The Role of Nucleophosmin 1 (NPM1) Mutation in the Diagnosis and Management of Myeloid Neoplasms
Abstract
1. Introduction
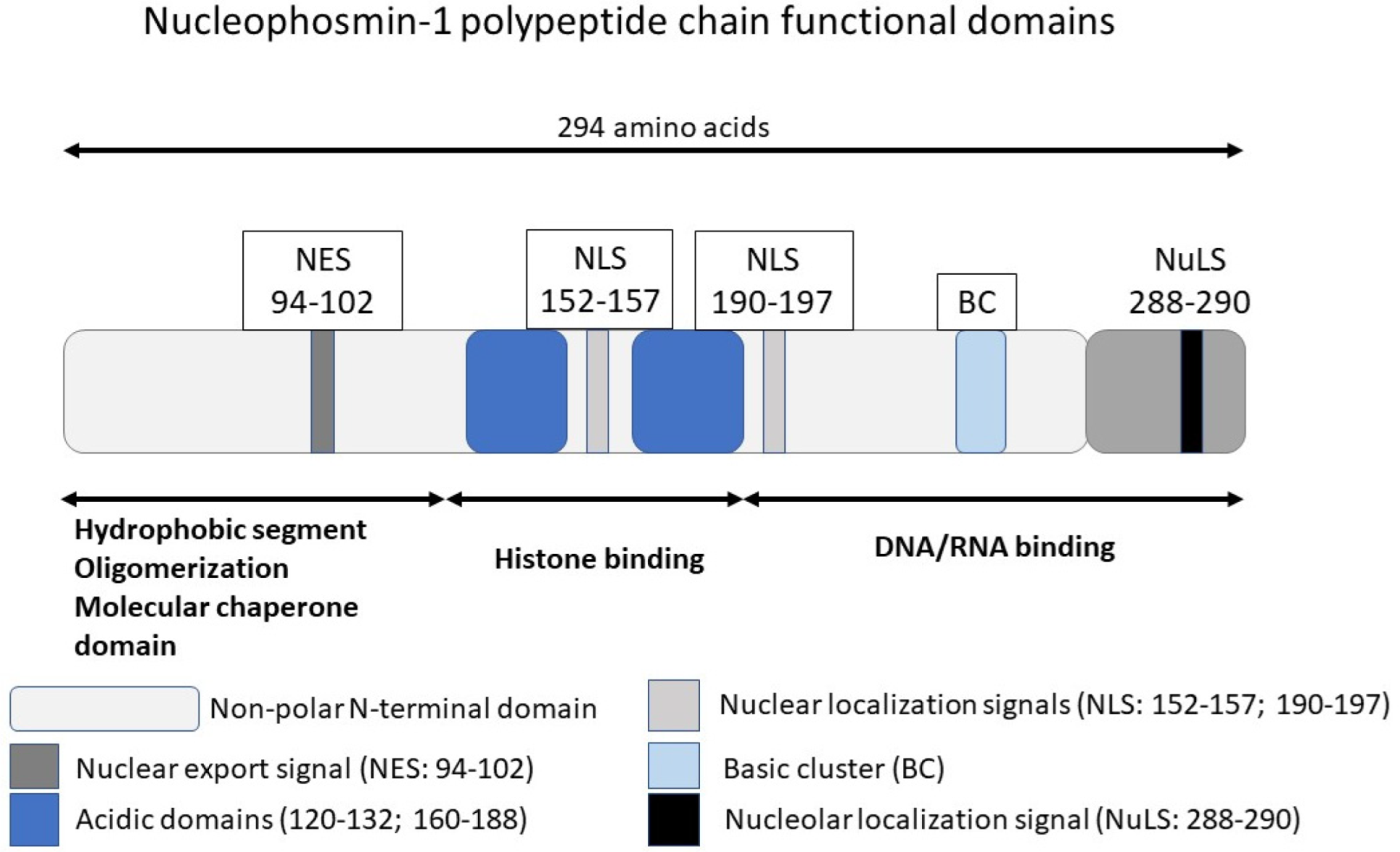
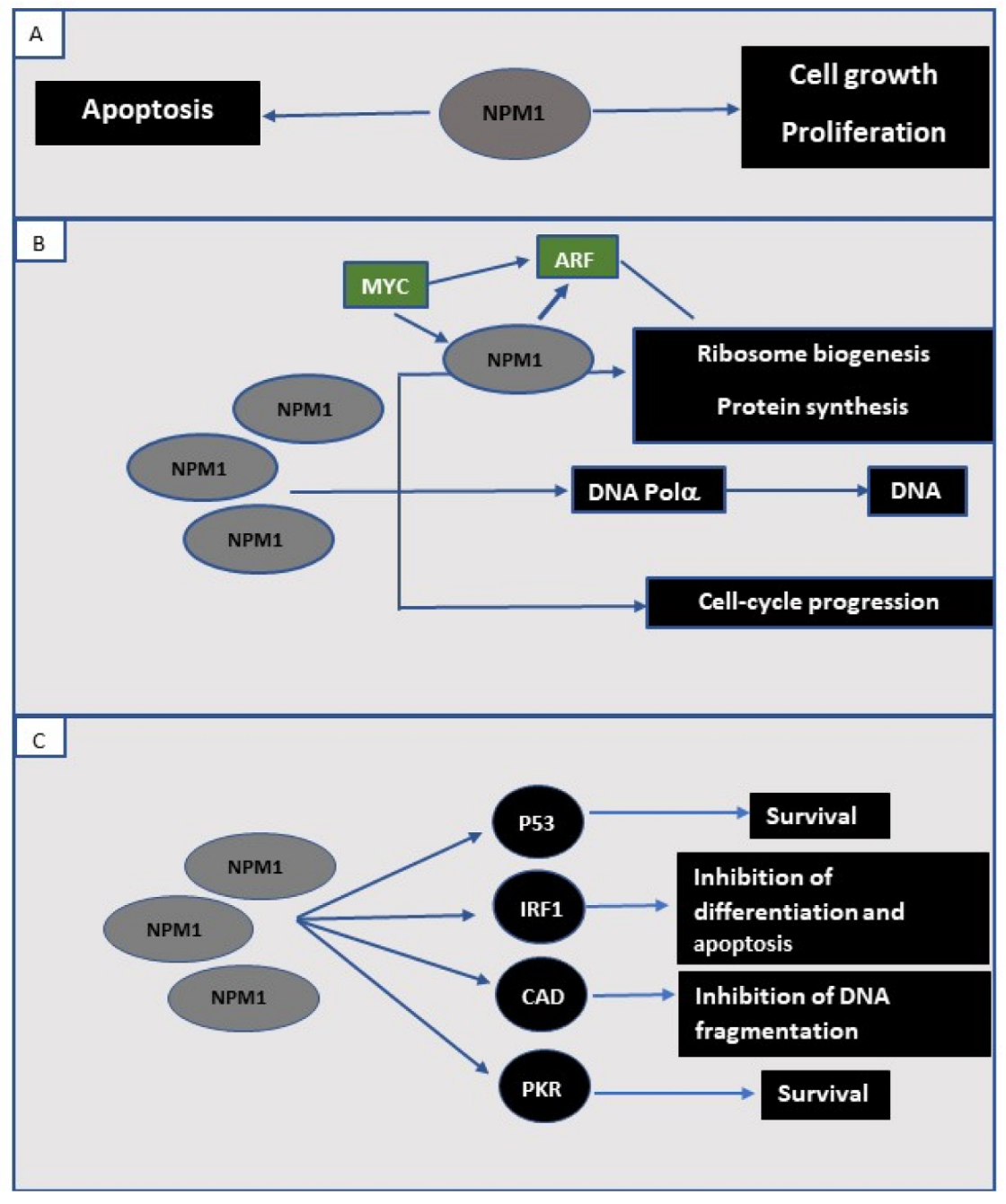
2. NPM1 Protein in the Normal Cell
3. NPM1 in Human Cancer
3.1. Acute Myeloid Leukemia (AML) with Mutated NPM1; A Myeloid Neoplasm with Unique Features
3.2. Prognosis of NPM1-Mutated AML
4. Minimal Residual Disease (MRD) Monitoring in NPM1-Mutated AML
5. NPM1 Mutations in Myeloid Neoplasms with <20% Blasts
6. Summary
Funding
Conflicts of Interest
References
- Kang, Y.J.; Olson, M.O.; Busch, H. Phosphorylation of acid-soluble proteins in isolated nucleoli of Novikoff hepatoma ascites cells. Effects of divalent cations. J. Biol. Chem. 1974, 249, 5580–5585. [Google Scholar] [CrossRef]
- Kang, Y.J.; Olson, M.O.; Jones, C.; Busch, H. Nucleolar phosphoproteins of normal rat liver and Novikoff hepatoma ascites cells. Cancer Res. 1975, 35, 1470–1475. [Google Scholar] [PubMed]
- Chan, W.Y.; Liu, Q.R.; Borjigin, J.; Busch, H.; Rennert, O.M.; Tease, L.A.; Chan, P.K. Characterization of the Cdna-Encoding Human Nucleophosmin and Studies of Its Role in Normal and Abnormal Growth. Biochemistry 1989, 28, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Grisendi, S.; Bernardi, R.; Rossi, M.; Cheng, K.; Khandker, L.; Manova, K.; Pandolfi, P.P. Role of nucleophosmin in embryonic development and tumorigenesis. Nature 2005, 437, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Mecucci, C.; Tiacci, E.; Alcalay, M.; Rosati, R.; Pasqualucci, L.; La Starza, R.; Diverio, D.; Colombo, E.; Santucci, A.; et al. Cytoplasmic nucleophosmin in acute myelogenous leukemia with a normal karyotype. N. Engl. J. Med. 2005, 352, 254–266. [Google Scholar] [CrossRef]
- Borer, R.A.; Lehner, C.F.; Eppenberger, H.M.; Nigg, E.A. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell 1989, 56, 379–390. [Google Scholar] [CrossRef]
- Yun, J.P.; Chew, E.C.; Liew, C.T.; Chan, J.Y.; Jin, M.L.; Ding, M.X.; Fai, Y.H.; Li, H.K.; Liang, X.M.; Wu, Q.L. Nucleophosmin/B23 is a proliferate shuttle protein associated with nuclear matrix. J. Cell Biochem. 2003, 90, 1140–1148. [Google Scholar] [CrossRef] [PubMed]
- Hingorani, K.; Szebeni, A.; Olson, M.O. Mapping the functional domains of nucleolar protein B23. J. Biol. Chem. 2000, 275, 24451–24457. [Google Scholar] [CrossRef]
- Szebeni, A.; Olson, M.O. Nucleolar protein B23 has molecular chaperone activities. Protein Sci. 1999, 8, 905–912. [Google Scholar] [CrossRef]
- Okuwaki, M.; Tsujimoto, M.; Nagata, K. The RNA binding activity of a ribosome biogenesis factor, nucleophosmin/B23, is modulated by phosphorylation with a cell cycle-dependent kinase and by association with its subtype. Mol. Biol. Cell 2002, 13, 2016–2030. [Google Scholar] [CrossRef] [PubMed]
- Okuwaki, M.; Matsumoto, K.; Tsujimoto, M.; Nagata, K. Function of nucleophosmin/B23, a nucleolar acidic protein, as a histone chaperone. FEBS Lett. 2001, 506, 272–276. [Google Scholar] [CrossRef]
- Swaminathan, V.; Kishore, A.H.; Febitha, K.K.; Kundu, T.K. Human histone chaperone nucleophosmin enhances acetylation-dependent chromatin transcription. Mol. Cell. Biol. 2005, 25, 7534–7545. [Google Scholar] [CrossRef]
- Falini, B.; Nicoletti, I.; Martelli, M.F.; Mecucci, C. Acute myeloid leukemia carrying cytoplasmic/mutated nucleophosmin (NPMc(+) AML): Biologic and clinical features. Blood 2007, 109, 874–885. [Google Scholar] [CrossRef]
- Falini, B.; Bolli, N.; Liso, A.; Martelli, M.P.; Mannucci, R.; Pileri, S.; Nicoletti, I. Altered nucleophosmin transport in acute myeloid leukaemia with mutated NPM1: Molecular basis and clinical implications. Leukemia 2009, 23, 1731–1743. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Baumann, A.; Szebeni, A.; Olson, M.O. The nucleic acid binding activity of nucleolar protein B23.1 resides in its carboxyl-terminal end. J. Biol. Chem. 1994, 269, 30994–30998. [Google Scholar] [CrossRef]
- Savkur, R.S.; Olson, M.O. Preferential cleavage in pre-ribosomal RNA byprotein B23 endoribonuclease. Nucleic Acids Res. 1998, 26, 4508–4515. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.H.; Olson, M.O. Structure of the gene for rat nucleolar protein B23. J. Biol. Chem. 1990, 265, 18227–18233. [Google Scholar] [CrossRef]
- Wang, D.; Umekawa, H.; Olson, M.O. Expression and subcellular locations of two forms of nucleolar protein B23 in rat tissues and cells. Cell Mol. Biol. Res. 1993, 39, 33–42. [Google Scholar]
- Herrera, J.E.; Correia, J.J.; Jones, A.E.; Olson, M.O. Sedimentation analyses of the salt- and divalent metal ion-induced oligomerization of nucleolar protein B23. Biochemistry 1996, 35, 2668–2673. [Google Scholar] [CrossRef]
- Namboodiri, V.M.; Akey, I.V.; Schmmmidt-Zachmann, M.S.; Head, J.F.; Akey, C.W. The structure and function of Xenopus NO38-core, a histone chaperone in the nucleolus. Structure 2004, 12, 2149–2160. [Google Scholar] [CrossRef]
- Tanaka, M.; Sasaki, H.; Kino, I.; Sugimura, T.; Terada, M. Genes preferentially expressed in embryo stomach are predominantly expressed in gastric cancer. Cancer Res. 1992, 52, 3372–3377. [Google Scholar]
- Nozawa, Y.; Van Belzen, N.; Van der Made, A.C.; Dinjens, W.N.; Bosman, F.T. Expression of nucleophosmin/B23 in normal and neoplastic colorectal mucosa. J. Pathol. 1996, 178, 48–52. [Google Scholar] [CrossRef]
- Shields, L.B.; Gercel-Taylor, C.; Yashar, C.M.; Wan, T.C.; Katsanis, W.A.; Spinnato, J.A.; Taylor, D.D. Induction of immune responses to ovarian tumor antigens by multiparity. J. Soc. Gynecol. Investig. 1997, 4, 298–304. [Google Scholar] [CrossRef]
- Subong, E.N.; Shue, M.J.; Epstein, J.I.; Briggman, J.V.; Chan, P.K.; Partin, A.W. Monoclonal antibody to prostate cancer nuclear matrix protein (PRO:4-216) recognizes nucleophosmin/B23. Prostate 1999, 39, 298–304. [Google Scholar] [CrossRef]
- Tsui, K.H.; Cheng, A.J.; Chang, P.; Pan, T.L.; Yung, B.Y. Association of nucleophosmin/B23 mRNA expression with clinical outcome in patients with bladder carcinoma. Urology 2004, 64, 839–844. [Google Scholar] [CrossRef]
- Redner, R.L.; Rush, E.A.; Faas, S.; Rudert, W.A.; Corey, S.J. The t(5;17) variant of acute promyelocytic leukemia expresses a nucleophosmin-retinoic acid receptor fusion. Blood 1996, 87, 882–886. [Google Scholar] [CrossRef]
- Morris, S.W.; Kirstein, M.N.; Valentine, M.B.; Dittmer, K.G.; Shapiro, D.N.; Saltman, D.L.; Look, A.T. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin’s lymphoma. Science 1994, 263, 1281–1284. [Google Scholar] [CrossRef] [PubMed]
- Raimondi, S.C.; Dube, I.D.; Valentine, M.B.; Mirro, J.; Jr Watt, H.J.; Larson, R.A.; Bitter, M.A.; Le Beau, M.M.; Rowley, J.D. Clinicopathologic manifestations and breakpoints of the t(3;5) in patients with acute nonlymphocytic leukemia. Leukemia 1989, 3, 42–47. [Google Scholar]
- Yoneda-Kato, N.; Look, A.T.; Kirstein, M.N.; Valentine, M.B.; Raimondi, S.C.; Cohen, K.J.; Carroll, A.J.; Morris, S.W. The t(3;5)(q25.1;q34) of myelodysplastic syndrome and acute myeloid leukemia produces a novel fusion gene, NPM-MLF1. Oncogene 1996, 12, 265–275. [Google Scholar]
- Dergunova, N.; Bulycheva, T.I.; Artemenko, E.G.; Shpakova, A.P.; Pegova, A.N.; Gemjian, E.G.; Dudnik, O.A.; Zatsepina, O.V.; Malashenko, O.S. A major nucleolar protein B23 as a marker of proliferation activity of human peripheral lymphocytes. Immunol. Lett. 2002, 83, 67–72. [Google Scholar] [CrossRef]
- Zeller, K.I.; Haggerty, T.J.; Barrett, J.F.; Guo, Q.; Wonsey, D.R.; Dang, C.V. Characterization of nucleophosmin (B23) as a Myc target by scanning chromatin immunoprecipitation. J. Biol. Chem. 2001, 276, 48285–48291. [Google Scholar] [CrossRef]
- Boon, K.; Caron, H.N.; van Asperen, R.; Valentijn, L.; Hermus, M.C.; van Sluis, P.; Roobeek, I.; Weis, I.; Voute, P.A.; Schwab, M.; et al. N-myc enhances the expression of a large set of genes functioning in ribosome biogenesis and protein synthesis. EMBO J. 2001, 20, 1383–1393. [Google Scholar] [CrossRef]
- Takemura, M.; Sato, K.; Nishio, M.; Akiyama, T.; Umekawa, H.; Yoshida, S. Nucleolar protein B23.1 binds to retinoblastoma protein and synergistically stimulates DNA polymerase alpha activity. J. Biochem. 1999, 125, 904–909. [Google Scholar] [CrossRef]
- Dumbar, T.S.; Gentry, G.A.; Olson, M.O. Interaction of nucleolar phosphoprotein B23 with nucleic acids. Biochemistry 1989, 28, 9495–9501. [Google Scholar] [CrossRef]
- Prestayko, A.W.; Klomp, G.R.; Schmoll, D.J.; Busch, H. Comparison of proteins of ribosomal subunits and nucleolar preribosomal particles from Novikoff hepatoma ascites cells by two-dimensional polyacrylamide gel electrophoresis. Biochemistry 1974, 13, 1945–1951. [Google Scholar] [CrossRef] [PubMed]
- Olson, M.O.; Wallace, M.O.; Herrera, A.H.; Marshall-Carlson, L.; Hunt, R.C. Preribosomal ribonucleoprotein particles are a major component of a nucleolar matrix fraction. Biochemistry 1986, 25, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Kondo, T.; Minamino, N.; Nagamura-Inoue, T.; Matsumoto, M.; Taniguchi, T.; Tanaka, N. Identification and characterization of nucleophosmin/B23/numatrin which binds the anti-oncogenic transcription factor IRF-1 and manifests oncogenic activity. Oncogene 1997, 15, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Bertwistle, D.; Sugimoto, M.; Sherr, C.J. Physical and functional interactions of the Arf tumor suppressor protein with nucleophosmin/B23. Mol. Cell. Biol. 2004, 24, 985–996. [Google Scholar] [CrossRef]
- Kuo, M.L.; den Besten, W.; Bertwistle, D.; Roussel, M.F.; Sherr, C.J. N-terminal polyubiquitination and degradation of the Arf tumor suppressor. Genes Dev. 2004, 18, 1862–1874. [Google Scholar] [CrossRef]
- Brady, S.N.; Yu, Y.; Maggi, L.B.; Weber, J.D. ARF impedes NPM/B23 shuttling in an Mdm2-sensitive tumor suppressor pathway. Mol. Cell. Biol. 2004, 24, 9327–9338. [Google Scholar] [CrossRef]
- Kurki, S.; Peltonen, K.; Latonen, L.; Kiviharju, T.M.; Ojala, P.M.; Meek, D.; Laiho, M. Nucleolar protein NPM interacts with HDM2 and protects tumor suppressor protein p53 from HDM2-mediated degradation. Cancer Cell 2004, 5, 465–475. [Google Scholar] [CrossRef]
- Oren, M. Regulation of the p53 tumor suppressor protein. J. Biol. Chem. 1999, 274, 36031–36034. [Google Scholar] [CrossRef]
- Gao, H.; Jin, S.Q.; Song, Y.M.; Fu, M.; Wang, M.R.; Liu, Z.H.; Wu, M.; Zhan, Q.M. B23 regulates GADD45a nuclear translocation and contributes to GADD45a-induced cell cycle G(2)-M arrest. J. Biol. Chem. 2005, 280, 10988–10996. [Google Scholar] [CrossRef]
- Wu, M.H.; Chang, J.H.; Yung, B.Y. Resistance to UV-induced cell-killing in nucleophosmin/B23 over-expressed NIH 3T3 fibroblasts: Enhancement of DNA repair and up-regulation of PCNA in association with nucleophosmin/B23 over-expression. Carcinogenesis 2002, 23, 93–100. [Google Scholar] [CrossRef]
- Okuda, M.; Horn, H.F.; Tarapore, P.; Tokuyama, Y.; Smulian, A.G.; Chan, P.K.; Knudsen, E.S.; Hofmann, I.A.; Snyder, J.D.; Bove, K.E.; et al. Nucleophosmin/B23 is a target of CDK2/cyclin E in centrosome duplication. Cell 2000, 103, 127–140. [Google Scholar] [CrossRef]
- Falini, B.; Brunetti, L.; Sportoletti, P.; Martelli, M.P. NPM1-mutated acute myeloid leukemia: From bench to bedside. Blood 2020, 136, 1707–1721. [Google Scholar] [CrossRef]
- Grimwade, D.; Ivey, A.; Huntly, B.J.P. Molecular landscape of acute myeloid leukemia in younger adults and its clinical relevance. Blood 2016, 127, 29–41. [Google Scholar] [CrossRef] [PubMed]
- Arber, D.A.; Brunning, R.D.; Le Beau, M.M.; Falini, B.; Vardiman, J.W.; Porwit, A.; Thiele, J.; Foucar, K.; Dohner, H.; Bloomfield, C.D. Acute myeloid leukaemia with recurrent genetic abnormalities. In WHO CLassification of Tumours of Haematopoietic and Lymphoid Tissue; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D.A., Hasserjian, R.P., Le Beau, M.M., et al., Eds.; International Agency for Research on Cancer: Lyon, France, 2017; pp. 141–142. [Google Scholar]
- Verhaak, R.G.W.; Goudswaard, C.S.; van Putten, W.; Bijl, M.A.; Sanders, M.A.; Hugens, W.; Uitterlinden, A.G.; Erpelinck, C.A.J.; Delwel, R.; Lowenberg, B.; et al. Mutations in nucleophosmin (NPM1) in acute myeloid leukemia (AML): Association with other gene abnormalities and previously established gene expression signatures and their favorable prognostic significance. Blood 2005, 106, 3747–3754. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, M.; Kameoka, Y.; Suzuki, R. Nucleophosmin in acute myelogenous leukemia. N. Engl. J. Med. 2005, 352, 1819–1820. [Google Scholar]
- Falini, B.; Bolli, N.; Shan, J.; Martelli, M.P.; Liso, A.; Pucciarini, A.; Bigerna, B.; Pasqualucci, L.; Mannucci, R.; Rosati, R.; et al. Both carboxy-terminus NES motif and mutated tryptophan(s) are crucial for aberrant nuclear export of nucleophosmin leukemic mutants in NPMc(+) AML. Blood 2006, 107, 4514–4523. [Google Scholar] [CrossRef] [PubMed]
- Grisendi, S.; Pandolfi, P.P. NPM mutations in acute myelogenous leukemia. N. Engl. J. Med. 2005, 352, 291–292. [Google Scholar] [CrossRef]
- Schnittger, S.; Schoch, C.; Kern, W.; Mecucci, C.; Tschulik, C.; Martelli, M.F.; Haferlach, T.; Hiddemann, W.; Falini, B. Nucleophosmin gene mutations are predictors of favorable prognosis in acute myelogenous leukemia with a normal karyotype. Blood 2005, 106, 3733–3739. [Google Scholar] [CrossRef] [PubMed]
- Dohner, K.; Schlenk, R.F.; Habdank, M.; Scholl, C.; Rucker, F.G.; Corbacioglu, A.; Bullinger, L.; Frohling, S.; Dohner, H. Mutant nucleophosmin (NPM1) predicts favorable prognosis in younger adults with acute myeloid leukemia and normal cytogenetics: Interaction with other gene mutations. Blood 2005, 106, 3740–3746. [Google Scholar] [CrossRef]
- Diaz-Beya, M.; Rozman, M.; Pratcorona, M.; Torrebadell, M.; Camos, M.; Aguilar, J.L.; Esteve, J. The prognostic value of multilineage dysplasia in de novo acute myeloid leukemia patients with intermediate-risk cytogenetics is dependent on NPM1 mutational status. Blood 2010, 116, 6147–6148. [Google Scholar] [CrossRef] [PubMed]
- Falini, B.; Macijewski, K.; Weiss, T. Multilineage dysplasia has no impact on biologic, clinicopathologic, and prognostic features of AML with mutated nucleophosmin (NPM1) (vol 115, pg 3776, 2010). Blood 2010, 116, 1017. [Google Scholar]
- Falini, B.; Martelli, M.P.; Bolli, N.; Sportoletti, P.; Liso, A.; Tiacci, E.; Haferlach, T. Acute myeloid leukemia with mutated nucleophosmin (NPM1): Is it a distinct entity? Blood 2011, 117, 1109–1120. [Google Scholar] [CrossRef] [PubMed]
- Hollein, A.; Meggendorfer, M.; Dicker, F.; Jeromin, S.; Nadarajah, N.; Kern, W.; Haferlach, C.; Haferlach, T. NPM1 mutated AML can relapse with wild-type NPM1: Persistent clonal hematopoiesis can drive relapse. Blood Adv. 2018, 2, 3118–3125. [Google Scholar] [CrossRef]
- Forghieri, F.; Comoli, P.; Marasca, R.; Potenza, L.; Luppi, M. Minimal/Measurable Residual Disease Monitoring in NPM1-Mutated Acute Myeloid Leukemia: A Clinical Viewpoint and Perspectives. Int. J. Mol. Sci. 2018, 19, 3492. [Google Scholar] [CrossRef]
- Brown, P.; McIntyre, E.; Rau, R.; Meshinchi, S.; Lacayo, N.; Dahl, G.; Alonzo, T.A.; Chang, M.; Arceci, R.J.; Small, D. The incidence and clinical significance of nucleophosmin mutations in childhood AML. Blood 2007, 110, 979–985. [Google Scholar] [CrossRef]
- Schlenk, R.F.; Dohner, K.; Krauter, J.; Frohling, S.; Corbacioglu, A.; Bullinger, L.; Habdank, M.; Spath, D.; Morgan, M.; Benner, A.; et al. Mutations and treatment outcome in cytogenetically normal acute myeloid leukemia. N. Engl. J. Med. 2008, 358, 1909–1918. [Google Scholar] [CrossRef]
- Thiede, C.; Koch, S.; Creutzig, E.; Steudel, C.; Illmer, T.; Schaich, M.; Ehninger, G. Prevalence and prognostic impact of NPM1 mutations in 1485 adult patients with acute myeloid leukemia (AML). Blood 2006, 107, 4011–4020. [Google Scholar] [CrossRef] [PubMed]
- Haferlach, C.; Mecucci, C.; Schnittger, S.; Kohlmann, A.; Mancini, M.; Cuneo, A.; Testoni, N.; Rege-Cambrin, G.; Santucci, A.; Vignetti, M.; et al. AML with mutated NPM1 carrying a normal or aberrant karyotype show overlapping biologic, pathologic, immunophenotypic, and prognostic features. Blood 2009, 114, 3024–3032. [Google Scholar] [CrossRef] [PubMed]
- Micol, J.B.; Boissel, N.; Renneville, A.; Castaigne, S.; Gardin, C.; Preudhomme, C.; Dombret, H. The role of cytogenetic abnormalities in acute myeloid leukemia with NPM1 mutations and no FLT3 internal tandem duplication. Blood 2009, 114, 4601–4602. [Google Scholar] [CrossRef] [PubMed]
- Gale, R.E.; Green, C.; Allen, C.; Mead, A.J.; Burnett, A.K.; Hills, R.K.; Linch, D.C. Medical Research Council Adult Leukaemia Working P: The impact of FLT3 internal tandem duplication mutant level, number, size, and interaction with NPM1 mutations in a large cohort of young adult patients with acute myeloid leukemia. Blood 2008, 111, 2776–2784. [Google Scholar] [CrossRef]
- Pratcorona, M.; Brunet, S.; Nomdedeu, J.; Ribera, J.M.; Tormo, M.; Duarte, R.; Escoda, L.; Guardia, R.; Queipo de Llano, M.P.; Salamero, O.; et al. Favorable outcome of patients with acute myeloid leukemia harboring a low-allelic burden FLT3-ITD mutation and concomitant NPM1 mutation: Relevance to post-remission therapy. Blood 2013, 121, 2734–2738. [Google Scholar] [CrossRef] [PubMed]
- Schlenk, R.F.; Kayser, S.; Bullinger, L.; Kobbe, G.; Casper, J.; Ringhoffer, M.; Held, G.; Brossart, P.; Lubbert, M.; Salih, H.R.; et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 2014, 124, 3441–3449. [Google Scholar] [CrossRef]
- Becker, H.; Marcucci, G.; Maharry, K.; Radmacher, M.D.; Mrozek, K.; Margeson, D.; Whitman, S.P.; Wu, Y.Z.; Schwind, S.; Paschka, P.; et al. Favorable prognostic impact of NPM1 mutations in older patients with cytogenetically normal de novo acute myeloid leukemia and associated gene- and microRNA-expression signatures: A Cancer and Leukemia Group B study. J. Clin. Oncol. 2010, 28, 596–604. [Google Scholar] [CrossRef]
- Loghavi, S.; Zuo, Z.; Ravandi, F.; Kantarjian, H.M.; Bueso-Ramos, C.; Zhang, L.; Singh, R.R.; Patel, K.P.; Medeiros, L.J.; Stingo, F.; et al. Clinical features of de novo acute myeloid leukemia with concurrent DNMT3A, FLT3 and NPM1 mutations. J. Hematol. Oncol. 2014, 7, 74. [Google Scholar] [CrossRef]
- Ossenkoppele, G.; Schuurhuis, G.J. MRD in AML: Does it already guide therapy decision-making? Hematol. Am. Soc. Hematol Educ. Program 2016, 2016, 356–365. [Google Scholar] [CrossRef] [PubMed]
- Gorello, P.; Cazzaniga, G.; Alberti, F.; Dell’Oro, M.G.; Gottardi, E.; Specchia, G.; Roti, G.; Rosati, R.; Martelli, M.F.; Diverio, D.; et al. Quantitative assessment of minimal residual disease in acute myeloid leukemia carrying nucleophosmin (NPM1) gene mutations. Leukemia 2006, 20, 1103–1108. [Google Scholar] [CrossRef]
- Papadaki, C.; Dufour, A.; Seibl, M.; Schneider, S.; Bohlander, S.K.; Zellmeier, E.; Mellert, G.; Hiddemann, W.; Spiekermann, K. Monitoring minimal residual disease in acute myeloid leukaemia with NPM1 mutations by quantitative PCR: Clonal evolution is a limiting factor. Br. J. Haematol. 2009, 144, 517–523. [Google Scholar] [CrossRef]
- Schnittger, S.; Kern, W.; Tschulik, C.; Weiss, T.; Dicker, F.; Falini, B.; Haferlach, C.; Haferlach, T. Minimal residual disease levels assessed by NPM1 mutation-specific RQ-PCR provide important prognostic information in AML. Blood 2009, 114, 2220–2231. [Google Scholar] [CrossRef] [PubMed]
- Hubmann, M.; Kohnke, T.; Hoster, E.; Schneider, S.; Dufour, A.; Zellmeier, E.; Fiegl, M.; Braess, J.; Bohlander, S.K.; Subklewe, M.; et al. Molecular response assessment by quantitative real-time polymerase chain reaction after induction therapy in NPM1-mutated patients identifies those at high risk of relapse. Haematologica 2014, 99, 1317–1325. [Google Scholar] [CrossRef] [PubMed]
- Alizad Ghandforoush, N.; Chahardouli, B.; Rostami, S.; Ghadimi, H.; Ghasemi, A.; Alimoghaddam, K.; Ghavamzadeh, A.; Nadali, F. Evaluation of Minimal Residual Disease in Acute Myeloid Leukemia with NPM1 Marker. Int. J. Hematol. Oncol. Stem. Cell Res. 2016, 10, 147–152. [Google Scholar]
- Balsat, M.; Renneville, A.; Thomas, X.; de Botton, S.; Caillot, D.; Marceau, A.; Lemasle, E.; Marolleau, J.P.; Nibourel, O.; Berthon, C.; et al. Postinduction Minimal Residual Disease Predicts Outcome and Benefit From Allogeneic Stem Cell Transplantation in Acute Myeloid Leukemia With NPM1 Mutation: A Study by the Acute Leukemia French Association Group. J. Clin. Oncol. 2017, 35, 185–193. [Google Scholar] [CrossRef]
- Patkar, N.; Kodgule, R.; Kakirde, C.; Raval, G.; Bhanshe, P.; Joshi, S.; Chaudhary, S.; Badrinath, Y.; Ghoghale, S.; Kadechkar, S.; et al. Clinical impact of measurable residual disease monitoring by ultradeep next generation sequencing in NPM1 mutated acute myeloid leukemia. Oncotarget 2018, 9, 36613–36624. [Google Scholar] [CrossRef]
- Schuurhuis, G.J.; Heuser, M.; Freeman, S.; Bene, M.C.; Buccisano, F.; Cloos, J.; Grimwade, D.; Haferlach, T.; Hills, R.K.; Hourigan, C.S.; et al. Minimal/measurable residual disease in AML: A consensus document from the European LeukemiaNet MRD Working Party. Blood 2018, 131, 1275–1291. [Google Scholar] [CrossRef]
- Shayegi, N.; Kramer, M.; Bornhauser, M.; Schaich, M.; Schetelig, J.; Platzbecker, U.; Rollig, C.; Heiderich, C.; Landt, O.; Ehninger, G.; et al. The level of residual disease based on mutant NPM1 is an independent prognostic factor for relapse and survival in AML. Blood 2013, 122, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hantel, A.; Stock, W.; Kosuri, S. Molecular Minimal Residual Disease Testing in Acute Myeloid Leukemia: A Review for the Practicing Clinician. Clin. Lymphoma Myeloma Leuk 2018, 18, 636–647. [Google Scholar] [CrossRef]
- Dohner, H.; Estey, E.; Grimwade, D.; Amadori, S.; Appelbaum, F.R.; Buchner, T.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Larson, R.A.; et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017, 129, 424–447. [Google Scholar] [CrossRef]
- Gu, X.; Ebrahem, Q.; Mahfouz, R.Z.; Hasipek, M.; Enane, F.; Radivoyevitch, T.; Rapin, N.; Przychodzen, B.; Hu, Z.; Balusu, R.; et al. Leukemogenic nucleophosmin mutation disrupts the transcription factor hub that regulates granulomonocytic fates. J. Clin. Investig. 2018, 128, 4260–4279. [Google Scholar] [CrossRef]
- Patel, S.S.; Kuo, F.C.; Gibson, C.J.; Steensma, D.P.; Soiffer, R.J.; Alyea, E.P.; 3rd Chen, Y.A.; Fathi, A.T.; Graubert, T.A.; Brunner, A.M.; et al. High NPM1-mutant allele burden at diagnosis predicts unfavorable outcomes in de novo AML. Blood 2018, 131, 2816–2825. [Google Scholar] [CrossRef]
- Tomlinson, B.; Lazarus, H.M. Enhancing acute myeloid leukemia therapy—Monitoring response using residual disease testing as a guide to therapeutic decision-making. Expert Rev. Hematol. 2017, 10, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Ravandi, F.; Walter, R.B.; Freeman, S.D. Evaluating measurable residual disease in acute myeloid leukemia. Blood Adv. 2018, 2, 1356–1366. [Google Scholar] [CrossRef]
- Chou, W.C.; Tang, J.L.; Wu, S.J.; Tsay, W.; Yao, M.; Huang, S.Y.; Huang, K.C.; Chen, C.Y.; Huang, C.F.; Tien, H.F. Clinical implications of minimal residual disease monitoring by quantitative polymerase chain reaction in acute myeloid leukemia patients bearing nucleophosmin (NPM1) mutations. Leukemia 2007, 21, 998–1004. [Google Scholar] [CrossRef] [PubMed]
- Barragan, E.; Pajuelo, J.C.; Ballester, S.; Fuster, O.; Cervera, J.; Moscardo, F.; Senent, L.; Such, E.; Sanz, M.A.; Bolufer, P. Minimal residual disease detection in acute myeloid leukemia by mutant nucleophosmin (NPM1): Comparison with WT1 gene expression. Clin. Chim. Acta 2008, 395, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Bacher, U.; Badbaran, A.; Fehse, B.; Zabelina, T.; Zander, A.R.; Kroger, N. Quantitative monitoring of NPM1 mutations provides a valid minimal residual disease parameter following allogeneic stem cell transplantation. Exp. Hematol. 2009, 37, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dvorakova, D.; Racil, Z.; Jeziskova, I.; Palasek, I.; Protivankova, M.; Lengerova, M.; Razga, F.; Mayer, J. Monitoring of minimal residual disease in acute myeloid leukemia with frequent and rare patient-specific NPM1 mutations. Am. J. Hematol. 2010, 85, 926–929. [Google Scholar] [CrossRef]
- Kristensen, T.; Moller, M.B.; Friis, L.; Bergmann, O.J.; Preiss, B. NPM1 mutation is a stable marker for minimal residual disease monitoring in acute myeloid leukaemia patients with increased sensitivity compared to WT1 expression. Eur. J. Haematol. 2011, 87, 400–408. [Google Scholar] [CrossRef]
- Kronke, J.; Schlenk, R.F.; Jensen, K.O.; Tschurtz, F.; Corbacioglu, A.; Gaidzik, V.I.; Paschka, P.; Onken, S.; Eiwen, K.; Habdank, M.; et al. Monitoring of minimal residual disease in NPM1-mutated acute myeloid leukemia: A study from the German-Austrian acute myeloid leukemia study group. J. Clin. Oncol. 2011, 29, 2709–2716. [Google Scholar] [CrossRef]
- Karas, M.; Steinerova, K.; Lysak, D.; Hrabetova, M.; Jungova, A.; Sramek, J.; Jindra, P.; Polivka, J.; Holubec, L. Pre-transplant Quantitative Determination of NPM1 Mutation Significantly Predicts Outcome of AIlogeneic Hematopoietic Stem Cell Transplantation in Patients with Normal Karyotype AML in Complete Remission. Anticancer Res. 2016, 36, 5487–5498. [Google Scholar] [CrossRef] [PubMed]
- Bill, M.; Grimm, J.; Jentzsch, M.; Kloss, L.; Goldmann, K.; Schulz, J.; Beinicke, S.; Hantschel, J.; Cross, M.; Vucinic, V.; et al. Digital droplet PCR-based absolute quantification of pre-transplant NPM1 mutation burden predicts relapse in acute myeloid leukemia patients. Ann. Hematol. 2018, 97, 1757–1765. [Google Scholar] [CrossRef] [PubMed]
- Delsing Malmberg, E.; Johansson Alm, S.; Nicklasson, M.; Lazarevic, V.; Stahlman, S.; Samuelsson, T.; Lenhoff, S.; Asp, J.; Ehinger, M.; Palmqvist, L.; et al. Minimal residual disease assessed with deep sequencing of NPM1 mutations predicts relapse after allogeneic stem cell transplant in AML. Leuk Lymphoma 2019, 60, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Kayser, S.; Benner, A.; Thiede, C.; Martens, U.; Huber, J.; Stadtherr, P.; Janssen, J.W.; Rollig, C.; Uppenkamp, M.J.; Bochtler, T.; et al. Pretransplant NPM1 MRD levels predict outcome after allogeneic hematopoietic stem cell transplantation in patients with acute myeloid leukemia. Blood Cancer J. 2016, 6, e449. [Google Scholar] [CrossRef] [PubMed]
- Getta, B.M.; Devlin, S.M.; Levine, R.L.; Arcila, M.E.; Mohanty, A.S.; Zehir, A.; Tallman, M.S.; Giralt, S.A.; Roshal, M. Multicolor Flow Cytometry and Multigene Next-Generation Sequencing Are Complementary and Highly Predictive for Relapse in Acute Myeloid Leukemia after Allogeneic Transplantation. Biol. Blood Marrow Transpl. 2017, 23, 1064–1071. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Othus, M.; Walter, R.B.; Estey, E.H.; Wu, D.; Wood, B.L. Deep NPM1 Sequencing Following Allogeneic Hematopoietic Cell Transplantation Improves Risk Assessment in Adults with NPM1-Mutated AML. Biol. Blood Marrow Transpl. 2018, 24, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Stahl, T.; Badbaran, A.; Kroger, N.; Klyuchnikov, E.; Zabelina, T.; Zeschke, S.; Schafhausen, P.; Schultz, W.; Asenova, S.; Smirnova, A.; et al. Minimal residual disease diagnostics in patients with acute myeloid leukemia in the post-transplant period: Comparison of peripheral blood and bone marrow analysis. Leuk Lymphoma 2010, 51, 1837–1843. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Savani, B.N.; Mohty, M.; Gorin, N.C.; Labopin, M.; Ruggeri, A.; Schmid, C.; Baron, F.; Esteve, J.; Giebel, S.; et al. Post-remission strategies for the prevention of relapse following allogeneic hematopoietic cell transplantation for high-risk acute myeloid leukemia: Expert review from the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transpl. 2019, 54, 519–530. [Google Scholar]
- Meloni, G.; Mancini, M.; Gianfelici, V.; Martelli, M.P.; Foa, R.; Falini, B. Late relapse of acute myeloid leukemia with mutated NPM1 after eight years: Evidence of NPM1 mutation stability. Haematologica 2009, 94, 298–300. [Google Scholar] [CrossRef]
- Bolli, N.; Galimberti, S.; Martelli, M.P.; Tabarrini, A.; Roti, G.; Mecucci, C.; Martelli, M.F.; Petrini, M.; Falini, B. Cytoplasmic nucleophosmin in myeloid sarcoma occurring 20 years after diagnosis of acute myeloid leukaemia. Lancet Oncol. 2006, 7, 350–352. [Google Scholar] [CrossRef]
- Ivey, A.; Hills, R.K.; Simpson, M.A.; Jovanovic, J.V.; Gilkes, A.; Grech, A.; Patel, Y.; Bhudia, N.; Farah, H.; Mason, J.; et al. Assessment of Minimal Residual Disease in Standard-Risk AML. N. Engl. J. Med. 2016, 374, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Thol, F.; Kolking, B.; Damm, F.; Reinhardt, K.; Klusmann, J.H.; Reinhardt, D.; von Neuhoff, N.; Brugman, M.H.; Schlegelberger, B.; Suerbaum, S.; et al. Next-generation sequencing for minimal residual disease monitoring in acute myeloid leukemia patients with FLT3-ITD or NPM1 mutations. Genes Chromosomes Cancer 2012, 51, 689–695. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Losada, C.; Serrano-Lopez, J.; Serrano-Lopez, J.; Noguera, N.I.; Garza, E.; Piredda, L.; Lavorgna, S.; Consalvo, M.A.I.; Ottone, T.; Alfonso, V.; et al. Clonal genetic evolution at relapse of favorable-risk acute myeloid leukemia with NPM1 mutation is associated with phenotypic changes and worse outcomes. Haematologica 2018, 103, e400–e403. [Google Scholar] [CrossRef] [PubMed]
- Kronke, J.; Bullinger, L.; Teleanu, V.; Tschurtz, F.; Gaidzik, V.I.; Kuhn, M.W.; Rucker, F.G.; Holzmann, K.; Paschka, P.; Kapp-Schworer, S.; et al. Clonal evolution in relapsed NPM1-mutated acute myeloid leukemia. Blood 2013, 122, 100–108. [Google Scholar] [CrossRef]
- Sockel, K.; Wermke, M.; Radke, J.; Kiani, A.; Schaich, M.; Bornhauser, M.; Ehninger, G.; Thiede, C.; Platzbecker, U. Minimal residual disease-directed preemptive treatment with azacitidine in patients with NPM1-mutant acute myeloid leukemia and molecular relapse. Haematologica 2011, 96, 1568–1570. [Google Scholar] [CrossRef]
- Bacher, U.; Porret, N.; Joncourt, R.; Sanz, J.; Aliu, N.; Wiedemann, G.; Jeker, B.; Banz, Y.; Pabst, T. Pitfalls in the molecular follow up of NPM1 mutant acute myeloid leukemia. Haematologica 2018, 103, e486–e488. [Google Scholar] [CrossRef]
- Schnittger, S.; Bacher, U.; Haferlach, C.; Alpermann, T.; Dicker, F.; Sundermann, J.; Kern, W.; Haferlach, T. Characterization of NPM1-mutated AML with a history of myelodysplastic syndromes or myeloproliferative neoplasms. Leukemia 2011, 25, 615–621. [Google Scholar] [CrossRef][Green Version]
- Courville, E.L.; Wu, Y.; Kourda, J.; Roth, C.G.; Brockmann, J.; Muzikansky, A.; Fathi, A.T.; de Leval, L.; Orazi, A.; Hasserjian, R.P. Clinicopathologic analysis of acute myeloid leukemia arising from chronic myelomonocytic leukemia. Mod. Pathol. 2013, 26, 751–761. [Google Scholar] [CrossRef]
- Fernandez-Mercado, M.; Yip, B.H.; Pellagatti, A.; Davies, C.; Larrayoz, M.J.; Kondo, T.; Perez, C.; Killick, S.; McDonald, E.J.; Odero, M.D.; et al. Mutation patterns of 16 genes in primary and secondary acute myeloid leukemia (AML) with normal cytogenetics. PLoS ONE 2012, 7, e42334. [Google Scholar]
- Lindsley, R.C.; Mar, B.G.; Mazzola, E.; Grauman, P.V.; Shareef, S.; Allen, S.L.; Pigneux, A.; Wetzler, M.; Stuart, R.K.; Erba, H.P.; et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015, 125, 1367–1376. [Google Scholar] [CrossRef]
- Wang, S.Y.; Cheng, W.Y.; Mao, Y.F.; Zhu, Y.M.; Liu, F.J.; Ma, T.T.; Shen, Y. Genetic alteration patterns and clinical outcomes of elderly and secondary acute myeloid leukemia. Hematol. Oncol. 2019, 37, 456–463. [Google Scholar] [CrossRef]
- Badar, T.; Szabo, A.; Sallman, D.; Komrojki, R.; Lancet, J.; Padron, E.; Song, J.; Hussaini, M.O. Interrogation of molecular profiles can help in differentiating between MDS and AML with MDS-related changes. Leuk Lymphoma 2020, 61, 1418–1427. [Google Scholar] [CrossRef]
- Forghieri, F.; Nasillo, V.; Paolini, A.; Bettelli, F.; Pioli, V.; Giusti, D.; Gilioli, A.; Colasante, C.; Acquaviva, G.; Riva, G.; et al. NPM1-Mutated Myeloid Neoplasms with <20% Blasts: A Really Distinct Clinico-Pathologic Entity? Int. J. Mol. Sci. 2020, 21, 8975. [Google Scholar] [CrossRef] [PubMed]
- Caudill, J.S.; Sternberg, A.J.; Li, C.Y.; Tefferi, A.; Lasho, T.L.; Steensma, D.P. C-terminal nucleophosmin mutations are uncommon in chronic myeloid disorders. Br. J. Haematol. 2006, 133, 638–641. [Google Scholar] [CrossRef]
- Oki, Y.; Jelinek, J.; Beran, M.; Verstovsek, S.; Kantarjian, H.M.; Issa, J.P. Mutations and promoter methylation status of NPM1 in myeloproliferative disorders. Haematologica 2006, 91, 1147–1148. [Google Scholar]
- Bains, A.; Luthra, R.; Medeiros, L.J.; Zuo, Z. FLT3 and NPM1 mutations in myelodysplastic syndromes: Frequency and potential value for predicting progression to acute myeloid leukemia. Am. J. Clin. Pathol. 2011, 135, 62–69. [Google Scholar] [CrossRef]
- Forghieri, F.; Paolini, A.; Morselli, M.; Bigliardi, S.; Bonacorsi, G.; Leonardi, G.; Coluccio, V.; Maccaferri, M.; Fantuzzi, V.; Faglioni, L.; et al. NPM1 mutations may reveal acute myeloid leukemia in cases otherwise morphologically diagnosed as myelodysplastic syndromes or myelodysplastic/myeloproliferative neoplasms. Leuk Lymphoma 2015, 56, 3222–3226. [Google Scholar] [CrossRef]
- Peng, J.; Zuo, Z.; Fu, B.; Oki, Y.; Tang, G.; Goswami, M.; Priyanka, P.; Muzzafar, T.; Medeiros, L.J.; Luthra, R.; et al. Chronic myelomonocytic leukemia with nucleophosmin (NPM1) mutation. Eur. J. Haematol. 2016, 96, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Vallapureddy, R.; Lasho, T.L.; Hoversten, K.; Finke, C.M.; Ketterling, R.; Hanson, C.; Gangat, N.; Tefferi, A.; Patnaik, M.M. Nucleophosmin 1 (NPM1) mutations in chronic myelomonocytic leukemia and their prognostic relevance. Am J. Hematol. 2017, 92, E614–E618. [Google Scholar] [CrossRef] [PubMed]
- Montalban-Bravo, G.; Kanagal-Shamanna, R.; Sasaki, K.; Patel, K.; Ganan-Gomez, I.; Jabbour, E.; Kadia, T.; Ravandi, F.; DiNardo, C.; Borthakur, G.; et al. NPM1 mutations define a specific subgroup of MDS and MDS/MPN patients with favorable outcomes with intensive chemotherapy. Blood Adv. 2019, 3, 922–933. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.M.; Kim, S.M.; Nam, Y.; Kim, J.; Kim, S.; Ahn, Y.O.; Park, Y.; Yoon, S.S.; Shin, S.; Kwon, S.; et al. Targeted sequencing aids in identifying clonality in chronic myelomonocytic leukemia. Leuk Res. 2019, 84, 106190. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.S.; Ho, C.; Ptashkin, R.N.; Sadigh, S.; Bagg, A.; Geyer, J.T.; Xu, M.L.; Prebet, T.; Mason, E.F.; Seegmiller, A.C.; et al. Clinicopathologic and genetic characterization of nonacute NPM1-mutated myeloid neoplasms. Blood Adv. 2019, 3, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Li, X.; Xu, F.; Wu, D.; He, Q.; Song, L.; Xiao, C.; Zhao, Y.; Zhang, Z.; Guo, J.; et al. NPM1 mutation with DNMT3A wild type defines a subgroup of MDS with particularly favourable outcomes after decitabine therapy. Br. J. Haematol. 2020, 189, 982–984. [Google Scholar] [CrossRef] [PubMed]

| Mutation | Sequence | Predicted Amino Acid |
|---|---|---|
| Wild type (NPM 1.1) | GAT CTC TGG CAG TGG AGG AAG TCT CTT TAA GAAAATAG | -DLWOWRKSL |
| Mutation A | GAT CTC TGT CTG GCA GTG GAG GAA GTC TCT TTA AGA AAA TAG | -DLCLAVEEVSLRK |
| Mutation B | GAT CTC TGC ATG GCA GTG GAG GAA GTC TCT TTA AGA AAA TAG | -DLCMAVEEVSLRK |
| Mutation D | GAT CTC TGC CTG GCA GTG GAG GAA GTC TCT TTA AGA AAA TAG | -DLCLAVEEVSLRK |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kelemen, K. The Role of Nucleophosmin 1 (NPM1) Mutation in the Diagnosis and Management of Myeloid Neoplasms. Life 2022, 12, 109. https://doi.org/10.3390/life12010109
Kelemen K. The Role of Nucleophosmin 1 (NPM1) Mutation in the Diagnosis and Management of Myeloid Neoplasms. Life. 2022; 12(1):109. https://doi.org/10.3390/life12010109
Chicago/Turabian StyleKelemen, Katalin. 2022. "The Role of Nucleophosmin 1 (NPM1) Mutation in the Diagnosis and Management of Myeloid Neoplasms" Life 12, no. 1: 109. https://doi.org/10.3390/life12010109
APA StyleKelemen, K. (2022). The Role of Nucleophosmin 1 (NPM1) Mutation in the Diagnosis and Management of Myeloid Neoplasms. Life, 12(1), 109. https://doi.org/10.3390/life12010109





