Ingestional Toxicity of Radiation-Dependent Metabolites of the Host Plant for the Pale Grass Blue Butterfly: A Mechanism of Field Effects of Radioactive Pollution in Fukushima
Abstract
1. Introduction
2. Materials and Methods
2.1. Egg Collection and Larval Rearing
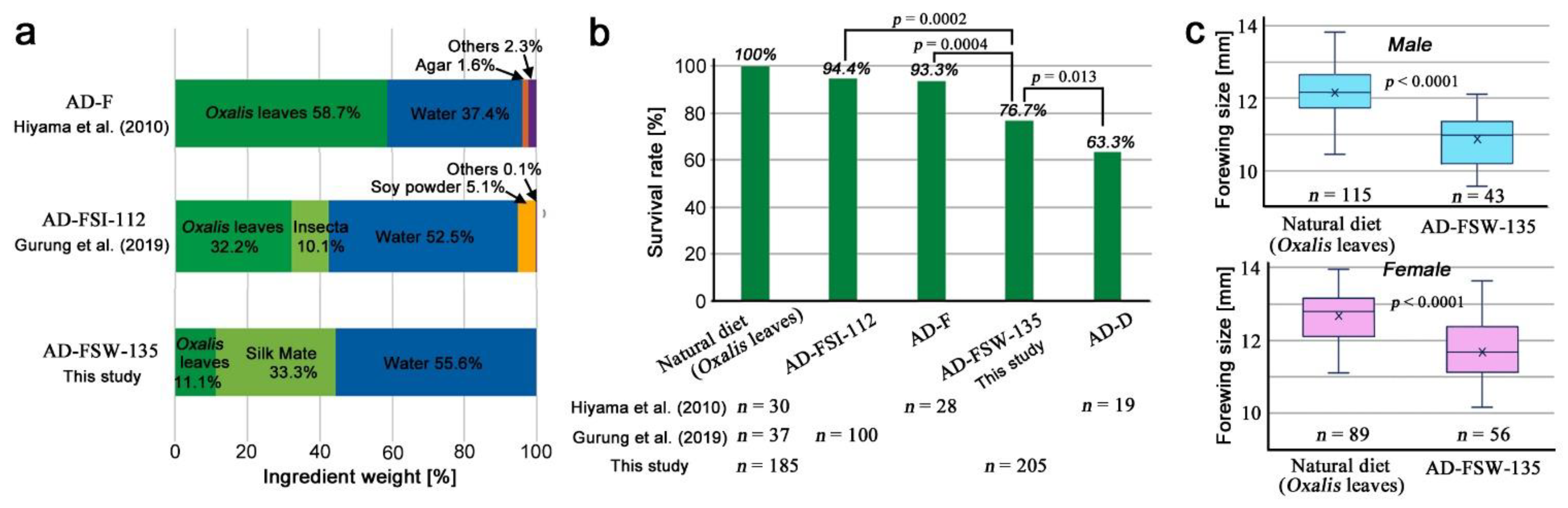
2.2. Artificial Diet Preparation
2.3. Lauric Acid, Alfuzosin, and Ikarugamycin
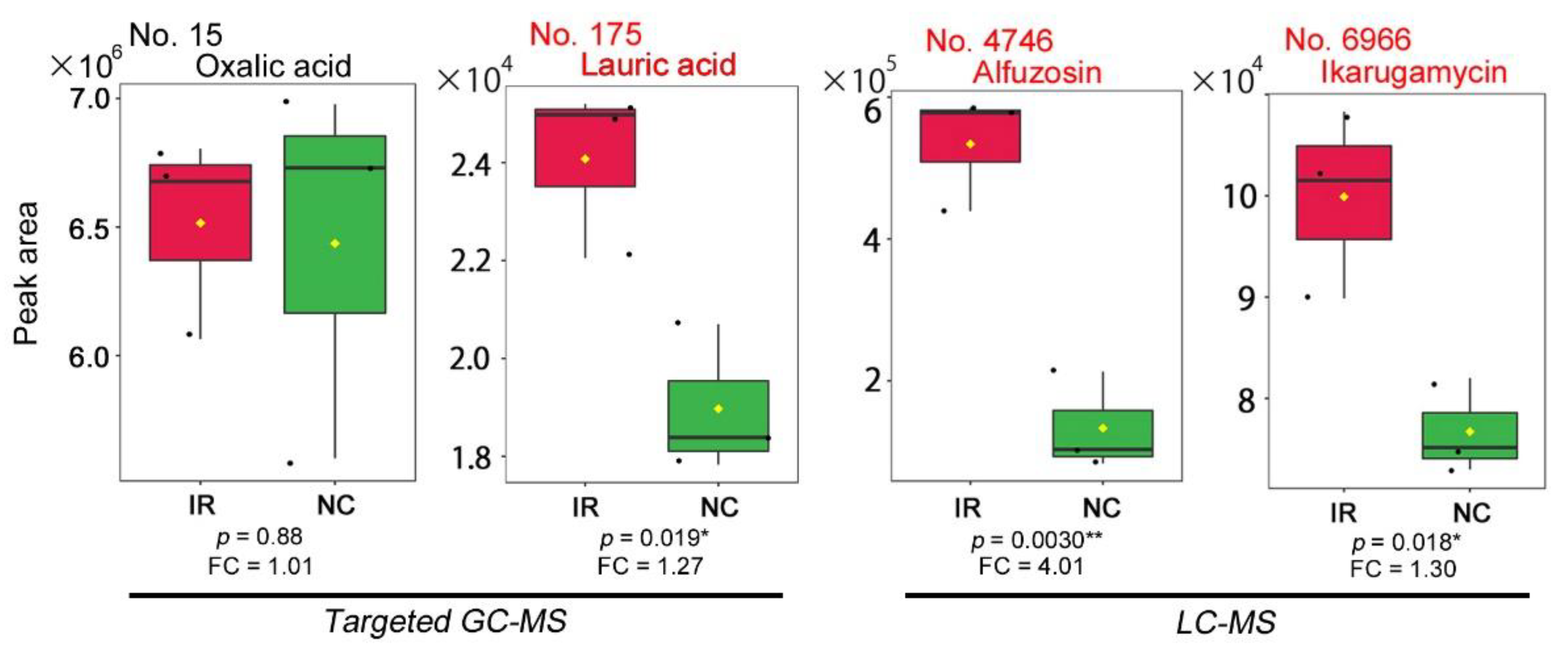
2.4. Concentration of Lauric Acid in Leaves
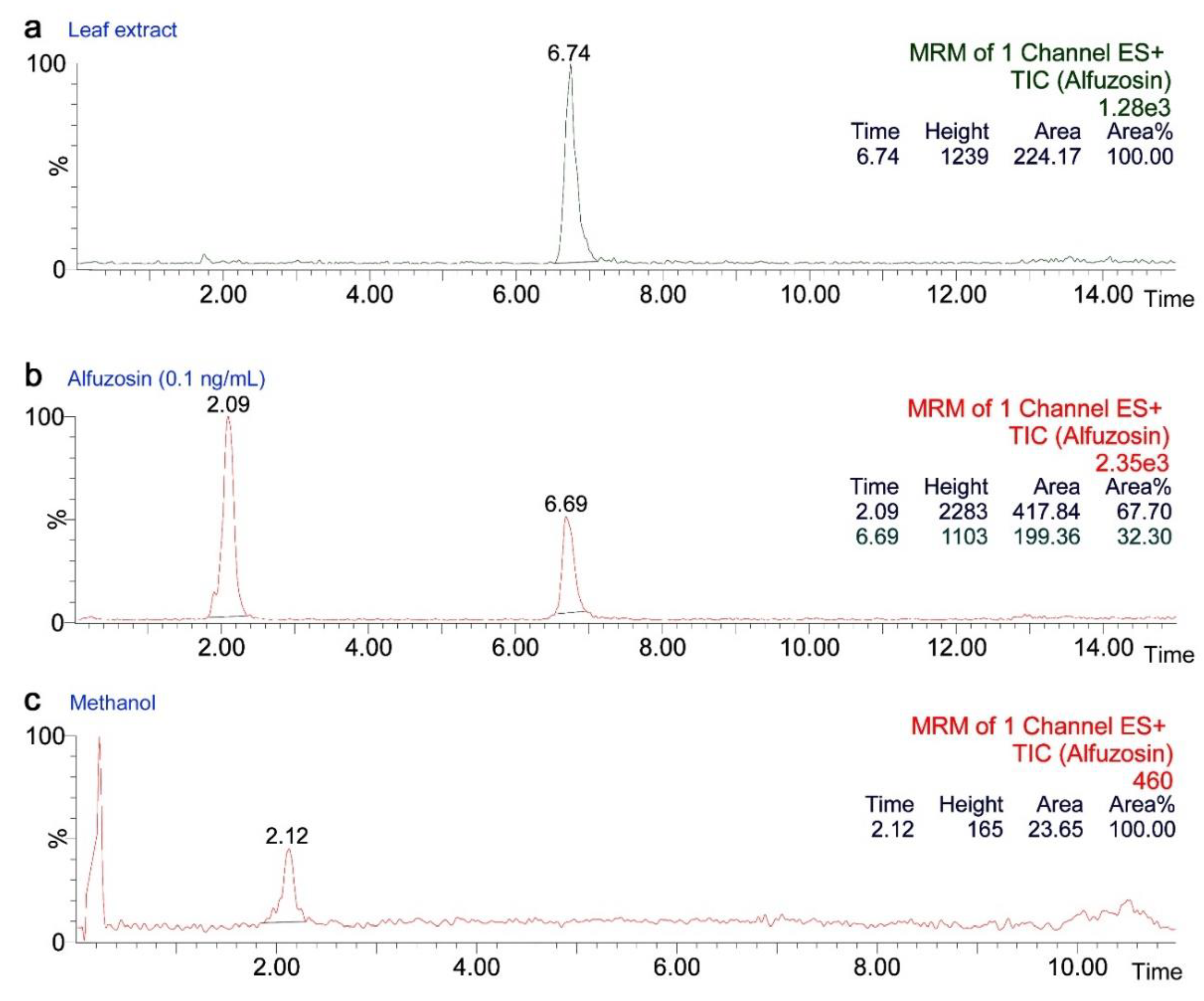
2.5. Concentration of the Alfuzosin-Related Compound in Leaves
2.6. Concentration of Ikarugamycin in Leaves
2.7. Toxicological Output Data
2.8. Statistical Analysis
3. Results
3.1. Performance of the Artificial Diet AD-FSW-135
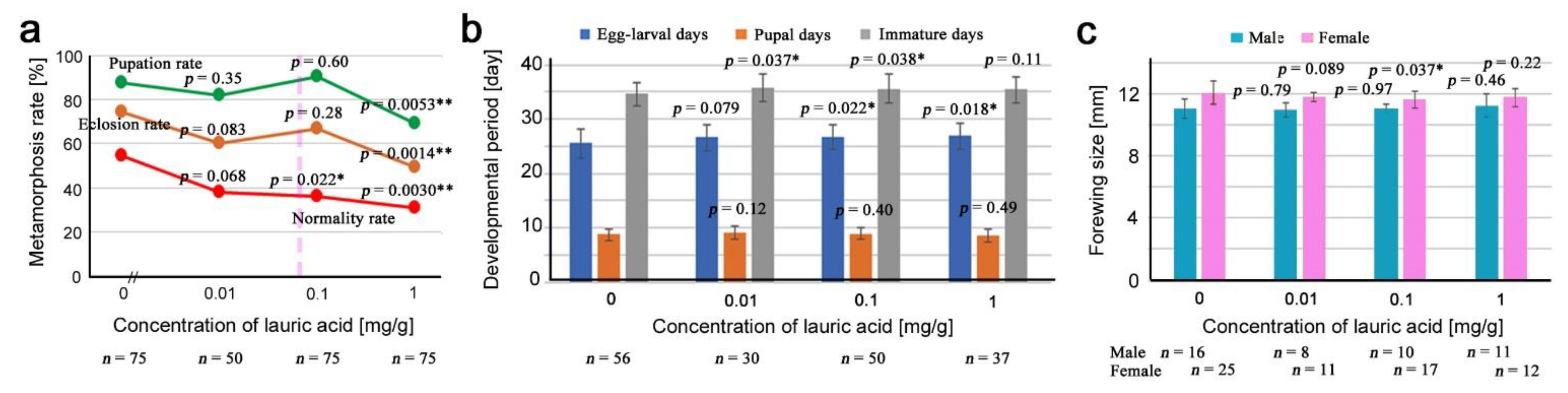
3.2. Lauric Acid
3.3. Alfuzosin
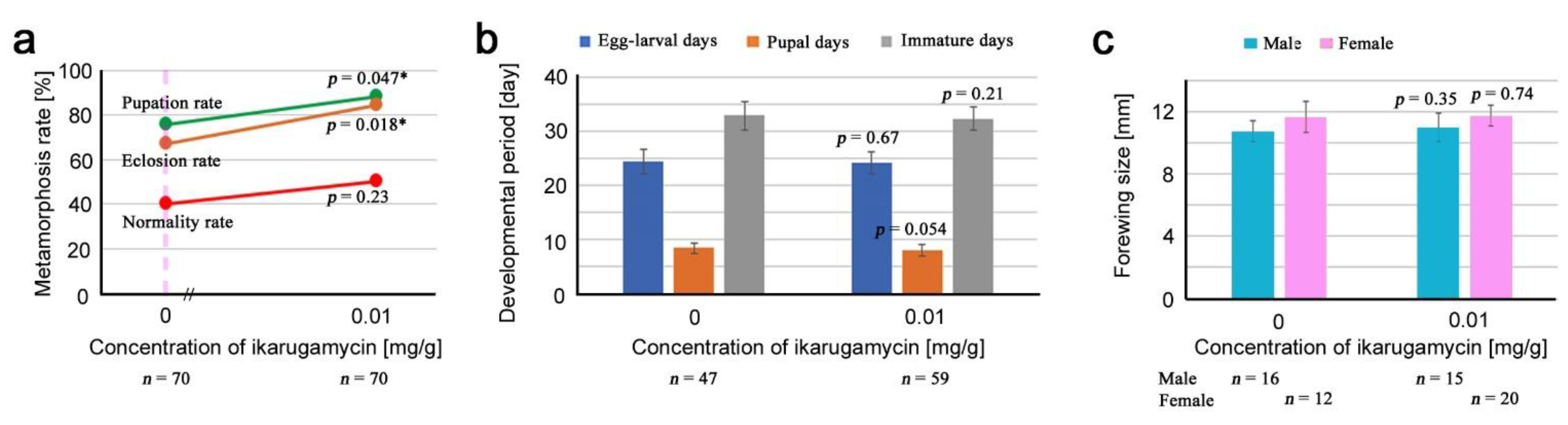
3.4. Ikarugamycin
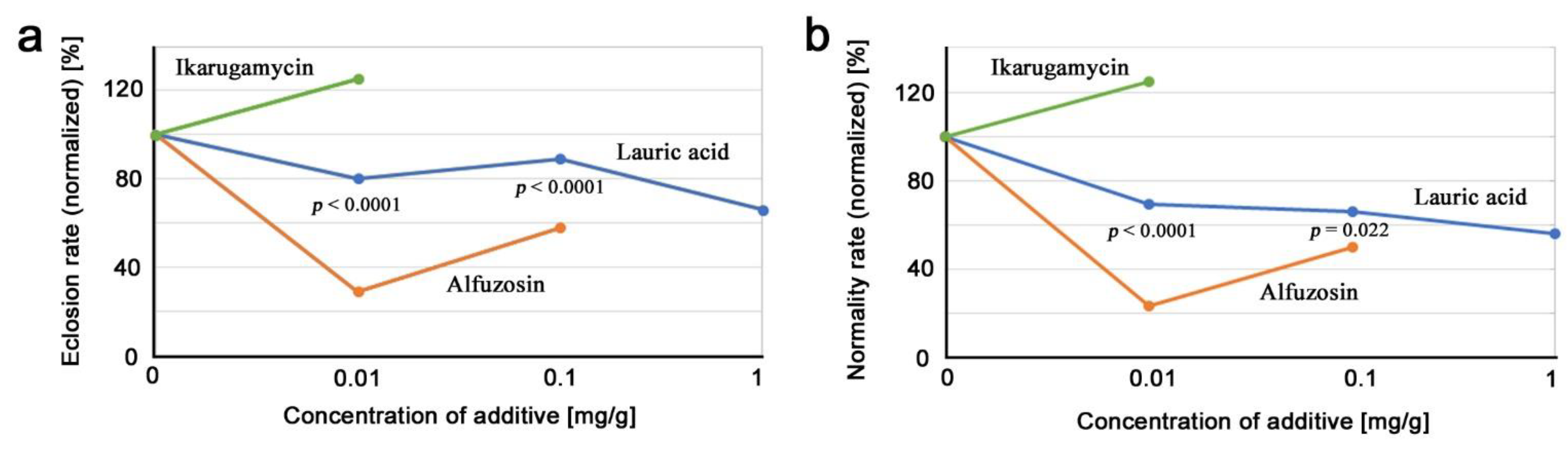
3.5. Comparison of Three Compounds
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| Number | Leaf | AD-FSW-135 |
|---|---|---|
| Number of starting individuals | 185 (100%) | 205 (100%) |
| Number of pupae (Pupation rate) | 168 (90.8%) | 165 (80.5%) |
| Number of eclosion (Eclosion rate) | 166 (89.7%) | 141 (68.7%) |
| Number of normal adults (Normality rate) | 156 (84.4%) | 99 (48.3%) |
| Number | 0 mg/g | 0.01 mg/g | 0.1 mg/g | 1 mg/g |
|---|---|---|---|---|
| Number of starting individuals | 75 (100%) | 50 (100%) | 75 (100%) | 75 (100%) |
| Number of pupae (Pupation rate) | 66 (88.0%) | 41 (82.0%) | 68 (90.7%) | 52 (69.3%) |
| Number of eclosion (Eclosion rate) | 56 (74.7%) | 30 (60.0%) | 50 (66.7%) | 37 (49.3%) |
| Number of normal adults (Normality rate) | 41 (54.7%) | 19 (38.0%) | 27 (36%) | 23 (30.7%) |
| Number | 0 mg/g | 0.01 mg/g | 0.1 mg/g |
|---|---|---|---|
| Number of starting larvae | 60 (100%) | 60 (100%) | 60 (100%) |
| Number of pupae (Pupation rate) | 46 (76.7%) | 17 (28.3%) | 26 (43.3%) |
| Number of eclosion (Eclosion rate) | 38 (63.3%) | 11 (18.3%) | 22 (36.7%) |
| Number of normal adults (Normality rate) | 30 (50.0%) | 7 (11.7%) | 15 (25.0%) |
| Number | 0 mg/g | 0.01 mg/g |
|---|---|---|
| Number of starting individuals | 70 (100%) | 70 (100%) |
| Number of pupae (Pupation rate) | 53 (75.7%) | 62 (88.6%) |
| Number of eclosion (Eclosion rate) | 47 (67.1%) | 59 (84.3%) |
| Number of normal adults (Normality rate) | 28 (40.0%) | 35 (50.0%) |
| Metabolite | 0 mg/g | 0.01 mg/g | 0.1 mg/g | 1 mg/g |
|---|---|---|---|---|
| Lauric acid | 100% | 80.3% | 89.3% | 66.0% |
| Alfuzosin | 100% | 28.9% | 58.0% | NA |
| Ikarugamycin | 100% | 125.6% | NA | NA |
| Metabolite | 0 mg/g | 0.01 mg/g | 0.1 mg/g | 1 mg/g |
|---|---|---|---|---|
| Lauric acid | 100% | 69.5% | 65.8% | 56.1% |
| Alfuzosin | 100% | 23.4% | 50.0% | NA |
| Ikarugamycin | 100% | 125.0% | NA | NA |
References
- D’Mello, J.P.F. Preface. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2020; pp. xxv–xxxvi. [Google Scholar]
- D’Mello, J.P.F. Unequivocal evidence associating environmental contaminants and pollutants with human morbidity and ecological degradation. In A Handbook of Environmental Toxicology: Human Disorders and Ecotoxicology; D’Mello, J.P.F., Ed.; CAB International: Wallingford, UK, 2020; pp. 587–610. [Google Scholar]
- Lokobauer, N.; Franić, Z.; Bauman, A.; Maračić, M.; Cesar, D.; Senčar, J. Radiation contamination after the Chernobyl nuclear accident and the effective dose received by the population of Croatia. J. Environ. Radioact. 1998, 41, 137–146. [Google Scholar] [CrossRef]
- Arapis, G.D.; Karandinos, M.G. Migration of 137Cs in the soil of sloping semi-natural ecosystems in Northern Greece. J. Environ. Radioact. 2004, 77, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Tahir, S.N.A.; Jamil, K.; Zaidi, J.H.; Arif, M.; Ahmed, N. Activity concentration of 137Cs in soil samples from Punjab province (Pakistan) and estimation of gamma-ray dose rate for external exposure. Radiat. Prot. Dosim. 2006, 118, 345–351. [Google Scholar] [CrossRef] [PubMed]
- Ambrosino, F.; Stellato, L.; Sabbarese, C. A case study on possible radiological contamination in the Lo Uttara landfill site (Caserta, Italy). J. Phys. Conf. Ser. 2020, 1548, 012001. [Google Scholar] [CrossRef]
- Endo, S.; Kimura, S.; Takatsuji, T.; Nanasawa, K.; Imanaka, T.; Shizuma, K. Measurement of soil contamination by radionuclides due to the Fukushima Dai-ichi Nuclear Ppower Plant accident and associated estimated cumulative external dose estimation. J. Environ. Radioact. 2021, 111, 18–27. [Google Scholar] [CrossRef]
- Thomas, J.A. Monitoring changes in the abundance and distribution of insects using butterflies and other indicator groups. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2005, 360, 339–357. [Google Scholar] [CrossRef]
- Vickery, M. Butterflies as indicators of climate change. Sci. Prog. 2008, 91, 193–201. [Google Scholar] [CrossRef]
- Warren, M.S.; Hill, J.K.; Thomas, J.A.; Asher, J.; Fox, R.; Huntley, B.; Roy, D.B.; Telfer, M.G.; Jeffcoate, S.; Harding, P.; et al. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 2001, 414, 65–69. [Google Scholar] [CrossRef]
- MacGregor, C.J.; Thomas, C.D.; Roy, D.B.; Beaumont, M.; Bell, J.; Brereton, T.; Bridle, J.; Dytham, C.; Fox, R.; Gotthard, K.; et al. Climate-induced phenology shifts linked to range expansions in species with multiple reproductive cycles per year. Nat. Commun. 2019, 10, 4455. [Google Scholar] [CrossRef]
- Halsch, C.A.; Shapiro, A.M.; Fordyce, J.A.; Nice, C.C.; Thorne, J.H.; Waetjen, D.P.; Forister, M.L. Insects and recent climate change. Proc. Natl. Acad. Sci. USA 2021, 118, e2002543117. [Google Scholar] [CrossRef]
- Forister, M.L.; Halsch, C.A.; Nice, C.C.; Fordyce, J.A.; Dilts, T.E.; Oliver, J.C.; Prudic, K.L.; Shapiro, A.M.; Wilson, J.K.; Glassberg, J. Fewer butterflies seen by community scientists across the warming and drying landscapes of the American West. Science 2021, 371, 1042–1045. [Google Scholar] [CrossRef] [PubMed]
- Forister, M.L.; McCall, A.C.; Sanders, N.J.; Fordyce, J.A.; Thorne, J.H.; O’Brien, J.; Waetjen, D.P.; Shapiro, A.M. Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl. Acad. Sci. USA 2010, 107, 2088–2092. [Google Scholar] [CrossRef] [PubMed]
- Rödder, D.; Schmitt, T.; Gros, P.; Ulrich, W.; Habel, J.C. Climate change drives mountain butterflies towards the summits. Sci. Rep. 2021, 11, 14382. [Google Scholar] [CrossRef] [PubMed]
- Zografou, K.; Swartz, M.T.; Adamidis, G.; Tilden, V.P.; McKinney, E.N.; Sewall, B.J. Species traits affect phenological responses to climate change in a butterfly community. Sci. Rep. 2021, 11, 3283. [Google Scholar] [CrossRef] [PubMed]
- Otaki, J.M.; Hiyama, A.; Iwata, M.; Kudo, T. Phenotypic plasticity in the range-margin population of the lycaenid butterfly Zizeeria maha. BMC Evol. Biol. 2010, 10, 252. [Google Scholar] [CrossRef] [PubMed]
- Buckley, J.; Butlin, R.; Bridle, J.R. Evidence for evolutionary change associated with the recent range expansion of the British butterfly, Aricia agestis, in response to climate change. Mol. Ecol. 2012, 21, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, J.; Buckley, L. Evolution of plasticity and adaptive responses to climate change along climate gradients. Proc. Biol. Sci. 2017, 284, 20170386. [Google Scholar] [CrossRef]
- Au, T.F.; Bonebrake, T. Increased suitability of poleward climate for a tropical butterfly (Euripus nyctelius) (Lepidoptera: Nymphalidae) accompanies its successful range expansion. J. Insect Sci. 2019, 19, 2. [Google Scholar] [CrossRef]
- Carnicer, J.; Stefanescu, C.; Vives-Ingla, M.; López, C.; Cortizas, S.; Wheat, C.; Vila, R.; Llusià, J.; Peñuelas, J. Phenotypic biomarkers of climatic impacts on declining insect populations: A key role for decadal drought, thermal buffering and amplication effects and host plant dynamics. J. Anim. Ecol. 2019, 88, 376–391. [Google Scholar] [CrossRef]
- Herremans, M.; Gielen, K.; Van Kerckhoven, J.; Vanormelingen, P.; Veraghtert, W.; Swinnen, K.R.R.; Maes, D. Abundant citizen science data reveal that the peacock butterfly Aglais io recently became bivoltine in Belgium. Insects 2021, 12, 683. [Google Scholar] [CrossRef]
- Wendt, M.; Senftleben, N.; Gros, P.; Schmitt, T. Coping with environmental extremes: Population ecology and behavioral adaptation of Erebia pronoe, an alpine butterfly species. Insects 2021, 12, 896. [Google Scholar] [CrossRef]
- Hiyama, A.; Iwata, M.; Otaki, J.M. Rearing the pale grass blue Zizeeria maha (Lepidoptera, Lycaenidae): Toward the establishment of a lycaenid model system for butterfly physiology and genetics. Entomol. Sci. 2010, 13, 293–302. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Otaki, J.M. Color-pattern evolution in response to environmental stress in butterflies. Front. Genet. 2012, 3, 15. [Google Scholar] [CrossRef]
- Taira, W.; Iwasaki, M.; Otaki, J.M. Body size distributions of the pale grass blue butterfly in Japan: Size rules and the status of the Fukushima population. Sci. Rep. 2015, 5, 12351. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Sakauchi, K.; Otaki, J.M. Sampling efficiency of the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae): A versatile indicator species for environmental risk assessment in Japan. J. Asia-Pac. Entomol. 2018, 21, 609–615. [Google Scholar] [CrossRef]
- Hiyama, A.; Otaki, J.M. Dispersibility of the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae) revealed by one-individual tracking in the field: Quantitative comparisons between subspecies and between sexes. Insects 2020, 11, 122. [Google Scholar] [CrossRef]
- Buckley, J.; Bridle, J.R.; Pomiankowski, A. Novel variation associated with species range expansion. BMC Evol. Biol. 2010, 10, 382. [Google Scholar] [CrossRef]
- Gilbert, S.; Epel, D. Ecological Developmental Biology; Sinauer Associates: Sunderland, MA, USA, 2016. [Google Scholar]
- Møller, A.P.; Hagiwara, A.; Matsui, S.; Kasahara, S.; Kawatsu, K.; Nishiumi, I.; Suzuki, H.; Mousseau, T.A. Abundance of birds in Fukushima as judges from Chernobyl. Environ. Pollut. 2012, 164, 36–39. [Google Scholar] [CrossRef]
- Bonisoli-Alquati, A.; Koyama, K.; Tedeschi, D.J.; Kitamura, W.; Sukuzi, H.; Ostermiller, S.; Arai, E.; Møller, A.P.; Mousseau, T.A. Abundance and genetic damage of barn swallows from Fukushima. Sci. Rep. 2015, 5, 9432. [Google Scholar] [CrossRef]
- Murase, K.; Murase, J.; Horie, R.; Endo, K. Effects of the Fukushima Daiichi nuclear accident on goshawk reproduction. Sci. Rep. 2015, 5, 9405. [Google Scholar] [CrossRef]
- Hiyama, A.; Nohara, C.; Kinjo, S.; Taira, W.; Gima, S.; Tanahara, A.; Otaki, J.M. The biological impacts of the Fukushima nuclear accident on the pale grass blue butterfly. Sci. Rep. 2012, 2, 570. [Google Scholar] [CrossRef]
- Hiyama, A.; Nohara, C.; Taira, W.; Kinjo, S.; Iwata, M.; Otaki, J.M. The Fukushima nuclear accident and the pale grass blue butterfly: Evaluating biological effects of long-term low-dose exposures. BMC Evol. Biol. 2013, 13, 168. [Google Scholar] [CrossRef]
- Nohara, C.; Hiyama, A.; Taira, W.; Tanahara, A.; Otaki, J.M. The biological impacts of ingested radioactive materials on the pale grass blue butterfly. Sci. Rep. 2014, 4, 4946. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Nohara, C.; Iwasaki, M.; Kinjo, S.; Iwata, M.; Otaki, J.M. Spatiotemporal abnormality dynamics of the pale grass blue butterfly: Three years of monitoring (2011–2013) after the Fukushima nuclear accident. BMC Evol. Biol. 2015, 15, 15. [Google Scholar] [CrossRef]
- Akimoto, S. Morphological abnormalities in gall-forming aphids in a radiation-contaminated area near Fukushima Daiichi: Selective impact of fallout? Ecol. Evol. 2014, 4, 355–369. [Google Scholar] [CrossRef]
- Akimoto, S.I.; Li, Y.; Imanaka, T.; Sato, H.; Ishida, K. Effects of radiation from contaminated soil and moss in Fukushima on embryogenesis and egg hatching of the aphid Prociphilus oriens. J. Hered. 2018, 109, 199–205. [Google Scholar] [CrossRef]
- Ochiai, K.; Hayama, S.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Uno, T.; Kato, T.; Konno, F.; Kawamoto, Y.; Tsuchida, S.; et al. Low blood cell counts in wild Japanese monkeys after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2014, 4, 5793. [Google Scholar] [CrossRef]
- Hayama, S.; Tsuchiya, M.; Ochiai, K.; Nakiri, S.; Nakanishi, S.; Ishii, N.; Kato, T.; Tanaka, A.; Konno, F.; Kawamoto, Y.; et al. Small head size and delayed body weight growth in wild Japanese monkey fetuses after the Fukushima Daiichi nuclear disaster. Sci. Rep. 2017, 7, 3528. [Google Scholar] [CrossRef]
- Urushihara, Y.; Suzuki, T.; Shimizu, Y.; Ohtaki, M.; Kuwahara, Y.; Suzuki, M.; Uno, T.; Fujita, S.; Saito, A.; Yamashiro, H.; et al. Haematological analysis of Japanese macaques (Macaca fuscata) in the area affected by the Fukushima Daiichi Nuclear Power Plant accident. Sci. Rep. 2018, 8, 16748. [Google Scholar] [CrossRef]
- Horiguchi, T.; Yoshii, H.; Mizuno, S.; Shiraishi, H. Decline in intertidal biota after the 2011 Great East Japan Earthquake and Tsunami and the Fukushima nuclear disaster: Field observations. Sci. Rep. 2016, 6, 20416. [Google Scholar] [CrossRef]
- Hayashi, G.; Shibato, J.; Imanaka, T.; Cho, K.; Kubo, A.; Kikuchi, S.; Satoh, K.; Kimura, S.; Ozawa, S.; Fukutani, S.; et al. Unraveling low-level gamma radiation-responsive changes in expression of early and late genes in leaves of rice seedlings at Iitate Village, Fukushima. J. Hered. 2014, 105, 723–738. [Google Scholar] [CrossRef] [PubMed]
- Rakwal, R.; Hayashi, G.; Shibato, J.; Deepak, S.A.; Gundimeda, S.; Simha, U.; Padmanaban, A.; Gupta, R.; Han, S.; Kim, S.T.; et al. Progress toward rice seed OMICS in low-level gamma radiation environment in Iitate Village, Fukushima. J. Hered. 2018, 109, 2089–2211. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Ichikawa, S.; Kubota, M.; Hoshino, J.; Kubota, Y.; Maruyama, K.; Fuma, S.; Kawaguchi, I.; Yoschenko, V.I.; Yoshida, S. Morphological defects in native Japanese fir trees around the Fukushima Daiichi Nuclear Power Plant. Sci. Rep. 2015, 5, 13232. [Google Scholar] [CrossRef] [PubMed]
- Yoschenko, V.; Nanba, K.; Yoshida, S.; Watanabe, Y.; Takase, T.; Sato, N.; Keitoku, K. Morphological abnormalities in Japanese red pine (Pinus densiflora) at the territories contaminated as a result of the accident at Fukushima Dai-ichi Nuclear Power Plant. J. Environ. Radioact. 2016, 165, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Sakauchi, K.; Taira, W.; Toki, M.; Tsuhako, M.; Umetsu, K.; Otaki, J.M. Nutrient imbalance of the host plant for larvae of the pale grass blue butterfly may mediate the field effect of low-dose radiation exposure in Fukushima: Dose-dependent changes in the sodium content. Insects 2021, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Sakauchi, K.; Taira, W.; Otaki, J.M. Metabolomic response of the creeping wood sorrel Oxalis corniculata to low-dose radiation exposure from Fukushima’s contaminated soil. Life 2021, 11, 990. [Google Scholar] [CrossRef]
- Sakauchi, K.; Taira, W.; Otaki, J.M. Metabolomic profiles of the creeping wood sorrel Oxalis corniculata in radioactivelt contaminated fields in Fukushima: Dose-dependent changes in key metabolites. Life 2022, 12, 115. [Google Scholar] [CrossRef]
- Yamanouchi, K.; Tsujiguchi, T.; Shiroma, Y.; Suzuki, T.; Tamakuma, Y.; Sakamoto, Y.; Hegedüs, K.; Iwaoka, K.; Hosoda, M.; Kashiwakura, I.; et al. Comparison of bacterial flora in river sediments from Fukushima and Aomori prefectures by 16S rDNA sequence analysis. Radiat. Prot. Dosim. 2019, 184, 504–509. [Google Scholar] [CrossRef]
- Nohara, C.; Taira, W.; Hiyama, A.; Tanahara, A.; Takatsuji, T.; Otaki, J.M. Ingestion of radioactively contaminated diets for two generations in the pale grass blue butterfly. BMC Evol. Biol. 2014, 14, 193. [Google Scholar] [CrossRef]
- Taira, W.; Hiyama, A.; Nohara, C.; Sakauchi, K.; Otaki, J.M. Ingestional and transgenerational effects of the Fukushima nuclear accident on the pale grass blue butterfly. J. Radiat. Res. 2015, 56, i2–i18. [Google Scholar] [CrossRef]
- Taira, W.; Nohara, C.; Hiyama, A.; Otaki, J.M. Fukushima’s biological impacts: The case of the pale grass blue butterfly. J. Hered. 2014, 105, 710–722. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Taira, W.; Toki, M.; Kakinohana, K.; Sakauchi, K.; Otaki, J.M. Developmental and hemocytological effects of ingesting Fukushima’s radiocesium on the cabbage white butterfly Pieris rapae. Sci. Rep. 2019, 9, 2625. [Google Scholar] [CrossRef] [PubMed]
- Nohara, C.; Hiyama, A.; Taira, W.; Otaki, J.M. Robustness and radiation resistance of the pale grass blue butterfly from radioactively contaminated areas: A possible case of adaptive evolution. J. Hered. 2018, 109, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Hancock, S.; Vo, N.T.K.; Omar-Nazir, L.; Batlle, J.V.I.; Otaki, J.M.; Hiyama, A.; Byun, S.H.; Seymour, C.B.; Mothersill, C. Transgenerational effects of historic radiation dose in pale grass blue butterflies around Fukushima following the Fukushima Dai-ichi Nuclear Power Plant meltdown accident. Environ. Res. 2019, 168, 230–240. [Google Scholar] [CrossRef]
- Sakauchi, K.; Taira, W.; Hiyama, A.; Imanaka, T.; Otaki, J.M. The pale grass blue butterfly in ex-evacuation zones 5.5 years after the Fukushima nuclear accident: Contributions of initial high-dose exposure to transgenerational effects. J. Asia-Pac. Entomol. 2020, 23, 242–252. [Google Scholar] [CrossRef]
- Otaki, J.M. Fukushima’s lessons from the blue butterfly: A risk assessment of the human living environment in the post-Fukushima era. Integr. Environ. Assess. Manag. 2016, 12, 667–672. [Google Scholar] [CrossRef]
- Otaki, J.M.; Taira, W. Current status of the blue butterfly in Fukushima research. J. Hered. 2018, 109, 178–187. [Google Scholar] [CrossRef]
- Otaki, J.M. The pale grass blue butterfly as an indicator for the biological effect of the Fukushima Daiichi Nuclear Power Plant accident. In Low-Dose Radiation Effects on Animals and Ecosystems; Fukumoto, M., Ed.; Springer: Singapore, 2020; pp. 239–247. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Iwasaki, M.; Sakauchi, K.; Iwata, M.; Otaki, J.M. Morphological abnormality rate of the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae) in southwestern Japan: A reference data set for environmental monitoring. J. Asia-Pac. Entomol. 2017, 20, 1333–1339. [Google Scholar] [CrossRef]
- Hiyama, A.; Taira, W.; Iwasaki, M.; Sakauchi, K.; Gurung, R.; Otaki, J.M. Geographical distribution of morphological abnormalities and wing color pattern modifications of the pale grass blue butterfly in northeastern Japan. Entomol. Sci. 2017, 20, 100–110. [Google Scholar] [CrossRef]
- Gurung, R.D.; Taira, W.; Sakauchi, K.; Iwata, M.; Hiyama, A.; Otaki, J.M. Tolerance of high oral doses of nonradioactive and radioactive caesium chloride in the pale grass blue butterfly Zizeeria maha. Insects 2019, 10, 290. [Google Scholar] [CrossRef]
- Garnier-Laplace, J.; Geras’kin, S.; Della-Vedova, C.; Beaugelin-Seiller, K.; Hinton, T.G.; Real, A.; Oudalova, A. Are radiosensitivity data derived from natural field conditions consistent with data from controlled exposures? A case study of Chernobyl wildlife chronically exposed to low dose rates. J. Environ. Radioact. 2013, 121, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Beaugelin-Seiller, K.; Della-Vedova, C.; Garnier-Laplace, J. Is non-human species radiosensitivity in the lab a good indicator of that in the field? Making the comparison more robust. J. Environ. Radioact. 2020, 211, 105870. [Google Scholar] [CrossRef]
- Otaki, J.M. Understanding low-dose exposure and field effects to resolve the field-laboratory paradox: Multifaceted biological effects from the Fukushima nuclear accident. In New Trends in Nuclear Science; Awwad, N.S., AlFaify, S.A., Eds.; IntechOpen: London, UK, 2018; pp. 49–71. [Google Scholar] [CrossRef]
- Tangwatcharin, P.; Khopaibool, P. Activity of virgin coconut oil, lauric acid or monolaurin in combination with lactic acid against Staphylococcus aureus. Southeast Asian J. Trop. Med. Public Health 2012, 43, 969–985. [Google Scholar]
- Herdiyati, Y.; Astrid, Y.; Shadrina, A.A.N.; Wiani, I.; Satari, M.H.; Kurnia, D. Potential fatty acid as antibacterial agent against oral bacteria of Streptococcus mutans and Streptococcus sanguinis from basil (Ocimum americanum): In vitro and in silico studies. Curr. Drug Discov. Technol. 2021, 18, 532–541. [Google Scholar] [CrossRef] [PubMed]
- Muniyan, R.; Gurunathan, J. Lauric acid and myristic acid from Allium sativum inhibit the growth of Mycobacterium tuberculosis H37Ra: In silico analysis reveals possible binding to protein kinase B Pharm. Biol. 2016, 54, 2814–2821. [Google Scholar] [CrossRef] [PubMed]
- Walters, D.R.; Walker, R.L.; Walker, K.C. Lauric acid exhibits antifungal activity against plant pathogenic fungi. J. Phytopathol. 2003, 151, 228–230. [Google Scholar] [CrossRef]
- Liang, C.; Gao, W.; Ge, T.; Tan, X.; Wang, J.; Liu, H.; Wang, Y.; Han, C.; Xu, Q.; Wang, Q. Lauric acid is a potent biological control agent that damages the cell membrane of Phytophthora sojae. Front. Microbiol. 2021, 12, 666761. [Google Scholar] [CrossRef]
- Kannathasan, K.; Senthilkumar, A.; Venkatesalu, V.; Chandrasekaran, M. Larvicidal activity of fatty acid methyl esters of Vitex species against Culex quinquefasciatus. Parasitol. Res. 2008, 103, 999–1001. [Google Scholar] [CrossRef]
- Wang, X.; Wang, S.; Yi, J.; Li, Y.; Liu, J.; Wang, J.; Xi, J. Three host plant volatiles, hexanal, lauric acid, and tetradecane, are detected by an antenna-biased expressed odorant receptor 27 in the dark black chafer Holotrichia parallela. J. Agric. Food Chem. 2020, 68, 7316–7323. [Google Scholar] [CrossRef]
- Langer, S.Z. History and nomenclature of α1-adrenoceptors. Eur. Urol. 1999, 36, 2–6. [Google Scholar] [CrossRef]
- MacDonald, R.; Wilt, T.J. Alfuzosin for treatment of lower urinary tract symptoms compatible with benign prostatic hyperplasia: A systematic review of efficacy and adverse effects. Urology 2005, 66, 780–788. [Google Scholar] [CrossRef] [PubMed]
- McKeage, K.; PLoSker, G.L. Alfuzosin: A review of the therapeutic use of the prolonged-release formulation given once daily in the management of benign prostatic hyperplasia. Drugs 2002, 62, 633–653. [Google Scholar] [CrossRef] [PubMed]
- Wilde, M.I.; Fitton, A.; McTavish, D. Alfuzosin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in benign prostatic hyperplasia. Drugs 1993, 45, 410–429. [Google Scholar] [CrossRef] [PubMed]
- Jomon, K.; Kuroda, Y.; Ajisaka, M.; Sakai, H. A new antibiotic, ikarugamycin. J. Antibiot. 1972, 25, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, X.; Zhao, J.; Liu, Q.; Wang, L.; Guan, X.; He, H.; Xiang, W. Streptomyces harbinensis sp. nov., an endophytic, ikarugamycin-producing actinomycete isolated from soybean root [Glycine max (L.) Merr]. Int. J. Syst. Evol. Microbiol. 2013, 63, 3579–3584. [Google Scholar] [CrossRef]
- Qin, S.; Xing, K.; Jiang, J.-H.; Xu, L.-H.; Li, W.-J. Biodiversity, bioactive natural products and biotechnological potential of plant-associated endophytic actinobacteria. Appl. Microbiol. Biotechnol. 2011, 89, 457–473. [Google Scholar] [CrossRef]
- Shimizu, M. Endophytic actinomycetes: Biocontrol agents and growth promoters. In Bacteria in Agrobiology: Plant Growth Responses; Maheshwari, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 201–220. [Google Scholar] [CrossRef]
- Golinska, P.; Wypij, M.; Agarkar, G.; Rathod, D.; Dahm, H.; Rai, M. Endophytic actinobacteria of medicinal plants: Diversity and bioactivity. Antonie van Leeuwenhoek 2015, 108, 267–289. [Google Scholar] [CrossRef]
- Grover, M.; Bodhankar, S.; Maheswari, M.; Srinivasarao, C. Actinomycetes as mitigators of climate change and abiotic stress. In Plant Growth Promoting Actinobacteria; Subramaniam, G., Arumugam, S., Rajendran, V., Eds.; Springer: Singapore, 2016; pp. 203–212. [Google Scholar] [CrossRef]
- Kuldau, G.; Bacon, C. Clavicipitaceous endophytes: Their ability to enhance resistance of grasses to multiple stresses. Biol. Control 2008, 46, 57–71. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; Henson, J.; Volkenburgh, E.V.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.-O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef]
- Peng, A.; Liu, J.; Gao, Y.; Chen, Z. Distribution of endophytic bacteria in Alopecurus aequalis Sobol and Oxalis corniculata L. from soils contaminated by polycyclic aromatic hydrocarbons. PLoS ONE 2013, 8, e83054. [Google Scholar] [CrossRef]
- Mufti, R.; Amna; Rafique, M.; Haq, F.; Munis, M.F.H.; Masood, S.; Mumtaz, A.S.; Chaudhary, H.J. Genetic diversity and metal resistance assessment of endophytes isolated from Oxalis corniculata. Soil Environ. 2015, 34, 89–99. [Google Scholar]
- Lacret, R.; Oves-Costales, D.; Gómez, C.; Diaz, C.; de la Cruz, M.; Pérez-Victoria, I.; Vicente, F.; Genilloud, O.; Reyes, F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar. Drugs 2014, 13, 128–140. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.I.; Aklilu, E.; Mohammedsalih, K.M.; Adekola, A.A.; Mergani, A.E.; Mohamad, M.; Kamaruzzaman, N.F. Antibacterial activity of ikarugamycin against intracellular Staphylococcus aureus in bovine mammary epithelial cells in vitro infection model. Biology 2021, 10, 985. [Google Scholar] [CrossRef] [PubMed]
- Elkin, S.R.; Oswald, N.W.; Reed, D.K.; Mettlen, M.; MacMillan, J.B.; Schmid, S. Ikarugamycin: A natural product inhibitor of clathrin-mediated endocytosis. Traffic 2016, 17, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, M.; Matsuyama, S.; Yamaji, K. Oxalic acid as a larval feeding stimulant for the pale grass blue butterfly Zizeeria maha (Lepidoptera: Lycaenidae). Appl. Entomol. Zool. 2016, 51, 91–98. [Google Scholar] [CrossRef]
- Ito, T. Nutritional requirements and artificial diets for the silkworm, Bombyx mori L. J. Sericult. Sci. Jpn. 1967, 36, 315–319. [Google Scholar] [CrossRef]
- Lin, K.; Yamada, H.; Kato, M. Free fatty acids promote feeding behavior of the silk-worm, Bombyx mori L. Memories Faculty Sci. Kyoto Univ. Ser. Biol. 1971, 4, 108–115. Available online: http://hdl.handle.net/2433/258804 (accessed on 29 March 2022).
- Furukawa, Y.; Sakiyama, T.; Wakamiya, K.; Ichikou, J.; Matsumura, T.; Mizokami, H. Comparison of oviposition and growth in response to the host plant differences in the pale grass blue butterfly. Chem. Biol. 2020, 58, 640–643. (In Japanese) [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; de Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.É.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucl. Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef]
- Nishida, R. Sequestration of plant secondary compounds by butterflies and moths. Chemoecology 1994, 5, 127–138. [Google Scholar] [CrossRef]
- Roeder, T. The control of metabolic traits by octopamine and tyramine in invertebrates. J. Exp. Biol. 2020, 223, jeb194282. [Google Scholar] [CrossRef] [PubMed]

| Metabolite | Leaf (w/o Radiation) | Leaf (with Radiation) *1 | Basal Level in AD-FSW-135 | Toxicity Test in AD-FSW-135 *2 | Coverage *3 | TD50 | LD50 |
|---|---|---|---|---|---|---|---|
| Lauric acid | 0.050 mg/g | 0.063 mg/g | 0.0055 mg/g | 0, 0.01, 0.1, 1 mg/g | Yes | 1.2 mg/g | 1.6 mg/g |
| Alfuzosin *4 | 0.40 ng/g | 1.6 ng/g | 0.044 ng/g | 0, 0.01, 0.1 mg/g | No | 0.079 mg/g | 0.13 mg/g |
| Ikarugamycin | 0.20 ng/g | 0.26 ng/g | 0.022 ng/g | 0, 0.01 mg/g | No | NA | NA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morita, A.; Sakauchi, K.; Taira, W.; Otaki, J.M. Ingestional Toxicity of Radiation-Dependent Metabolites of the Host Plant for the Pale Grass Blue Butterfly: A Mechanism of Field Effects of Radioactive Pollution in Fukushima. Life 2022, 12, 615. https://doi.org/10.3390/life12050615
Morita A, Sakauchi K, Taira W, Otaki JM. Ingestional Toxicity of Radiation-Dependent Metabolites of the Host Plant for the Pale Grass Blue Butterfly: A Mechanism of Field Effects of Radioactive Pollution in Fukushima. Life. 2022; 12(5):615. https://doi.org/10.3390/life12050615
Chicago/Turabian StyleMorita, Akari, Ko Sakauchi, Wataru Taira, and Joji M. Otaki. 2022. "Ingestional Toxicity of Radiation-Dependent Metabolites of the Host Plant for the Pale Grass Blue Butterfly: A Mechanism of Field Effects of Radioactive Pollution in Fukushima" Life 12, no. 5: 615. https://doi.org/10.3390/life12050615
APA StyleMorita, A., Sakauchi, K., Taira, W., & Otaki, J. M. (2022). Ingestional Toxicity of Radiation-Dependent Metabolites of the Host Plant for the Pale Grass Blue Butterfly: A Mechanism of Field Effects of Radioactive Pollution in Fukushima. Life, 12(5), 615. https://doi.org/10.3390/life12050615







