2018, 9, 51. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.S., Jr.; Francini, R. Evolutionary strategies of chemical defense in aposematic butterflies: Cyanogenesis in Asteraceae-feeding American Acraeinae. Chemoecology 1990, 1, 52–56. [Google Scholar] [CrossRef]
- Sakurai, N.; Ara, T.; Enomoto, M.; Motegi, T.; Morishita, Y.; Kurabayashi, A.; Iijima, Y.; Ogata, Y.; Nakajima, D.; Suzuki, H.; et al. Tools and databases of the KOMICS web portal for preprocessing, mining, and dissemination of metabolomics data. BioMed Res. Int. 2014, 2014, 194812. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Shibata, D. Tools and databases for an integrated metabolite annotation environment for liquid chromatography-mass spectrometry-based untargeted metabolomics. Carotenoid Sci. 2017, 22, 16–22. [Google Scholar]
- Sakurai, N.; Narise, T.; Sim, J.-S.; Lee, C.-M.; Ikeda, C.; Akimoto, N.; Kanaya, S. UC2 search: Using unique connectivity of uncharged compounds for metabolite annotation by database searching in mass-spectrometry-based metabolomics. Bioinformatics 2018, 34, 698–700. [Google Scholar] [CrossRef]
- Afendi, F.M.; Okada, T.; Yamazaki, M.; Hirai-Morita, A.; Nakamura, Y.; Nakamura, K.; Ikeda, S.; Takahashi, H.; Altaf-Ul-Amin, M.; Darusman, L.K.; et al. KNApSAcK family databases: Integrated metabolite-plant species databases for multifaceted plant research. Plant Cell Physiol. 2021, 53, e1. [Google Scholar] [CrossRef]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S. HMDB: The Human Metabolome Database. Nucl. Acid Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef]
- Wishard, D.S.; Feunang, Y.D.; Marcu, A.; Guo, A.C.; Liang, K.; Vázquez-Fresno, R.; Sajed, T.; Johnson, D.; Li, C.; Karu, N. HMDB 4.0: The human metabolome database for 2018. Nucl. Acids Res. 2018, 46, D608–D617. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, N.; Ara, T.; Kanaya, S.; Nakamura, Y.; Iijima, Y.; Enomoto, M.; Motegi, T.; Aoki, K.; Suzuki, H.; Shibata, D. An application of a relational database system for high-throughput prediction of elemental compositions from accurate mass values. Bioinformatics 2013, 29, 290–291. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Web-based inference of biological patterns, functions and pathways from metabolomic data using MetaboAnalyst. Nat. Protocol. 2011, 6, 743–760. [Google Scholar] [CrossRef]
- Xia, J.; Sinelnikov, I.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucl. Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucl. Acids Res. 2021, 49, gkab382. [Google Scholar] [CrossRef]
- Che, Y.; Wang, Q.; Xiao, R.; Zhang, J.; Zhang, Y.; Gu, W.; Rao, G.; Wang, C.; Kuang, H. Kudinoside-D, a triterpenoid saponin derived from Ilex kudingcha suppresses adipogenesis through modulation of the AMPK pathway in 3T3-L1 adipocytes. Fitoterapia 2018, 125, 208–216. [Google Scholar] [CrossRef]
- Mill, S.; Hootelé, C. Alkaloids of Andrachne aspera. J. Nat. Prod. 2000, 63, 762–764. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xiong, L.; Stevenson, B.; Lu, T.; Zhu, J.-K. The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. Plant Cell 2002, 14, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Titiz, O.; Tambasco-Studart, M.; Warzych, E.; Apel, K.; Amrheim, N.; Laloi, C.; Fitzpatrick, T.B. PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J. 2006, 48, 933–946. [Google Scholar] [CrossRef]
- González, E.; Danehower, D.; Daub, M.E. Vitamer levels, stress response, enzyme activity, and gene regulation of Arabidopsis lines mutant in the pyridoxine/pyridoxamine 5′-phosphate oxidase (PDX3) and pyridoxal kinase (SOS4) genes involved in the vitamin B6 salvage pathway. Plant Physiol. 2007, 145, 985–996. [Google Scholar] [CrossRef]
- Mahajan, S.; Pandey, G.K.; Tuteja, N. Calcium- and salt-stress signaling in plants: Shedding light on SOS pathway. Arch. Biochem. Biophys. 2008, 471, 146–158. [Google Scholar] [CrossRef]
- Hussain, S.; Huang, J.; Zhu, C.; Zhu, L.; Cao, X.; Hussain, S.; Ashraf, M.; Khaskheli, M.A.; Kong, Y.; Jin, Q.; et al. Phyridoxal 5′-phosphate enhances the growth and morpho-physiological characteristics of rice cultivars by mitigating the ethylene accumulation under salinity stress. Plant Physiol. Biochem. 2020, 154, 782–795. [Google Scholar] [CrossRef]
- You, J.; Hu, H.; Xiong, L. An ornithine δ-aminotransferase gene OsOAT confers drought and oxidative stress tolerance in rice. Plant Sci. 2021, 197, 59–69. [Google Scholar] [CrossRef]
- Singh, R.P.; Shelke, G.M.; Kumar, A.; Jha, P.N. Biochemistry and genetics of ACC deaminase: A weapon to “stress ethylene” produced in plants. Front. Microbiol. 2015, 6, 937. [Google Scholar] [CrossRef] [PubMed]
- Czégény, G.; Kőrösi, L.; Strid, Å.; Hideg, É. Multiple roles for vitamin B6 in plant acclimation to UV-B. Sci. Rep. 2019, 9, 1259. [Google Scholar] [CrossRef]
- Havaux, M.; Ksas, B.; Szewczyk, A.; Rumeau, D.; Franck, F.; Caffarri, S.; Triantaphylidès, C. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 2009, 9, 130. [Google Scholar] [CrossRef] [PubMed]
- Vanderschuren, H.; Boycheva, S.; Li, K.-T.; Szydlowski, N.; Gruissem, W.; Fitzpatrick, T.B. Strategies for vitamin B6 biofortification of plants: A dual role as a micronutrient and a stress protectant. Front. Plant Sci. 2013, 4, 143. [Google Scholar] [CrossRef] [PubMed]
- Komiyama, K.; Okada, K.; Tomisaka, S.; Umezawa, I.; Hamamoto, T.; Beppu, T. Antitumor activity of leptomycin B. J. Antibiotics 1985, 38, 427–429. [Google Scholar] [CrossRef] [PubMed]
- Tunac, J.B.; Graham, B.D.; Dobson, W.E.; Lenzini, M.D. Novel antitumor antibiotics, CI-940 (PD 114,720) and PD 114,721. Taxonomy, fermentation and biological activity. J. Antibiotics 1985, 38, 460–465. [Google Scholar] [CrossRef]
- Hamamoto, T.; Uozumi, T.; Beppu, T. Leptomycins A and B, new antifungal antibiotics. III. Mode of action of leptomycin B on Schizosaccharomyces pombe. J. Antibiotics 1985, 38, 1573–1580. [Google Scholar] [CrossRef] [PubMed]
- Mizobuchi, S.; Mochizuki, J.; Soga, H.; Tanba, H.; Inoue, H. Aldgamycin G, a new macrolide antibiotic. J. Antibiotics 1986, 39, 1776–1778. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Bui, H.T.N.; Jansen, R.; Pham, H.T.L.; Mundt, S. Carbamidocyclophanes A-E, chlorinated paracyclophanes with cytotoxic and antibiotic activity from the Vietnamese cyanobacterium Nostoc sp. J. Nat. Prod. 2007, 70, 499–503. [Google Scholar] [CrossRef]
- Sugawara, T.; Tanaka, A.; Nagai, K.; Suzuki, K.; Okada, G. New member of the trichothecene family. J. Antibiotics 1997, 50, 778–780. [Google Scholar] [CrossRef][Green Version]
- Yoshikawa, M.; Shimada, H.; Saka, M.; Yoshizumi, S.; Yamahara, J.; Matsuda, H. Medicinal foodstuffs. V. Moroheiya. (1): Absolute stereostructures of corchoionosides A, B, and C, histamine release inhibitors from the leaves of Vietnamese Corchorus olitorius L. (Tiliaceae). Chem. Pharm. Bull. 1997, 45, 464–469. [Google Scholar] [CrossRef]
- Hyun, S.K.; Kang, S.S.; Son, K.H.; Chung, H.Y.; Choi, J.S. Biflavone glucosides from Ginkgo biloba yellow leaves. Chem. Pharm. Bull. 2005, 53, 1200–1201. [Google Scholar] [CrossRef][Green Version]
- Zhao, Y.; Wu, Y.; Wang, M. Bioactive substances of plant origin. In Handbook of Food Chemistry; Cheung, P.C.K., Mehta, B.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 967–1008. [Google Scholar] [CrossRef]
- Tan, N.-H.; Zhou, J. Plant cyclopeptides. Chem. Rev. 2006, 106, 840–895. [Google Scholar] [CrossRef]
- Ammar, S.; Mahjoub, M.A.; Charfi, N.; Skandarani, I.; Chekir-Ghedira, L.; Mighri, Z. Mutagenic, antimutagenic and antioxidant activities of a new class of β-glucoside hydroxyhydroquinone from Anagallis monelli growing in Tunisia. Chem. Pharm. Bull. 2007, 55, 385–388. [Google Scholar] [CrossRef][Green Version]
- Kim, M.; Jang, Y. Phytochemical analysis of Clerodendron trichotomum by UHPLC-ESI-MS. Planta Med. 2013, 79, PL20. [Google Scholar] [CrossRef]
- He, D.-H.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Aramoto, M.; Bando, M.; Takeda, Y. Tricalysiosides H-O: Ent-kaurane glucosides from the leaves of Tricalysia dubia. Phytochemisty 2005, 66, 2857–2864. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Feng, X.Z.; Wu, L.J.; Wang, B.X.; Ikejima, T. Two new steroidal saponins from the rhizomes of Dioscorea panthaica and their cytotoxic activity. Planta Med. 2001, 67, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Bosisio, E.; Benelli, C.; Pirola, O. Effect of the flavonolignans of Silybum marianum L. on lipid peroxidation in rat liver microsomes and freshly isolated hepatocytes. Pharmacol. Res. 1992, 25, 147–165. [Google Scholar] [CrossRef]
- Li, X.; Lee, S.M.; Choi, H.D.; Kang, J.S.; Son, B.W. Microbial transformation of terreusione, an ultraviolet-A (UV-A) protecting dipyrroloquinone, by Streptomyces sp. Chem. Pharm. Bull. 2003, 51, 1458–1459. [Google Scholar] [CrossRef]
- Fiedler, H.-P.; Rohr, J.; Zeeck, A. Minor congeners of the elloramycin producer Streptomyces olivaceus. J. Antibiot. 1986, 39, 856–859. [Google Scholar] [CrossRef][Green Version]
- Itazaki, H.; Nagashima, K.; Sugita, K.; Yoshida, H.; Kawamura, Y.; Yasuda, Y.; Matsumoto, K.; Ishii, K.; Uotani, N.; Nakai, H.; et al. Isolation and structural elucidation of new cyclotetrapeptides, trapoxins A and B, having detransformation activities as antitumor agents. J. Antibiotics 1990, 43, 1524–1532. [Google Scholar] [CrossRef]
- Wang, X.; Lin, M.; Xu, D.; Lai, D.; Zhou, L. Structural diversity and biological activities of fungal cyclic peptides, excluding cyclodipeptides. Molecules 2017, 22, 2069. [Google Scholar] [CrossRef]
- Lydiard, R.B.; Gelenberg, A.J. Amoxapine—An antidepressant with some neuroleptic properties?: A review of its chemistry, animal pharmacology and toxicology, human pharmacology, and clinical efficacy. Pharmacotherapy 1981, 1, 163–178. [Google Scholar] [CrossRef]
- Wozel, G.; Blasum, C. Dapsone in dermatology and beyond. Arch. Dermatol. Res. 2014, 306, 103–124. [Google Scholar] [CrossRef]
- Smith, H.S.; Cox, L.R.; Smith, B.R. Dopamine receptor antagonists. Ann. Palliat. Med. 2012, 1, 137–142. [Google Scholar] [CrossRef]
- Yelnosky, J.; Katz, R.; Dietrich, E.V. A study of some of the pharmacologic actions of droperidol. Toxicol. Appl. Pharmacol. 1964, 6, 37–47. [Google Scholar] [CrossRef]
- Freye, E.; Kuschinsky, E. Effects of fentanyl and droperidol on the dopamine metabolism of the rat striatum. Pharmacology 1976, 14, 1–7. [Google Scholar] [CrossRef]
- Bradshaw, H.; Pleuvry, B.J.; Sharma, H.L. Effect of droperidol on dopamine-induced increase in effective retinal plasma flow in dogs. British J. Anaesthesia 1980, 52, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucl. Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. KEGG bioinformatics resource for plant genomics and metabolomics. Methods Mol. Biol. 2016, 1374, 55–70. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating viruses and cellular organisms. Nucl. Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef] [PubMed]
- Araújo, W.L.; Martins, A.O.; Femie, A.R.; Tohge, T. 2-Oxoglutarate: Linking TCA cycle function with amino acid, glucosinolate, flavonoid, alkaloid, and gibberellin. Front. Plant Sci. 2014, 5, 552. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, X.; Sun, T.; Tian, Q.; Zhang, W.-H. Glutamate receptor homolog3.4 is involved in regulation of seed germination under salt stress in Arabidopsis. Plant Cell Physiol. 2018, 59, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-G.; Ye, X.-Y.; Qiu, X.-M. Glutamate signaling enhances the heat tolerance of maize seedling by plant glutamate receptor-like channels-mediated calcium signaling. Protoplasma 2019, 256, 1165–1169. [Google Scholar] [CrossRef]
- Yamasaki, H.; Ogura, M.P.; Kingjoe, K.A.; Cohen, M.F. D-Cysteine-induced rapid root abscission in the water fern Azolla pinnata: Implications for the linkage between D-amino acid and reactive sulfur species (RSS) in plant environmental responses. Antioxidants 2019, 8, 411. [Google Scholar] [CrossRef]
- Qiu, X.-M.; Sun, Y.-Y.; Ye, X.-Y.; Li, Z.G. Signaling role of glutamate in plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Sakihama, Y.; Yamasaki, H. Phytochemical antioxidants: Past, present and future. In Antioxidants—Benefits, Sources, Mechanisms of Action; Waisundara, V., Ed.; IntechOpen: London, UK, 2021. [Google Scholar] [CrossRef]
- Qin, S.; Xing, K.; Jiang, J.-H.; Xu, L.-H.; Li, W.-J. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl. Microbiol. Biotechnol. 2011, 89, 457–473. [Google Scholar] [CrossRef]
- Shimizu, M. Endophytic actinomycetes: Biocontrol agents and growth promoters. In Bacteria in Agrobiology: Plant Growth Responses; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 201–220. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie Leeuwenhoek 2015, 108, 267–289. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Bodhankar, S.; Maheswari, M.; Srinivasarao, C. Actinomycetes as mitigators of climate change and abiotic stress. In Plant Growth Promoting Actinobacteria; Subramaniam, G., Arumugam, S., Rajendran, V., Eds.; Springer: Singapore, 2016; pp. 203–212. [Google Scholar] [CrossRef]
- Peng, A.; Liu, J.; Gao, Y.; Chen, Z. Distribution of endophytic bacteria in Alopecurus aequalis Sobol and Oxalis corniculata L. from soils contaminated by polycyclic aromatic hydrocarbons. PLoS ONE 2013, 8, e83054. [Google Scholar] [CrossRef]
- Mufti, R.; Amna Rafique, M.; Haq, F.; Munis, M.F.H.; Masood, S.; Mumtaz, A.S.; Chaudhary, H.J. Genetic diversity and metal resistance assessment of endophytes isolated from Oxalis corniculata. Soil Environ. 2015, 34, 89–99. [Google Scholar]
- Kuldau, G.; Bacon, C. Clavicipitaceous endophytes: Their ability to enhance resistance of grasses to multiple stresses. Biol. Control 2008, 46, 57–71. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Volkenburgh, E.V.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.-O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Roje, S. S-Adenosyl-L-methionine: Beyond the universal methyl group donor. Phytochemistry 2006, 67, 1686–1698. [Google Scholar] [CrossRef] [PubMed]
- Anjum, N.A.; Gill, R.; Kaushik, M.; Hasanuzzaman, M.; Pereira, E.; Ahmad, I.; Tuteja, N.; Gill, S.S. ATP-sulfurylase, sulfur-compounds, and plant stress tolerance. Front. Plant Sci. 2015, 6, 210. [Google Scholar] [CrossRef]
- Pattyn, J.; Vaughan-Hirsch, J.; Van de Poel, B. The regulation of ethylene biosynthesis: A complex multilevel control circuitry. New Phytol. 2021, 229, 770–782. [Google Scholar] [CrossRef]
- Severns, P.M.; Guzman-Martinez, M. Plant pathogen invasion modifies the eco-evolutionary host plant interactions of an endangered checkerspot butterfly. Insects 2021, 12, 246. [Google Scholar] [CrossRef] [PubMed]
- Tambasco-Studart, M.; Titiz, O.; Raschle, T.; Forster, G.; Amrhein, N.; Fitzpatrick, T.B. Vitamin B6 biosynthesis in higher plants. Proc. Natl. Acad. Sci. USA 2005, 102, 13687–13692. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, S.C.; Chanda, B.; Vaillancourt, L.; Kachroo, A.; Kachroo, P. The common metabolite glycerol-3-phosphate is a novel regulator of plant defense signaling. Plant Signal. Behav. 2009, 4, 746–749. [Google Scholar] [CrossRef] [PubMed]
- Mandal, M.K.; Chanda, B.; Xia, Y.; Yu, K.; Sekine, K.; Gao, Q.; Selote, D.; Kachroo, A.; Kachroo, P. Glycerol-3-phosphate and systemic immunity. Plant Signal. Behav. 2011, 6, 1871–1874. [Google Scholar] [CrossRef] [PubMed]
- Hoque, T.; Hossain, M.A.; Mostofa, M.G.; Burritt, D.J.; Fujita, M.; Tran, L.-S.P. Methylglyoxal: An emerging signaling molecule in plant abiotic stress responses and tolerance. Front. Plant Sci. 2016, 7, 1341. [Google Scholar] [CrossRef]
- Prouty, C.; Barriga, P.; Davis, A.K.; Krischik, V.; Altizer, S. Host plant species mediates impact of neonicotinoid exposure to Monarch butterflies. Insects 2021, 12, 999. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M. Fukushima’s lessons from the blue butterfly: A risk assessment of the human living environment in the post-Fukushima era. Integr. Environ. Assess. Manag. 2016, 12, 667–672. [Google Scholar] [CrossRef]
- Otaki, J.M.; Taira, W. Current status of the blue butterfly in Fukushima research. J. Hered. 2018, 109, 178–187. [Google Scholar] [CrossRef]
- Otaki, J.M. Fukushima Nuclear Accident: Potential health effects inferred from butterfly and human cases. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2020; pp. 497–514. [Google Scholar]
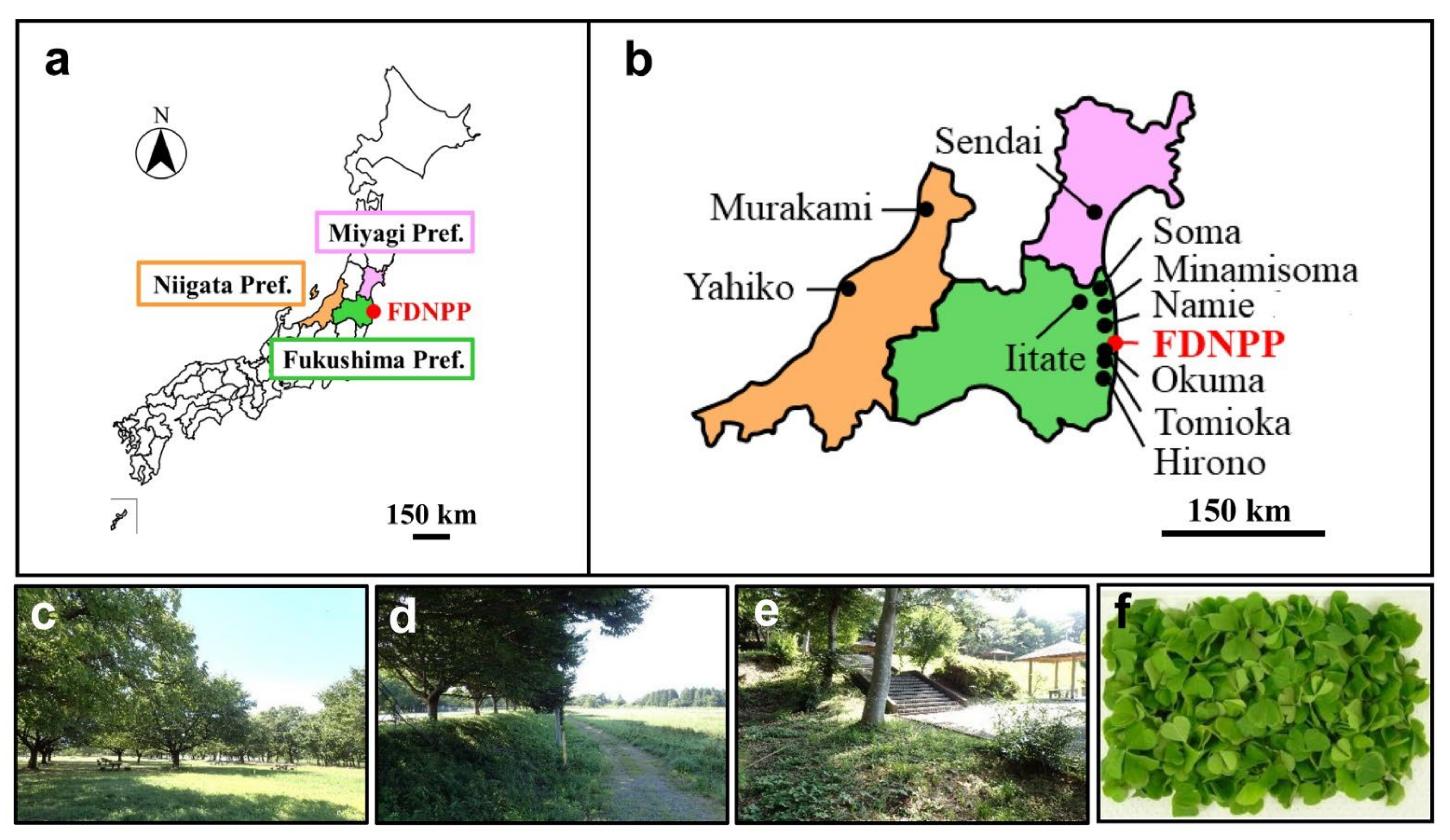




| Sample Name | Sampling Locality | Date (2018) | Ground Dose Rate [μSv/h] (*1) | 137Cs Radioactivity Concentration [Bq/kg] |
|---|---|---|---|---|
| OC01 | Murakami City, Niigata Pref. | 29 Jul | 0.07 (B) | 0 |
| OC02 | Yahiko Village, Niigata Pref. | 30 Jul | 0.04 (B) | 0 |
| OC03 | Sendai City, Miyagi Pref. | 31 Jul | 0.04 (B) | 4.54 |
| OC04 | Soma City, Fukushima Pref. | 31 Jul | 0.10 (L) | 74.45 |
| OC06 | Minamisoma City, Fukushima Pref. (Minamisoma-2) | 31 Jul | 0.42 (L) | 84.27 |
| OC07 | Hirono Town, Fukushima Pref. | 1 Aug | 0.11 (L) | 7.96 |
| OC08 | Namie Town, Fukushima Pref. (Namie-1) | 1 Aug | 0.97 (L) | 64.10 |
| OC09 | Namie Town, Fukushima Pref. (Namie-2) | 1 Aug | 2.45 (H) | 551.16 |
| OC10 | Okuma Town, Fukushima Pref. | 1 Aug | 0.60 (L) | 424.45 |
| OC11 | Tomioka Town, Fukushima Pref. | 1 Aug | 0.27 (L) | 135.98 |
| OC13 | Iitate Village, Fukushima Pref. (Iitate-1) | 17 Sep | 3.50 (H) | 213.72 |
| OC14 | Iitate Village, Fukushima Pref. (Iitate-2) | 17 Sep | 2.94 (H) | 494.74 |
| OC15 | Namie Town, Fukushima Pref. (Namie-3) | 17 Sep | 4.55 (H) | 717.65 |
| OC16 | Minamisoma City, Fukushima Pref. (Minamisoma-3) | 17 Sep | 1.46 (H) | 175.23 |
| No. | Formula | Exact Mass | Annotation (Compound Name) | Possible Function | Origin |
|---|---|---|---|---|---|
| 9321 | C33H48O6 | 540.345 | Antibiotic Cl 940, Antibiotic CL 1957A, Cl 940, Elactocin, Leptomycin B, Mantuamycin | Antibiotics | Streptomyces sp. (Microbe-derived) |
| 9368 | C47H72O17 | 908.477 | Kudinoside D | Triterpenoid saponin; Adipogenesis suppressor | Ilex kudingucha (Plant-derived) |
| 8451 | C38H56O8N2Cl2 | 738.341 | Carbamidocyclophane C, (+)-Carbamidocyclophane C | Cytotoxic compound | Nostoc sp. (Cyanobacteria) (Microbe-derived) |
| 7968 | C37H56O15 | 740.362 | Aldgamycin G | Antibiotics | Streptomyces sp. (Microbe-derived) |
| 8935 | C13H25O2N | 227.189 | Andrachcinidine, (-)-Andrachcinidine | Alkaloid | Andrachne aspera (Plant-derived) |
| 7563 | C33H46O11 | 618.304 | YM 47525 | Trichothecene; Fungicide | Fungus (Microbe-derived) |
| 178 | C8H10O6NP | 247.025 | Pyridoxal phosphate | Activated vitamin B6 | Plants and microbes (Plant-derived) (*1) |
| No. | Formula | Exact Mass | Annotation (Compound Name) | Possible Function | Origin |
|---|---|---|---|---|---|
| 4887 | C19H28O9 | 400.173 | Corchoionoside B | Fatty acyl glucoside; Membrane stabilizer | Corchorus olitorius (Plant-derived) |
| 7156 | C38H32O15 | 728.174 | Isoginkgetin-7-O-β-D-glucopyranoside | Bioflavone glucoside | Ginkgo biloba (Plant-derived) |
| 6296 | C18H22O5N2 | 346.153 | Terreusinol, (+)-Terreusinol | Antibiotics | Streptomyces sp. (Microbe-derived) |
| 3152 | C17H16ON3Cl | 313.098 | Amoxapine | GPCR (G-protein-coupled receptor) inhibitor | (Others, synthetic) |
| 3073 | C32H34O15 | 658.190 | Elloramycin E | Antibiotics | Streptomyces sp. (Microbe-derived) |
| 8925 | C31H42O6N4 | 566.310 | Sanjoinine A dialdehyde | Alkaloid (Cyclopeptide) | Zizyphus lotus (Plant-derived) (*1) |
| 609 | C14H21O8N | 331.127 | Zinolol | Antioxidant | Anagallis onellin (Plant-derived) |
| 2963 | C28H28O17 | 636.133 | Acacetin-7-glucuronosyl-(1→2)-glucuronide | Flavonoid | Clerodendron trichotomum (Plant-derived) |
| 3171 | C12H12O3N2S | 264.057 | Dapsone hydroxylamine | Dermatologically used drug | (Others, synthetic) |
| 8804 | C34H42O6N4 | 602.310 | RF 1023A, Trapoxin A | Cyclic peptide; histone deacetylase inhibitor | Helicoma ambiens RF-1023 (Fungus) (Microbe-derived) |
| 7781 | C28H46O11 | 558.304 | Tricalysioside N, (-)-Tricalysioside N | Ent-kaurane glucoside | Tricalysia dubia (Plant-derived) |
| 9091 | C39H60O15 | 768.393 | Pregnadienolone-3-O-β-D-chacotrioside | Saponin | Dioscorea panthaica (Plant-derived) |
| 4347 | C21H26ON3SCl | 403.149 | Perphenazine | Dopamine receptor D2 antagonist | (Others, synthetic) |
| 3836 | C25H24O10 | 484.137 | Silidianin | Flavonolignan | Silybum marianum (Plant-derived) |
| 8800 | C22H22O2N3F | 379.170 | Droperidol | Dopamine receptor antagonist | (Others, synthetic) |
| No. | Brief Annotation | Ground Radiation Dose Rate [μSv/h] | Radioactivity Concentration of 137Cs [Bq/kg] |
|---|---|---|---|
| 9321 | Leptomycin B | r = 0.75, p = 0.0021 ** (linear) | r = 0.66, p = 0.0100 * (linear) |
| 9368 | Kudinoside D | r = 0.84, p = 0.0002 *** (linear) | r = 0.59, p = 0.027 * (linear) |
| 8451 | Carbamidocyclophane C | r = 0.72, p = 0.0037 ** (linear) r = 0.78, p = 0.0009 *** (logarithmic) | r = 0.51, p = 0.062 (linear) |
| 7968 | Aldgamycin G | r = 0.58, p = 0.030 * (linear) r = 0.72, p = 0.0037 ** (logarithmic) | r = 0.53, p = 0.051 (linear) |
| 8935 | Andrachcinidine | r = 0.83, p = 0.003 *** (linear) r = 0.84, p = 0.0001 *** (logarithmic) | r = 0.66, p = 0.0097 ** (linear) |
| 7563 | YM 47525 | r = 0.87, p < 0.0001 *** (linear) | r = 0.72, p = 0.0035 ** (linear) |
| 178 | Pyridoxal phosphate | r = 0.69, p = 0.0088 ** (linear) | r = 0.42, p = 0.14 (linear) |
| No. | Brief Annotation | Ground Radiation Dose Rate [μSv/h] | Radioactivity Concentration of 137Cs [Bq/kg] |
|---|---|---|---|
| 4887 | Corchoionoside B | r = −0.55, p = 0.041 * (linear) r = −0.76, p = 0.0017 ** (logarithmic) | r = −0.51, p = 0.065 (linear) |
| 7156 | Isoginkgetin-7-O-β-D- glucopyranoside | r = −0.41, p = 0.14 (linear) r = −0.66, p = 0.0102 * (logarithmic) | r = −0.45, p = 0.10 (linear) |
| 6296 | Terreusinol | r = −0.41, p = 0.15 (linear) r = −0.65, p = 0.012 * (logarithmic) | r = −0.45, p = 0.10 (linear) |
| 3152 | Amoxapine | r = −0.74, p = 0.0026 ** (linear) r = −0.92, p < 0.0001 *** (logarithmic) | r = −0.72, p = 0.0031 ** (linear) |
| 3073 | Elloramycin E | r = −0.62, p = 0.018 * (linear) r = −0.80, p = 0.0006 *** (logarithmic) | r = −0.51, p = 0.060 (linear) |
| 8925 | Sanjoinine A dialdehyde | r = −0.56, p = 0.039 * (linear) r = −0.76, p = 0.0015 ** (logarithmic) | r = −0.54, p = 0.046 (linear) |
| 609 | Zinolol | r = −0.34, p = 0.24 (linear) r = −0.59, p = 0.028 * (logarithmic) | r = −0.38, p = 0.18 (linear) |
| 2963 | Acacetin-7-glucuronosyl-(1→2)- glucuronide | r = −0.61, p = 0.020 * (linear) r = −0.79, p = 0.0009 ***(logarithmic) | r = −0.41, p = 0.14 (linear) |
| 3171 | Dapsone hydroxylamine | r = −0.39, p = 0.16 (linear) r = −0.60, p = 0.023 * (logarithmic) | r = −0.36, p = 0.21 (linear) |
| 8804 | Trapoxin A | r = −0.50, p = 0.066 (linear) r = −0.70, p = 0.0053 ** (logarithmic) | r = −0.54, p = 0.047 * (linear) |
| 7781 | Tricalysioside N | r = −0.65, p = 0.012 * (linear) r = −0.74, p = 0.0023 ** (logarithmic) | r = −0.59, p = 0.026 * (linear) |
| 9091 | Pregnadienolone-3-O-β-D- chacotrioside | r = −0.66, p = 0.0098 ** (linear) r = −0.84, p = 0.0102 * (logarithmic) | r = −0.68, p = 0.0079 ** (linear) |
| 4347 | Perphenazine | r = −0.52, p = 0.059 (linear) r = −0.66, p = 0.0002 *** (logarithmic) | r = −0.52, p = 0.056 (linear) |
| 3836 | Silidianin | r = −0.59, p = 0.027 * (linear) r = −0.72, p = 0.0034 ** (logarithmic) | r = −0.53, p = 0.052 (linear) |
| 8800 | Droperidol | r = −0.75, p = 0.0020 ** (linear) | r = −0.61, p = 0.019 * (linear) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sakauchi, K.; Taira, W.; Otaki, J.M. Metabolomic Profiles of the Creeping Wood Sorrel Oxalis corniculata in Radioactively Contaminated Fields in Fukushima: Dose-Dependent Changes in Key Metabolites. Life 2022, 12, 115. https://doi.org/10.3390/life12010115
Sakauchi K, Taira W, Otaki JM. Metabolomic Profiles of the Creeping Wood Sorrel Oxalis corniculata in Radioactively Contaminated Fields in Fukushima: Dose-Dependent Changes in Key Metabolites. Life. 2022; 12(1):115. https://doi.org/10.3390/life12010115
Chicago/Turabian StyleSakauchi, Ko, Wataru Taira, and Joji M. Otaki. 2022. "Metabolomic Profiles of the Creeping Wood Sorrel Oxalis corniculata in Radioactively Contaminated Fields in Fukushima: Dose-Dependent Changes in Key Metabolites" Life 12, no. 1: 115. https://doi.org/10.3390/life12010115
APA StyleSakauchi, K., Taira, W., & Otaki, J. M. (2022). Metabolomic Profiles of the Creeping Wood Sorrel Oxalis corniculata in Radioactively Contaminated Fields in Fukushima: Dose-Dependent Changes in Key Metabolites. Life, 12(1), 115. https://doi.org/10.3390/life12010115







