Abstract
Myeloid neoplasms with germline predisposition have recently been added as distinct provisional entities in the 2017 revision of the World Health Organization’s classification of tumors of hematopoietic and lymphatic tissue. Individuals with germline predisposition have increased risk of developing myeloid neoplasms—mainly acute myeloid leukemia and myelodysplastic syndrome. Although the incidence of myeloid neoplasms with germline predisposition remains poorly defined, these cases provide unique and important insights into the biology and molecular mechanisms of myeloid neoplasms. Knowledge of the regulation of the germline genes and their interactions with other genes, proteins, and the environment, the penetrance and clinical presentation of inherited mutations, and the longitudinal dynamics during the process of disease progression offer models and tools that can further our understanding of myeloid neoplasms. This knowledge will eventually translate to improved disease sub-classification, risk assessment, and development of more effective therapy. In this review, we will use examples of these disorders to illustrate the key molecular pathways of myeloid neoplasms.
1. Introduction
Myeloid neoplasms unite a group of heterogeneous hematologic diseases that harbor a set of specific genetic variations, often occurring as a result of stepwise accumulation of genetic alterations in the hematopoietic stem cells or progenitor cells. Germline contributions, initially noted in families with enrichments of hematologic malignancies in first-degree relatives, eventually gained recognition as a provisional category named ‘myeloid neoplasms with germline predisposition’ in the most recent update of WHO’s Classification of Tumours of Haematopoietic and Lymphoid Tissues [1]. This category includes myeloid neoplasms with preceding cytopenias and platelet disorders (e.g., inherited variants in GATA2 (GATA-binding protein 2), RUNX1 (runt-related transcription factor 1), and ETV6 (ETS Variant Transcription Factor 6)), myeloid neoplasms lacking other preexisting dysfunctions (e.g., inherited variants in CEBPA (CCAAT enhancer binding protein alpha) and DDX41 (DEAD-Box Helicase 41)), and myeloid neoplasms in the setting of bone marrow failure syndromes (e.g., Fanconi anemia) (Table 1). There is a growing list of genes identified in recent years that may be included in this category. The incidence of germline mutations predisposing to hematologic neoplasms in the general population remains poorly defined; however, the current estimate in populations of patients with myeloid neoplasms varies significantly from 4.4% to 18% [2,3].

Table 1.
WHO classification of myeloid neoplasms with germline predisposition [1].
The study of germline mutations in myeloid neoplasms provides profound insights into leukemogenesis and a potential direction for therapeutic intervention. We do not intend to provide a comprehensive review of all of the described genes that are associated with germline predisposition to hematologic malignancies. The goal of this review is to use several specific examples of these disorders to illustrate the key molecular pathways leading to myeloid neoplasms. In the list of germline mutations with predisposition to myeloid neoplasms, many encode known master transcription factors, such as CEBPA, RUNX1, and GATA2. Somatic mutations in these genes are frequent in myeloid neoplasms—particularly acute myeloid leukemia (AML). Other genes that have been added more recently have more diverse and elusive biological functions. For example, DDX41, SAMD9 (Sterile Alpha Motif Domain Containing 9), and SAMD9L (Sterile Alpha Motif Domain Containing 9 Like) have been described as being involved in innate immunity, antiviral responses, and RNA processing and endocytosis. These genes may uncover new pathways that confer genetic predisposition. Aberrant activation of important pathways is a common finding in hematologic malignancies. Therefore, gain-of-function germline mutations in genes, such as NRAS (NRAS Proto-Oncogene, GTPase), KRAS (KRAS Proto-Oncogene, GTPase), CBL (Cbl Proto-Oncogene), PTPN1 (Protein Tyrosine Phosphatase Non-Receptor Type 11), and SH2B3 (SH2B Adaptor Protein 3), result in aberrant activation of corresponding signaling pathways, resulting in myeloid neoplasms, such as Juvenile myelomonocytic leukemia (JMML) or myeloproliferative neoplasm (MPN). Predisposition to myeloid neoplasms can also occur in the setting of inherited disorders, such as bone marrow failure syndromes (i.e., Fanconi anemia, Diamond–Blackfan anemia, Dyskeratosis congenita, etc.), constitutional mismatch repair deficiency syndrome, or Li–Fraumeni syndrome due to germline TP53 mutations.
2. Transcription Control
Transcription factors recognize and bind to their specific consensus sequence elements and regulate downstream gene expression, which is critical for normal hematopoiesis. The dysregulation of transcription factors caused by gene mutations, chromosomal aberrations, or aberrant expression is an important mechanism for developing cancer, including AML. Mutations in transcription factors such as CEBPA, RUNX1, and GATA2 can be seen in myeloid neoplasms, both de novo or with germline predisposition.
2.1. CEBPA
CEBPA encodes a key hematopoietic transcription factor, C/EBP-α, which is involved in lineage-specific myeloid differentiation. Mutations in CEBPA occur in 5–10% of AML. AML with biallelic CEBPA mutations accounts for 2–15% of de novo AML and is a provisional entity in the WHO Classification of Tumours of Haematopoietic and Lymphoid tissues. Patients diagnosed with AML with biallelic CEBPA gene mutations have a longer overall survival and event-free survival compared to those with a monoallelic CEBPA mutation or CEBPA-wild-type, therefore justifying the recognition of this group as a distinct provisional entity. Normally, C/EBP-α has a full-length 42 kDa (p42) isoform. The biallelic combination of mutations is defined when one N-terminal mutation and one C-terminal mutation, which either block C/EBP-α dimerization or block DNA binding, are both present. Almost all familial cases of CEBPA-mutated AML have biallelic mutations, with the germline CEBPA mutation typically being a stop-gain frameshift variants clustered in the N terminus, resulting in a truncated 30 kDa (p30) isoform. The second mutation is typically acquired and is either a missense variant or in-frame insertion or deletion occurring in the C-terminal bZIP region and resulting in abolished DNA binding or dimerization. Recent studies revealed that in addition to sequence variants, CEBPA promoter methylation also results in silencing of the gene; thus, epigenetic control of expression of genes crucial for hematopoiesis may also contribute to the pathogenesis of AML or MDS [4,5]. Another interesting finding in AML patients with germline CEBPA mutations is that the genetic profile of the clone at the time of relapse is often distinct from the one at the time of diagnosis, including different somatic CEBPA mutations [6]. Tawana et al. reported a cumulative incidence of relapse in familial AML with biallelic CEBPA at the rate of 56% by 10 years, with some patients having more than three relapse occurrences over a period of 17 to 20 years [6]. This discovery highlights a unique pattern of disease development and progression in familial cases of AML with bi-allelic CEBPA. The most common co-occurring mutations in AML with biallelic CEBPA mutations are variants in GATA2, WT1 (WT1 transcription factor), TET2 (Tet Methylcytosine Dioxygenase 2), and CSF3R (Colony Stimulating Factor 3 Receptor). Other genes commonly mutated in myeloid neoplasms, such as FLT3 (fms like tyrosine kinase 3), DNMT3A (DNA Methyltransferase 3 Alpha), IDH1/2 (Isocitrate Dehydrogenase (NADP(+)) 1/2), NPM1 (Nucleophosmin 1), and RUNX1, are rare and almost mutually exclusive [7]. Although limited, the available literature reported high penetrance of germline CEBPA mutations, leading to almost 100% lifetime risk of developing subsequent AML [6], in contrast to other genes with frequent heritable mutations, such as RUNX1, which confers almost ~35% lifetime risk of myeloid neoplasm.
2.2. RUNX1
RUNX1 encodes the DNA-binding subunit of the core-binding factor complex and a master–regulator transcription factor (TF) involved in hematopoiesis. RUNX1 mutations occur in ~10% of adult AML cases. Although most RUNX1 mutations in AML are acquired, germline RUNX1 variants are not rare and represent 8–10% of RUNX1-mutated AML cases [8,9,10,11]. In contrast to AML with germline mutations in CEBPA, which often do not have precedent hematologic abnormalities, individuals with pathogenic germline RUNX1 variants may present as autosomal-dominant familial platelet disorder (FPD) with a high propensity for development of hematologic malignancies. Approximately 44% of individuals with RUNX1-FPD develop AML or MDS with a median age of onset of 33 years [12,13]. Germline mutations in RUNX1 encompass a whole spectrum ranging from missense, stop-gain, and frameshift variants to partial and whole gene deletions. Most missense RUNX1 germline variants cluster in the RUNT domain. However, missense mutations outside of the RUNT domain—in particular, in consensus splicing sites or exon-level deletions—have also been reported [8]. The mechanisms of pathogenicity of different RUNX1 mutations may be different; thus, missense variants in the RUNT domain may cause loss of binding activity, whereas stop-gain or frameshift variants or whole gene deletions result in protein loss-of-function with haploinsufficiency. In patients with germline RUNX1 mutations, a second allele often acquires somatic mutation at the time of progression to an overt myeloid neoplasm, likely as a result of an impaired DNA repair pathway and an increased mutagenicity. Interestingly, unlike in the CEBPA example, biallelic RUNX1 mutations are rarely seen in sporadic AML cases. Other co-mutations often seen in myeloid neoplasms with germline RUNX1 are in DNMT3A, FLT3, GATA2, PHF6 (PHD finger protein 6), BCOR (BCL6 Corepressor), WT1, and TET2 [14,15,16].
2.3. GATA2
GATA2 encodes a transcription factor with several functionally important domains, including the N-terminal and C-terminal zinc finger (ZF1 and ZF2) domains, a nuclear localization signal, and poorly defined transactivation domains. Gata2 binds to the consensus sequence W/GATA/R (W = A or T and R = A or G) in the promoter/enhancer regions of many target genes, such as PU.1, LMO2, TAL1, FLI1, and RUNX1, which regulate self-renewal of hematopoietic stem cells and promote differentiation to more mature blood cells. It is also involved in the development of the lymphatic system. If this gene is mutated, the disease penetrance is high. It was estimated that individuals with germline GATA2 mutations have almost 90% risk of developing clinical symptoms and 50–70% risk of developing myeloid neoplasms [17,18]. Pathogenic GATA2 germline variants often occur in the exonic and intronic/regulatory regions of the gene and include deletions, missense, nonsense, frameshift, and splice-site changes and alterations in intronic regulatory elements. Most pathogenic variants lead to haploinsufficiency due to an inability of non-functional proteins to bind DNA or other transcription factor partners. Acquisition of ASXL1 mutations may cooperate with germline GATA2 mutations as a driver of leukemogenesis [19]. Other concurrent mutations have been reported in genes, including RUNX1, TP53, STAG2, SETBP1 (SET Binding Protein 1), CBL, EZH2 (Enhancer of Zeste 2 Polycomb Repressive Complex 2 Subunit), NRAS/KRAS, JAK3 (Janus Kinase 3), and PTPN11 [20]. At the same time, SF3B1 (Splicing Factor 3b Subunit 1), U2AF1 (U2 Small Nuclear RNA Auxiliary Factor 1), NPM1, and FLT3 are rarely mutated in GATA2-mutated myeloid neoplasms [21,22].
3. Splicing and Signal Transduction Control
The dysregulation of alternative splicing has emerged as an important mechanism for myeloid neoplasms. Recent studies have shown that alternative pre-mRNA splicing is common in myeloid neoplasms, although the functional relevance of the splicing differences remains elusive. Most of the mutations in splicing factors are somatic; however, rare germline variants, such as DDX41 mutations, have recently been added to the list of contributors to germline predisposition. Another new addition to the list is SAMD9/SAMD9L, which is involved in endocytosis, growth factor signaling, and the antiviral inflammatory process. The functional mechanism in the pathogenesis of myeloid neoplasms is largely uncharacterized and is an area for active research. In contrast, it is well established that dysregulated cytokine and growth factor signaling pathways are common in myeloid leukemia. Germline variants involving genes along the RAS/MAPK pathway are associated with a diverse group of congenital disorders with high risk of development of myeloproliferative neoplasms—most notably, juvenile myelomonocytic leukemia. These entities are grouped as “juvenile myelomonocytic leukemia associated with neurofibromatosis, Noonan syndrome, or Noonan syndrome-like disorders” in the most recent update of the WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues.
3.1. DDX41
Germline DDX41 mutations have recently been added to the list of genes involved in familial MDS and AML. DDX41 (DEAD-Box Helicase 41) is located on chromosome 5q35 and encodes an RNA helicase protein with a function in RNA splicing. Mutations of spliceosomes are common in myeloid neoplasms, but are generally mutually exclusive with DDX41 mutations [23]. Deep total RNA sequencing revealed that DDX41 defects are associated with either more avid exon skipping or more exon retention [23]. Reports of germline DDX41 mutations have indicated specific mutational hot-spots with ethnic associations; thus, p.M1I and p.D140Gfs*2 germline variants are enriched in Caucasian populations, while the p.A500Cfs*9 germline change has been reported in families of Asian descent [23,24]. Acquisition of the p.R525H variant in DDX41 was reported as the most frequent somatic event in tumors. Germline DDX41 mutations strongly predispose individuals to an acquired mutation of the second DDX41 allele. Myeloid neoplasms with germline DDX41 mutations are frequently present with hypocellular bone marrow, erythroid dysplasia, and high-risk MDS or AML. Quesada et al. reported that approximately 60% of mutant DDX41-driven AML arose from antecedent MDS [25]. The additional concurrent somatic mutations in ASXL1 (ASXL Transcriptional Regulator 1), EZH2, SRSF2 (Serine And Arginine Rich Splicing Factor 2), CUX1 (Cut like homeobox 1), and SETBP1 have also been reported as being strongly associated with secondary AML rather than de novo AML [26]. DDX41 defects led to loss of tumor suppressor function due to altered pre-mRNA splicing and RNA processing. Germline DDX41 mutations reportedly represented 2.4% of a large cohort of 1385 unselected adult patients with MDS and AML [27]. In contrast to other genes with strong predisposition to familial myeloid neoplasms, such as GATA2, RUNX1, or CEBPA, which are frequently mutated in sporadic MDS/AML cases, somatic DDX41 variants are exceedingly rare (0.4%) in the absence of predisposing germline DDX41 variants and were reported only in five out of 1385 unselected patients with MDS or AML [27]. In addition, quite differently from other genes with germline predisposition to myeloid neoplasms with a younger age of disease onset, the median age at the time of diagnosis of hematologic malignancy for DDX41-mutated cases was 69 years, which is not significantly different from that of sporadic MDS/AML [27].
3.2. SMAD9 and SMAD9L
SMAD9 and SMAD9L are two interferon-inducible genes located on the long arm of chromosome 7 (7q21.3). The function of the SAMD9 and SAMD9L proteins in hematopoiesis is not entirely clear, but they appear to be involved in endocytosis and cytokine signaling [28]. Monosomy 7 and interstitial deletions of 7q (-7/7q-) are well-known abnormalities that are frequently identified in MDS and AML. Haploinsufficiency of SAMD9L and/or SAMD9 genes (both located on 7q) due to -7/7q- may contribute to development of myeloid neoplasms. Activating mutations in SAMD9 and SAMD9L genes have initially been described as being associated with a clinical spectrum of disorders, including the MIRAGE (myelodysplasia, infection, restriction of growth, adrenal hypoplasia, genital phenotypes, and enteropathy) syndrome, ataxia-pancytopenia syndrome, and myelodysplasia and leukemia syndrome with monosomy 7 syndrome [29]. These germline mutations cause gain of function of SAMD9 and SAMD9L, which normally suppress myeloid proliferation and inhibit cell cycle progression, leading to pancytopenias, as well as other organ hypoplasias and growth restrictions [30]. Schwatz et al. reported that germline variants of SAMD9 or SAMD9L were present in 17% of pediatric MDS patients [31]. Most of these variants are missense mutations and tend to cluster in the second half of the proteins within or near a putative P-loop nucleoside triphosphatase domain. The germline nature could be obscured due to the lower variant allele frequencies depending on the extent of the -7/7q-. Interestingly, studies have shown acquired loss-of-function mutations in SAMD9 or SAMD9L as a rescue from the deleterious effects of the gain-of-function germline effect. It has also been proposed that selective loss of chromosome 7, which harbors the mutant allele, occurs as a cellular adaptive mechanism to the germline SAMD9 and SAMD9L variants [29,31]. This haploinsufficiency of numerous other genes located in this region, including EZH2, CUX1, and KMT2C/MLL3 (Lysine Methylatransferase 2C/mixed-lineage leukemia 3), may also contribute and eventually lead to MDS and AML. These relevant or cooperating genetic alterations may influence the clinical phenotype of the individuals.
3.3. RAS/MAPK Pathway
The RAS superfamily of proteins includes HRAS, NRAS, and KRAS, which are small GTPases that play an important role in the control of various cell signaling pathways, including the MAPK pathway. RAS is active in the guanosine triphosphate (GTP)-bound state, controlling downstream signaling pathways, and is inactive in the guanosine diphosphate (GDP)-bound state. The downstream MAPK pathway has been reported as crucial for many cellular processes, such as proliferation and differentiation [32,33]. While the somatic variants in KRAS, NRAS, PTPN1, NF1 (Neurofibromin 1), and CBL involved in RAS/MAPK pathway are frequent in different types of cancers, germline variants in these genes are associated with a diverse group of congenital disorders (neurofibromatosis type 1, cardiofaciocutaneous syndrome, Costello syndrome, Noonan syndrome, and Noonan syndrome with multiple lentigines), often referred to as ‘RASopathies’. These conditions are characterized by a high risk of development of myeloproliferative neoplasms—most notably, juvenile myelomonocytic leukemia (JMML) [34,35,36]. JMML is an aggressive pediatric myeloid neoplasm with a dysregulated RAS/MAPK signaling pathway due to, most frequently, either heterozygous somatic gain-of-function mutations in KRAS, NRAS, or PTPN1 or germline RASopathy mutations in NF1 or CBL tumor suppressors with subsequent biallelic inactivation in hematopoietic cells, resulting in JMML [37]. Germline mutations in CBL in patients with JMML are predominantly located in intron 7 and exons 8 and 9 (linker and RING finger domain); chromosome 11q isodisomy, also known as copy-neutral loss of heterozygosity (CN-LOH), was reported as the most frequent mechanism resulting in the homozygous state of CBL mutations with no other secondary genetic abnormalities [38,39,40]. Germline mutations in the tumor suppressor gene NF1 are recurrent in JMML and are found in approximately 10% of patients. NF1 encodes neurofibromin, a GTPase-activating protein and a known repressor of RAS; thus, loss of one copy of NF1 due to germline mutation and subsequent loss of the second NF1 allele in JMML result in RAS hyperactivity in leukemia cells. Three main mechanisms may lead to the loss of a second NF1 in JMML: CN-LOH affecting chromosome 17q, somatic interstitial deletions, or a second NF1 mutation [41].
4. Bone Marrow Failure Syndromes and Other Inherited Disorders
Myeloid neoplasms associated with bone marrow failure syndromes, telomere biology disorders, Down syndrome, and other inherited disorders are grouped in the category of “myeloid neoplasm with germline predisposition and other organ dysfunction” in the most recent update of the WHO classification. Their clinical manifestations, molecular mechanisms, diagnostic modalities, and clinical outcomes are diverse. The respective pathologic pathways involve DNA repair (Fanconi anemia), telomere biology (Dyskeratosis congenita), ribosome biogenesis (Diamond–Blackfan anemia and Shwachman–Diamond syndrome), DNA repair, and genomic instability (Li–Fraumeni syndrome). The current classification emphasizes the importance of approaching these hematologic disorders in the context of a diverse genetic and clinical spectrum.
4.1. Fanconi Anemia (FA)
The risk of developing a myeloid neoplasm is increased in patients with bone marrow failure syndromes, including Fanconi anemia (FA), severe congenital neutropenia, dyskeratosis congenita, Shwachman–Diamond syndrome, and Diamond–Blackfan anemia. Although the molecular mechanisms of these disorders have not entirely been elucidated, the concept of dysfunctional DNA repair being responsible for the main pathophysiology of FA is well accepted. As a result, cells from patients with FA display hypersensitivity to DNA cross-linking agents, such as mitomycin C (MMC) and diepoxybutane (DEB), revealing an increased rate of chromosome breakage upon exposure to one of these two agents. The chromosome breakage test has been developed as a clinical diagnostic test for patients with clinical suspicion of having FA. If positive, next-generation sequencing testing with a panel of FA genes is recommended to detect mutations and affected genes associated with FA for further family studies in order to identify mutation carriers. Many FA genes have been identified and grouped into broad categories: the FA core complex, ID2 complex proteins (FANCD2 (FA Complementation Group D2), FANCI (FA Complementation Group I)), and a group of proteins in the downstream functional units. Proteins in the FA core complex work together to activate the ID2 complex and downstream proteins to bring in DNA repair proteins. Mutations in any of the genes involved in the FA pathway impair DNA repair, especially the homologous recombination repair of double-strand DNA damage. Hematopoietic elements are particularly sensitive to this defect. According to the International Fanconi Anemia Registry Study, the risk of developing either MDS or AML before the age of 20 is 27%, and it rapidly increases to 52% by the age of 40 [42]. The mechanism of leukemogenesis in FA is thought to be due to emerged malignant clones harboring mutations that allow them to evade cell cycle regulation and apoptosis, leading to MDS and AML [43].
4.2. Dyskeratosis Congenita (DC)
Another inherited bone marrow failure disorder is DC, one of a spectrum of telomere biology disorders based on shared genetic causes and telomere defects. Pathogenic variants in genes encoding core telomerase components (TERT (Telomerase Reverse Transcriptase) and TERC (Telomerase RNA Component)), or genes involved in telomere maintenance and function, have been identified in 60–70% of patients with clinical features of DC. DC is also a cancer predisposition syndrome, likely due to the genomic instability that can arise in the context of very short telomeres. Although the exact mechanism for developing myeloid neoplasms in patients with DC is still unclear, it was observed that somatic mutations in typical MDS-related genes are rare events in adult DC patients [44]. Therefore, accumulation of myeloid-associated clonal mutations does not seem to be the predominant mechanism for the initiation of myeloid neoplasms in DC patients. Other mechanisms, including the role of chromosomal instability due to critically short telomeres, may contribute to malignant transformation and expansion.
4.3. Diamond–Blackfan Anemia (DBA)
Similarly to FA and DC, DBA is also a cancer predisposition syndrome, although the cancer risk appears lower. DBA is the prototypic disorder linked to defects in ribosome synthesis known as ribosomopathies. The ribosomal protein genes harbor heterozygous loss-of-function mutations, resulting in haploinsufficiency. A study with a large cohort of DBA patients demonstrated an increased risk of developing MDS, AML, and solid tumors. There is accumulating evidence that ribosome dysfunction can drive malignant transformation due to the stimulation of protein synthesis. Recent studies also supported a role for p53 activation via ribosome stress signaling in leukemogenesis.
4.4. Li–Fraumeni Syndrome
Li–Fraumeni syndrome is a genetic disorder with a high risk of a wide range of malignancies, including hematologic neoplasms. As a classic cancer predisposition disorder, Li–Fraumeni syndrome is commonly associated with germline mutations of the p53 tumor suppressor gene, resulting in defective regulatory control over cell proliferation and homeostasis, the cell cycle, DNA repair, and genomic instability. Although hypodiploid acute lymphoblastic leukemia is the most recurrent leukemia in Li–Fraumeni patients, neoplasms of myeloid origin, including MDS and AML, are also frequent, typically developing as a therapy-related complication after the treatment of primary cancer, whereas the frequency of germline TP53 variants in de novo AML was reported at only 1.1% [45,46].
5. Conclusions
Myeloid neoplasms with germline predisposition have been increasingly recognized in clinical practice. Table 2 includes genes with a recognized inherited predisposition to myeloid neoplasms, but the rapidly expanding list of genes is well beyond the ones presented in this review. Although the incidence of myeloid neoplasms with germline predisposition is still poorly defined and the molecular pathogenesis has not entirely been elucidated for many of these entities, these cases provide unique and important insights into the biology and molecular mechanisms. An oversimplified molecular mechanism of myeloid neoplasms with germline predisposition is summarized in Figure 1. We continue to gain knowledge about the regulation of the germline genetic defects and their interactions with other genes and proteins, the role of the bone marrow microenvironment, the genotype/phenotype correlations, the clinical presentations, and the longitudinal dynamics during the process of disease progression. This group of inherited hematologic conditions offers a unique model for a better understanding of the mechanism of the development of myeloid neoplasms and disease progression. This knowledge will eventually translate into improved sub-classification, risk assessment, and development of effective therapies beyond standard options.

Table 2.
Genes with recognized associations with familial predisposition to myeloid neoplasms.
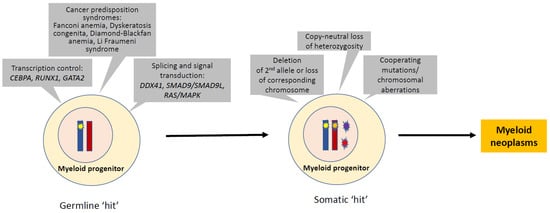
Figure 1.
Molecular pathogenesis in myeloid neoplasms with germline predisposition. The pathogenesis of myeloid neoplasms with germline predisposition serves as a model for multi-step leukemogenesis of MDS or AML. The germline aberrations in transcription control, splicing and signal transduction, bone marrow failure, or other inherited disorders often provide the first hit. Many somatically acquired secondary events may promote a transformation that leads to overt MDS and AML. These secondary events include deletion/loss of the second allele, copy-neutral loss of heterozygosity, and acquired cooperative mutations or chromosomal aberrations.
Author Contributions
Conceptualization, J.G., Y.C. and M.S.; writing—J.G., Y.C. and M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Peterson, L.C.; Bloomfield, C.D.; Niemeyer, C.M.; Dohner, H.; Godley, L.A. Myeloid Neoplasms with Germline Predisposition. In WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D., Hasserjian, R.P., Le Beau, M.M., Orazi, A., Siebert, R., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; pp. 122–129. [Google Scholar]
- Zhang, J.; Nichols, K.E.; Downing, J.R. Germline Mutations in Predisposition Genes in Pediatric Cancer. N. Engl. J. Med. 2016, 374, 1391. [Google Scholar] [CrossRef]
- DiNardo, C.D.; Bannon, S.A.; Routbort, M.; Franklin, A.; Mork, M.; Armanios, M.; Mace, E.M.; Orange, J.S.; Jeff-Eke, M.; Churpek, J.E.; et al. Evaluation of Patients and Families With Concern for Predispositions to Hematologic Malignancies Within the Hereditary Hematologic Malignancy Clinic (HHMC). Clin. Lymphoma Myeloma Leuk 2016, 16, 417–428.e2. [Google Scholar] [CrossRef]
- Kimura, Y.; Iwanaga, E.; Iwanaga, K.; Endo, S.; Inoue, Y.; Tokunaga, K.; Nagahata, Y.; Masuda, K.; Kawamoto, H.; Matsuoka, M. A regulatory element in the 3′-untranslated region of CEBPA is associated with myeloid/NK/T-cell leukemia. Eur. J. Haematol. 2021, 106, 327–339. [Google Scholar] [CrossRef] [PubMed]
- Wen, X.M.; Hu, J.B.; Yang, J.; Qian, W.; Yao, D.M.; Deng, Z.Q.; Zhang, Y.Y.; Zhu, X.W.; Guo, H.; Lin, J.; et al. CEBPA methylation and mutation in myelodysplastic syndrome. Med. Oncol. 2015, 32, 192. [Google Scholar] [CrossRef] [PubMed]
- Tawana, K.; Wang, J.; Renneville, A.; Bodor, C.; Hills, R.; Loveday, C.; Savic, A.; Van Delft, F.W.; Treleaven, J.; Georgiades, P.; et al. Disease evolution and outcomes in familial AML with germline CEBPA mutations. Blood 2015, 126, 1214–1223. [Google Scholar] [CrossRef] [PubMed]
- Wilhelmson, A.S.; Porse, B.T. CCAAT enhancer binding protein alpha (CEBPA) biallelic acute myeloid leukaemia: Cooperating lesions, molecular mechanisms and clinical relevance. Br. J. Haematol. 2020, 190, 495–507. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.P.T.; Kavelaars, F.G.; Lowenberg, B.; Valk, P.J.M.; Raaijmakers, M. RUNX1 germline variants in RUNX1-mutant AML: How frequent? Blood 2021, 137, 1428–1431. [Google Scholar] [CrossRef]
- Mendler, J.H.; Maharry, K.; Radmacher, M.D.; Mrozek, K.; Becker, H.; Metzeler, K.H.; Schwind, S.; Whitman, S.P.; Khalife, J.; Kohlschmidt, J.; et al. RUNX1 mutations are associated with poor outcome in younger and older patients with cytogenetically normal acute myeloid leukemia and with distinct gene and MicroRNA expression signatures. J. Clin. Oncol. 2012, 30, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Gaidzik, V.I.; Teleanu, V.; Papaemmanuil, E.; Weber, D.; Paschka, P.; Hahn, J.; Wallrabenstein, T.; Kolbinger, B.; Kohne, C.H.; Horst, H.A.; et al. RUNX1 mutations in acute myeloid leukemia are associated with distinct clinico-pathologic and genetic features. Leukemia 2016, 30, 2160–2168. [Google Scholar] [CrossRef] [PubMed]
- Drazer, M.W.; Kadri, S.; Sukhanova, M.; Patil, S.A.; West, A.H.; Feurstein, S.; Calderon, D.A.; Jones, M.F.; Weipert, C.M.; Daugherty, C.K.; et al. Prognostic tumor sequencing panels frequently identify germ line variants associated with hereditary hematopoietic malignancies. Blood Adv. 2018, 2, 146–150. [Google Scholar] [CrossRef]
- Godley, L.A. Inherited predisposition to acute myeloid leukemia. Semin. Hematol. 2014, 51, 306–321. [Google Scholar] [CrossRef]
- Brown, A.L.; Churpek, J.E.; Malcovati, L.; Dohner, H.; Godley, L.A. Recognition of familial myeloid neoplasia in adults. Semin. Hematol. 2017, 54, 60–68. [Google Scholar] [CrossRef]
- Brown, A.L.; Arts, P.; Carmichael, C.L.; Babic, M.; Dobbins, J.; Chong, C.E.; Schreiber, A.W.; Feng, J.; Phillips, K.; Wang, P.P.S.; et al. RUNX1-mutated families show phenotype heterogeneity and a somatic mutation profile unique to germline predisposed AML. Blood Adv. 2020, 4, 1131–1144. [Google Scholar] [CrossRef]
- Antony-Debre, I.; Duployez, N.; Bucci, M.; Geffroy, S.; Micol, J.B.; Renneville, A.; Boissel, N.; Dhedin, N.; Rea, D.; Nelken, B.; et al. Somatic mutations associated with leukemic progression of familial platelet disorder with predisposition to acute myeloid leukemia. Leukemia 2016, 30, 999–1002. [Google Scholar] [CrossRef]
- Churpek, J.E.; Pyrtel, K.; Kanchi, K.L.; Shao, J.; Koboldt, D.; Miller, C.A.; Shen, D.; Fulton, R.; O’Laughlin, M.; Fronick, C.; et al. Genomic analysis of germ line and somatic variants in familial myelodysplasia/acute myeloid leukemia. Blood 2015, 126, 2484–2490. [Google Scholar] [CrossRef]
- Collin, M.; Dickinson, R.; Bigley, V. Haematopoietic and immune defects associated with GATA2 mutation. Br. J. Haematol. 2015, 169, 173–187. [Google Scholar] [CrossRef]
- Spinner, M.A.; Sanchez, L.A.; Hsu, A.P.; Shaw, P.A.; Zerbe, C.S.; Calvo, K.R.; Arthur, D.C.; Gu, W.; Gould, C.M.; Brewer, C.C.; et al. GATA2 deficiency: A protean disorder of hematopoiesis, lymphatics, and immunity. Blood 2014, 123, 809–821. [Google Scholar] [CrossRef] [PubMed]
- Micol, J.B.; Abdel-Wahab, O. Collaborating constitutive and somatic genetic events in myeloid malignancies: ASXL1 mutations in patients with germline GATA2 mutations. Haematologica 2014, 99, 201–203. [Google Scholar] [CrossRef]
- Wang, X.; Muramatsu, H.; Okuno, Y.; Sakaguchi, H.; Yoshida, K.; Kawashima, N.; Xu, Y.; Shiraishi, Y.; Chiba, K.; Tanaka, H.; et al. GATA2 and secondary mutations in familial myelodysplastic syndromes and pediatric myeloid malignancies. Haematologica 2015, 100, e398. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.E.; Griffin, H.; Bigley, V.; Reynard, L.N.; Hussain, R.; Haniffa, M.; Lakey, J.H.; Rahman, T.; Wang, X.N.; McGovern, N.; et al. Exome sequencing identifies GATA-2 mutation as the cause of dendritic cell, monocyte, B and NK lymphoid deficiency. Blood 2011, 118, 2656–2658. [Google Scholar] [CrossRef] [PubMed]
- Dickinson, R.E.; Milne, P.; Jardine, L.; Zandi, S.; Swierczek, S.I.; McGovern, N.; Cookson, S.; Ferozepurwalla, Z.; Langridge, A.; Pagan, S.; et al. The evolution of cellular deficiency in GATA2 mutation. Blood 2014, 123, 863–874. [Google Scholar] [CrossRef]
- Polprasert, C.; Schulze, I.; Sekeres, M.A.; Makishima, H.; Przychodzen, B.; Hosono, N.; Singh, J.; Padgett, R.A.; Gu, X.; Phillips, J.G.; et al. Inherited and Somatic Defects in DDX41 in Myeloid Neoplasms. Cancer Cell 2015, 27, 658–670. [Google Scholar] [CrossRef]
- Lewinsohn, M.; Brown, A.L.; Weinel, L.M.; Phung, C.; Rafidi, G.; Lee, M.K.; Schreiber, A.W.; Feng, J.; Babic, M.; Chong, C.E.; et al. Novel germ line DDX41 mutations define families with a lower age of MDS/AML onset and lymphoid malignancies. Blood 2016, 127, 1017–1023. [Google Scholar] [CrossRef]
- Quesada, A.E.; Routbort, M.J.; DiNardo, C.D.; Bueso-Ramos, C.E.; Kanagal-Shamanna, R.; Khoury, J.D.; Thakral, B.; Zuo, Z.; Yin, C.C.; Loghavi, S.; et al. DDX41 mutations in myeloid neoplasms are associated with male gender, TP53 mutations and high-risk disease. Am. J. Hematol. 2019, 94, 757–766. [Google Scholar] [CrossRef]
- Lindsley, R.C.; Mar, B.G.; Mazzola, E.; Grauman, P.V.; Shareef, S.; Allen, S.L.; Pigneux, A.; Wetzler, M.; Stuart, R.K.; Erba, H.P.; et al. Acute myeloid leukemia ontogeny is defined by distinct somatic mutations. Blood 2015, 125, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Sebert, M.; Passet, M.; Raimbault, A.; Rahme, R.; Raffoux, E.; Sicre de Fontbrune, F.; Cerrano, M.; Quentin, S.; Vasquez, N.; Da Costa, M.; et al. Germline DDX41 mutations define a significant entity within adult MDS/AML patients. Blood 2019, 134, 1441–1444. [Google Scholar] [CrossRef] [PubMed]
- Nagamachi, A.; Matsui, H.; Asou, H.; Ozaki, Y.; Aki, D.; Kanai, A.; Takubo, K.; Suda, T.; Nakamura, T.; Wolff, L.; et al. Haploinsufficiency of SAMD9L, an endosome fusion facilitator, causes myeloid malignancies in mice mimicking human diseases with monosomy 7. Cancer Cell 2013, 24, 305–317. [Google Scholar] [CrossRef] [PubMed]
- Narumi, S.; Amano, N.; Ishii, T.; Katsumata, N.; Muroya, K.; Adachi, M.; Toyoshima, K.; Tanaka, Y.; Fukuzawa, R.; Miyako, K.; et al. SAMD9 mutations cause a novel multisystem disorder, MIRAGE syndrome, and are associated with loss of chromosome 7. Nat. Genet. 2016, 48, 792–797. [Google Scholar] [CrossRef]
- Davidsson, J.; Puschmann, A.; Tedgard, U.; Bryder, D.; Nilsson, L.; Cammenga, J. SAMD9 and SAMD9L in inherited predisposition to ataxia, pancytopenia, and myeloid malignancies. Leukemia 2018, 32, 1106–1115. [Google Scholar] [CrossRef]
- Schwartz, J.R.; Ma, J.; Lamprecht, T.; Walsh, M.; Wang, S.; Bryant, V.; Song, G.; Wu, G.; Easton, J.; Kesserwan, C.; et al. The genomic landscape of pediatric myelodysplastic syndromes. Nat. Commun. 2017, 8, 1557. [Google Scholar] [CrossRef]
- Drosten, M.; Dhawahir, A.; Sum, E.Y.; Urosevic, J.; Lechuga, C.G.; Esteban, L.M.; Castellano, E.; Guerra, C.; Santos, E.; Barbacid, M. Genetic analysis of Ras signalling pathways in cell proliferation, migration and survival. EMBO J. 2010, 29, 1091–1104. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, P. Ras-MAP kinase signaling pathways and control of cell proliferation: Relevance to cancer therapy. Crit. Rev. Clin. Lab. Sci. 2002, 39, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Strullu, M.; Caye, A.; Lachenaud, J.; Cassinat, B.; Gazal, S.; Fenneteau, O.; Pouvreau, N.; Pereira, S.; Baumann, C.; Contet, A.; et al. Juvenile myelomonocytic leukaemia and Noonan syndrome. J. Med. Genet. 2014, 51, 689–697. [Google Scholar] [CrossRef]
- Perez, B.; Mechinaud, F.; Galambrun, C.; Ben Romdhane, N.; Isidor, B.; Philip, N.; Derain-Court, J.; Cassinat, B.; Lachenaud, J.; Kaltenbach, S.; et al. Germline mutations of the CBL gene define a new genetic syndrome with predisposition to juvenile myelomonocytic leukaemia. J. Med. Genet. 2010, 47, 686–691. [Google Scholar] [CrossRef]
- Martinelli, S.; De Luca, A.; Stellacci, E.; Rossi, C.; Checquolo, S.; Lepri, F.; Caputo, V.; Silvano, M.; Buscherini, F.; Consoli, F.; et al. Heterozygous germline mutations in the CBL tumor-suppressor gene cause a Noonan syndrome-like phenotype. Am. J. Hum. Genet. 2010, 87, 250–257. [Google Scholar] [CrossRef]
- Baumann, I.; Niemeyer, C.M.; Thiele, J. Juvenile Myelomonocytic Leukaemia. In WHO Classification of Tumors of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Arber, D., Hasserjian, R.P., Le Beau, M.M., Orazi, A., et al., Eds.; International Agency for Research on Cancer (IARC): Lyon, France, 2017; pp. 89–92. [Google Scholar]
- Loh, M.L.; Sakai, D.S.; Flotho, C.; Kang, M.; Fliegauf, M.; Archambeault, S.; Mullighan, C.G.; Chen, L.; Bergstraesser, E.; Bueso-Ramos, C.E.; et al. Mutations in CBL occur frequently in juvenile myelomonocytic leukemia. Blood 2009, 114, 1859–1863. [Google Scholar] [CrossRef]
- Caye, A.; Strullu, M.; Guidez, F.; Cassinat, B.; Gazal, S.; Fenneteau, O.; Lainey, E.; Nouri, K.; Nakhaei-Rad, S.; Dvorsky, R.; et al. Juvenile myelomonocytic leukemia displays mutations in components of the RAS pathway and the PRC2 network. Nat. Genet. 2015, 47, 1334–1340. [Google Scholar] [CrossRef]
- Stieglitz, E.; Taylor-Weiner, A.N.; Chang, T.Y.; Gelston, L.C.; Wang, Y.D.; Mazor, T.; Esquivel, E.; Yu, A.; Seepo, S.; Olsen, S.; et al. The genomic landscape of juvenile myelomonocytic leukemia. Nat. Genet. 2015, 47, 1326–1333. [Google Scholar] [CrossRef]
- Steinemann, D.; Arning, L.; Praulich, I.; Stuhrmann, M.; Hasle, H.; Stary, J.; Schlegelberger, B.; Niemeyer, C.M.; Flotho, C. Mitotic recombination and compound-heterozygous mutations are predominant NF1-inactivating mechanisms in children with juvenile myelomonocytic leukemia and neurofibromatosis type 1. Haematologica 2010, 95, 320–323. [Google Scholar] [CrossRef] [PubMed]
- Butturini, A.; Gale, R.P.; Verlander, P.C.; Adler-Brecher, B.; Gillio, A.P.; Auerbach, A.D. Hematologic abnormalities in Fanconi anemia: An International Fanconi Anemia Registry study. Blood 1994, 84, 1650–1655. [Google Scholar] [CrossRef] [PubMed]
- Lensch, M.W.; Rathbun, R.K.; Olson, S.B.; Jones, G.R.; Bagby, G.C., Jr. Selective pressure as an essential force in molecular evolution of myeloid leukemic clones: A view from the window of Fanconi anemia. Leukemia 1999, 13, 1784–1789. [Google Scholar] [CrossRef][Green Version]
- Kirschner, M.; Maurer, A.; Wlodarski, M.W.; Ventura Ferreira, M.S.; Bouillon, A.S.; Halfmeyer, I.; Blau, W.; Kreuter, M.; Rosewich, M.; Corbacioglu, S.; et al. Recurrent somatic mutations are rare in patients with cryptic dyskeratosis congenita. Leukemia 2018, 32, 1762–1767. [Google Scholar] [CrossRef]
- Zebisch, A.; Lal, R.; Muller, M.; Lind, K.; Kashofer, K.; Girschikofsky, M.; Fuchs, D.; Wolfler, A.; Geigl, J.B.; Sill, H. Acute myeloid leukemia with TP53 germ line mutations. Blood 2016, 128, 2270–2272. [Google Scholar] [CrossRef]
- McNerney, M.E.; Godley, L.A.; Le Beau, M.M. Therapy-related myeloid neoplasms: When genetics and environment collide. Nat. Rev. Cancer 2017, 17, 513–527. [Google Scholar] [CrossRef]
- Geyer, J.T. Myeloid Neoplasms with Germline Predisposition. Pathobiology 2019, 86, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Noris, P.; Perrotta, S.; Seri, M.; Pecci, A.; Gnan, C.; Loffredo, G.; Pujol-Moix, N.; Zecca, M.; Scognamiglio, F.; De Rocco, D.; et al. Mutations in ANKRD26 are responsible for a frequent form of inherited thrombocytopenia: Analysis of 78 patients from 21 families. Blood 2011, 117, 6673–6680. [Google Scholar] [CrossRef]
- Saliba, J.; Saint-Martin, C.; Di Stefano, A.; Lenglet, G.; Marty, C.; Keren, B.; Pasquier, F.; Valle, V.D.; Secardin, L.; Leroy, G.; et al. Germline duplication of ATG2B and GSKIP predisposes to familial myeloid malignancies. Nat. Genet. 2015, 47, 1131–1140. [Google Scholar] [CrossRef] [PubMed]
- Plo, I.; Bellanne-Chantelot, C.; Vainchenker, W. ATG2B and GSKIP: 2 new genes predisposing to myeloid malignancies. Mol. Cell Oncol. 2016, 3, e1094564. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Niemeyer, C.M.; Kang, M.W.; Shin, D.H.; Furlan, I.; Erlacher, M.; Bunin, N.J.; Bunda, S.; Finklestein, J.Z.; Gorr, T.A.; Mehta, P.; et al. Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat. Genet. 2010, 42, 794–800. [Google Scholar] [CrossRef] [PubMed]
- Mohapatra, B.; Ahmad, G.; Nadeau, S.; Zutshi, N.; An, W.; Scheffe, S.; Dong, L.; Feng, D.; Goetz, B.; Arya, P.; et al. Protein tyrosine kinase regulation by ubiquitination: Critical roles of Cbl-family ubiquitin ligases. Biochim. Biophys. Acta 2013, 1833, 122–139. [Google Scholar] [CrossRef]
- Smith, M.L.; Cavenagh, J.D.; Lister, T.A.; Fitzgibbon, J. Mutation of CEBPA in familial acute myeloid leukemia. N. Engl. J. Med. 2004, 351, 2403–2407. [Google Scholar] [CrossRef]
- Noetzli, L.; Lo, R.W.; Lee-Sherick, A.B.; Callaghan, M.; Noris, P.; Savoia, A.; Rajpurkar, M.; Jones, K.; Gowan, K.; Balduini, C.; et al. Germline mutations in ETV6 are associated with thrombocytopenia, red cell macrocytosis and predisposition to lymphoblastic leukemia. Nat. Genet. 2015, 47, 535–538. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Churpek, J.E.; Keel, S.B.; Walsh, T.; Lee, M.K.; Loeb, K.R.; Gulsuner, S.; Pritchard, C.C.; Sanchez-Bonilla, M.; Delrow, J.J.; et al. Germline ETV6 mutations in familial thrombocytopenia and hematologic malignancy. Nat. Genet. 2015, 47, 180–185. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Metzger, M.L.; Wu, G.; Nishii, R.; Qian, M.; Devidas, M.; Yang, W.; Cheng, C.; Cao, X.; Quinn, E.; et al. Germline genetic variation in ETV6 and risk of childhood acute lymphoblastic leukaemia: A systematic genetic study. Lancet Oncol. 2015, 16, 1659–1666. [Google Scholar] [CrossRef]
- Hsu, A.P.; Sampaio, E.P.; Khan, J.; Calvo, K.R.; Lemieux, J.E.; Patel, S.Y.; Frucht, D.M.; Vinh, D.C.; Auth, R.D.; Freeman, A.F.; et al. Mutations in GATA2 are associated with the autosomal dominant and sporadic monocytopenia and mycobacterial infection (MonoMAC) syndrome. Blood 2011, 118, 2653–2655. [Google Scholar] [CrossRef]
- Hahn, C.N.; Chong, C.E.; Carmichael, C.L.; Wilkins, E.J.; Brautigan, P.J.; Li, X.C.; Babic, M.; Lin, M.; Carmagnac, A.; Lee, Y.K.; et al. Heritable GATA2 mutations associated with familial myelodysplastic syndrome and acute myeloid leukemia. Nat. Genet. 2011, 43, 1012–1017. [Google Scholar] [CrossRef]
- Wlodarski, M.W.; Hirabayashi, S.; Pastor, V.; Stary, J.; Hasle, H.; Masetti, R.; Dworzak, M.; Schmugge, M.; van den Heuvel-Eibrink, M.; Ussowicz, M.; et al. Prevalence, clinical characteristics, and prognosis of GATA2-related myelodysplastic syndromes in children and adolescents. Blood 2016, 127, 1387–1397; quiz 1518. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Kozyra, E.J.; Wlodarski, M.W. Germline predisposition in myeloid neoplasms: Unique genetic and clinical features of GATA2 deficiency and SAMD9/SAMD9L syndromes. Best Pract. Res. Clin. Haematol. 2020, 33, 101197. [Google Scholar] [CrossRef] [PubMed]
- Sanders, M.A.; Chew, E.; Flensburg, C.; Zeilemaker, A.; Miller, S.E.; Al Hinai, A.S.; Bajel, A.; Luiken, B.; Rijken, M.; McLennan, T.; et al. MBD4 guards against methylation damage and germ line deficiency predisposes to clonal hematopoiesis and early-onset AML. Blood 2018, 132, 1526–1534. [Google Scholar] [CrossRef] [PubMed]
- Niihori, T.; Ouchi-Uchiyama, M.; Sasahara, Y.; Kaneko, T.; Hashii, Y.; Irie, M.; Sato, A.; Saito-Nanjo, Y.; Funayama, R.; Nagashima, T.; et al. Mutations in MECOM, Encoding Oncoprotein EVI1, Cause Radioulnar Synostosis with Amegakaryocytic Thrombocytopenia. Am. J. Hum. Genet. 2015, 97, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Ripperger, T.; Hofmann, W.; Koch, J.C.; Shirneshan, K.; Haase, D.; Wulf, G.; Issing, P.R.; Karnebogen, M.; Schmidt, G.; Auber, B.; et al. MDS1 and EVI1 complex locus (MECOM): A novel candidate gene for hereditary hematological malignancies. Haematologica 2018, 103, e55–e58. [Google Scholar] [CrossRef]
- Germeshausen, M.; Ancliff, P.; Estrada, J.; Metzler, M.; Ponstingl, E.; Rutschle, H.; Schwabe, D.; Scott, R.H.; Unal, S.; Wawer, A.; et al. MECOM-associated syndrome: A heterogeneous inherited bone marrow failure syndrome with amegakaryocytic thrombocytopenia. Blood Adv. 2018, 2, 586–596. [Google Scholar] [CrossRef] [PubMed]
- Morgan, N.V.; Daly, M.E. Gene of the issue: RUNX1 mutations and inherited bleeding. Platelets 2017, 28, 208–210. [Google Scholar] [CrossRef] [PubMed]
- Buonocore, F.; Kuhnen, P.; Suntharalingham, J.P.; Del Valle, I.; Digweed, M.; Stachelscheid, H.; Khajavi, N.; Didi, M.; Brady, A.F.; Blankenstein, O.; et al. Somatic mutations and progressive monosomy modify SAMD9-related phenotypes in humans. J. Clin. Investig. 2017, 127, 1700–1713. [Google Scholar] [CrossRef] [PubMed]
- Tesi, B.; Davidsson, J.; Voss, M.; Rahikkala, E.; Holmes, T.D.; Chiang, S.C.C.; Komulainen-Ebrahim, J.; Gorcenco, S.; Rundberg Nilsson, A.; Ripperger, T.; et al. Gain-of-function SAMD9L mutations cause a syndrome of cytopenia, immunodeficiency, MDS, and neurological symptoms. Blood 2017, 129, 2266–2279. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, M.; Walne, A.J.; Plagnol, V.; Velangi, M.; Ho, A.; Hossain, U.; Vulliamy, T.; Dokal, I. Exome sequencing identifies autosomal-dominant SRP72 mutations associated with familial aplasia and myelodysplasia. Am. J. Hum. Genet. 2012, 90, 888–892. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).