Seminal Plasma Protein N-Glycan Peaks Are Potential Predictors of Semen Pathology and Sperm Chromatin Maturity in Men
Abstract
:1. Introduction
2. Results
2.1. Descriptive Characteristics of Study Participants
2.2. Association of Sperm DNA Fragmentation and Semen Parameters
2.3. Association of Sperm Chromatin Maturity and Semen Parameters
2.4. N-Glycan Composition in Normozoospermic and Pathological Diagnosis
2.5. Structural Characterization of SPGP14
2.6. Association of N-Glycans with Semen Parameters
2.7. Association between N-Glycans and Sperm Chromatin Maturity
3. Discussion
4. Materials and Methods
4.1. Study Sample Collection
4.2. Semen Parameter Analysis by CASA (Computer-Assisted Semen Analysis)
4.3. DNA Fragmentation Assay
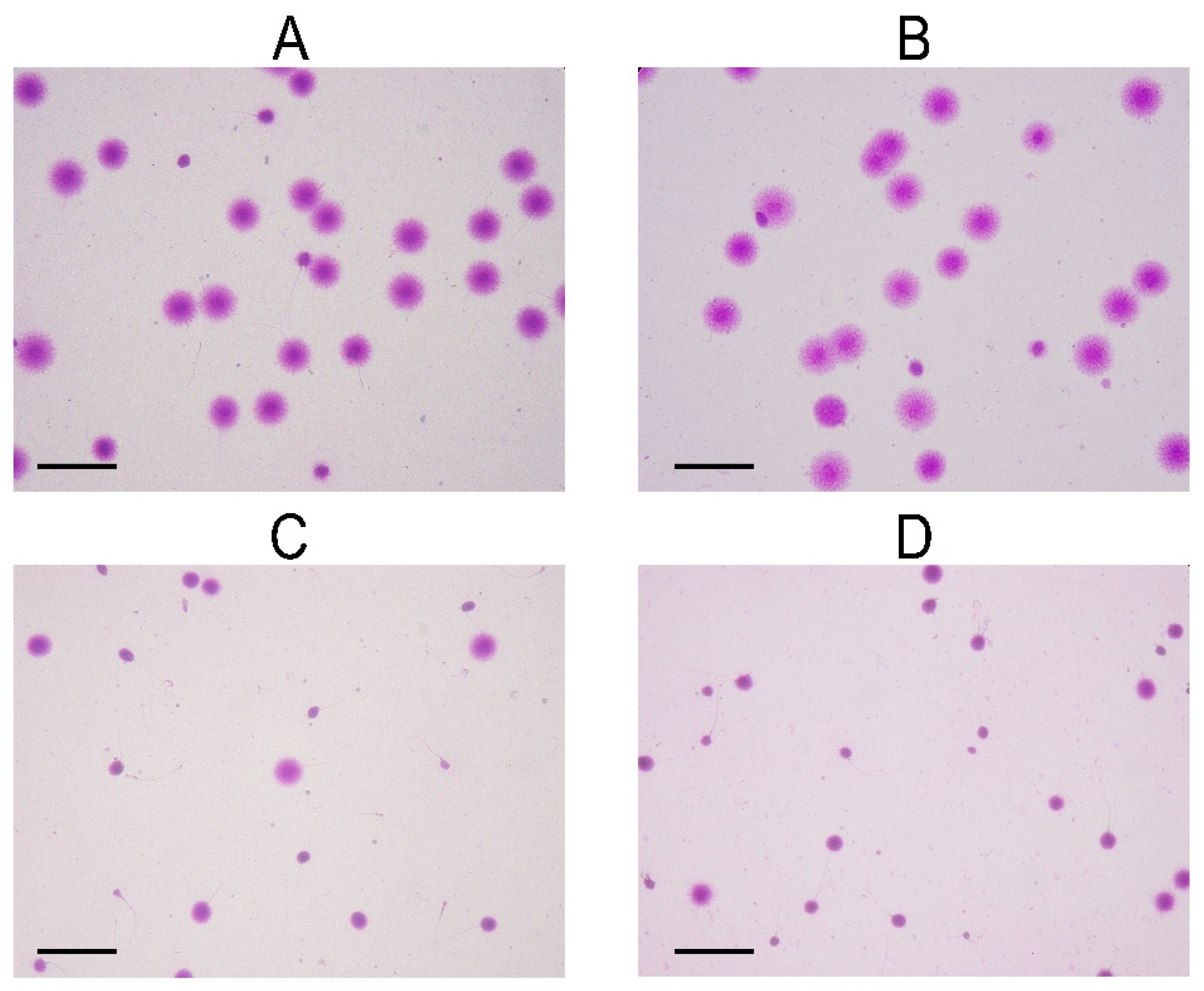
4.4. Aniline Blue Assay
4.5. N-Glycan Analysis from Total Seminal Plasma Proteins
4.6. Detection and Measurement of N-Glycans
4.7. Structural Characterization of SPGP14
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Levine, H.; Jorgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal trends in sperm count: A systematic review and meta-regression analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Krausz, C. Male infertility: Pathogenesis and clinical diagnosis. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 271–285. [Google Scholar] [CrossRef]
- Jungwirth, A.; Giwercman, A.; Tournaye, H.; Diemer, T.; Kopa, Z.; Dohle, G.; Krausz, C.; EAU Working Group on Male Infertility. European Association of Urology guidelines on Male Infertility: The 2012 update. Eur. Urol. 2012, 62, 324–332. [Google Scholar] [CrossRef]
- Rehman, I.; Ahmad, G.; Alshahrani, S. Lifestyle, environment, and male reproductive health: A lesson to learn. Bioenviron. Issues Affect. Men’s Reprod. Sex. Health 2018, 157–171. [Google Scholar] [CrossRef]
- Marić, T.; Fučić, A.; Aghayanian, A. Environmental and occupational exposures associated with male infertility. Arch. Ind. Hyg. Toxicol. 2021, 72, 101–113. [Google Scholar] [CrossRef]
- World Health Organization. WHO laboratory manual for the examination and processing of human semen. World Health Organ. 2010, 16, 867–871. [Google Scholar]
- Wang, C.; Swerdloff, R.S. Limitations of semen analysis as a test of male fertility and anticipated needs from newer tests. Fertil. Steril. 2014, 102, 1502–1507. [Google Scholar] [CrossRef] [Green Version]
- Oehninger, S.; Ombelet, W. Limits of current male fertility testing. Fertil. Steril. 2019, 111, 835–841. [Google Scholar] [CrossRef]
- Jedrzejczak, P.; Taszarek-Hauke, G.; Hauke, J.; Pawelczyk, L.; Duleba, A.J. Prediction of spontaneous conception based on semen parameters. Int. J. Androl. 2008, 31, 499–507. [Google Scholar] [CrossRef]
- Drabovich, A.P.; Saraon, P.; Jarvi, K.; Diamandis, E.P. Seminal plasma as a diagnostic fluid for male reproductive system disorders. Nat. Rev. Urol. 2014, 11, 278–288. [Google Scholar] [CrossRef]
- Rodriguez-Martinez, H.; Martinez, E.A.; Calvete, J.J.; Peña Vega, F.J.; Roca, J. Seminal Plasma: Relevant for Fertility? Int. J. Mol. Sci. 2021, 22, 4368. [Google Scholar] [CrossRef] [PubMed]
- Aebi, M. N-linked protein glycosylation in the ER. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2013, 1833, 2430–2437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, R.; Xin, M.; Hao, Z.; You, S.; Xu, Y.; Wu, J.; Dang, L.; Zhang, X.; Sun, S. Biological Functions and Large-Scale Profiling of Protein Glycosylation in Human Semen. J. Proteome Res. 2020, 19, 3877–3889. [Google Scholar] [CrossRef] [PubMed]
- Seppälä, M.; Koistinen, H.; Koistinen, R.; Chiu, P.C.N.; Yeung, W.S.B. Glycosylation related actions of glycodelin: Gamete, cumulus cell, immune cell and clinical associations. Hum. Reprod. Update 2007, 13, 275–287. [Google Scholar] [CrossRef] [Green Version]
- Pang, P.C.; Tissot, B.; Drobnis, E.Z.; Morris, H.R.; Dell, A.; Clark, G.F. Analysis of the human seminal plasma glycome reveals the presence of immunomodulatory carbohydrate functional groups. J. Proteome Res. 2009, 8, 4906–4915. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wu, Y.; Zhou, T.; Guo, Y.; Zheng, B.; Wang, J.; Bi, Y.; Liu, F.; Zhou, Z.; Guo, X.; et al. Mapping of the N-linked glycoproteome of human spermatozoa. J. Proteome Res. 2013, 12, 5750–5759. [Google Scholar] [CrossRef]
- Saraswat, M.; Joenvaara, S.; Tomar, A.K.; Singh, S.; Yadav, S.; Renkonen, R. N-Glycoproteomics of Human Seminal Plasma Glycoproteins. J. Proteome Res. 2016, 15, 991–1001. [Google Scholar] [CrossRef]
- Pang, P.-C.; Tissot, B.; Drobnis, E.Z.; Sutovsky, P.; Morris, H.R.; Clark, G.F.; Dell, A. Expression of Bisecting Type and Lewisx/Lewisy Terminated N-Glycans on Human Sperm. J. Biol. Chem. 2007, 282, 36593–36602. [Google Scholar] [CrossRef] [Green Version]
- Katnik-Prastowska, I.; Kratz, E.M.; Faundez, R.; Chełmońska-Soyta, A. Lower expression of the alpha2,3-sialylated fibronectin glycoform and appearance of the asialo-fibronectin glycoform are associated with high concentrations of fibronectin in human seminal plasma with abnormal semen parameters. Clin. Chem. Lab. Med. 2006, 44, 1119–1125. [Google Scholar] [CrossRef]
- Kratz, E.M.; Faundez, R.; Katnik-Prastowska, I. Fucose and sialic acid expressions in human seminal fibronectin and α{1} -acid glycoprotein associated with leukocytospermia of infertile men. Dis. Markers 2011, 31, 317–325. [Google Scholar] [CrossRef]
- Kratz, E.M.; Kaluza, A.; Zimmer, M.; Ferens-Sieczkowska, M. The analysis of sialylation, N-glycan branching, and expression of O-glycans in seminal plasma of infertile men. Dis. Markers 2015, 2015, 941871. [Google Scholar] [CrossRef] [Green Version]
- Kaluza, A.; Jarzab, A.; Gamian, A.; Kratz, E.M.; Zimmer, M.; Ferens-Sieczkowska, M. Preliminary MALDI-TOF-MS analysis of seminal plasma N-glycome of infertile men. Carbohydr. Res. 2016, 435, 19–25. [Google Scholar] [CrossRef]
- Kratz, E.M.; Ferens-Sieczkowska, M. Association of IgA secretory component sialylation with leucocytospermia of infertile men—a pilot study. Andrologia 2014, 46, 1200–1202. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Emery, B.R.; Carrell, D.T. Review: Diagnosis and impact of sperm DNA alterations in assisted reproduction. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 44, 38–56. [Google Scholar] [CrossRef]
- Agarwal, A.; Majzoub, A.; Baskaran, S.; Panner Selvam, M.K.; Cho, C.L.; Henkel, R.; Finelli, R.; Leisegang, K.; Sengupta, P.; Barbarosie, C.; et al. Sperm DNA Fragmentation: A New Guideline for Clinicians. World J. Mens Health 2020, 38, 412–471. [Google Scholar] [CrossRef]
- Nicopoullos, J.; Vicens-Morton, A.; Lewis, S.E.M.; Lee, K.; Larsen, P.; Ramsay, J.; Yap, T.; Minhas, S. Novel use of COMET parameters of sperm DNA damage may increase its utility to diagnose male infertility and predict live births following both IVF and ICSI. Hum. Reprod. 2019, 34, 1915–1923. [Google Scholar] [CrossRef]
- Fernandez, J.L.; Muriel, L.; Goyanes, V.; Segrelles, E.; Gosalvez, J.; Enciso, M.; LaFromboise, M.; De Jonge, C. Simple determination of human sperm DNA fragmentation with an improved sperm chromatin dispersion test. Fertil. Steril. 2005, 84, 833–842. [Google Scholar] [CrossRef] [PubMed]
- Balhorn, R. The protamine family of sperm nuclear proteins. Genome Biol. 2007, 8, 227. [Google Scholar] [CrossRef]
- Boskovic, A.; Torres-Padilla, M.E. How mammals pack their sperm: A variant matter. Genes Dev. 2013, 27, 1635–1639. [Google Scholar] [CrossRef] [Green Version]
- Champroux, A.; Torres-Carreira, J.; Gharagozloo, P.; Drevet, J.R.; Kocer, A. Mammalian sperm nuclear organization: Resiliencies and vulnerabilities. Basic Clin. Androl. 2016, 26, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.-S.; Kang, M.J.; Kim, S.A.; Oh, S.K.; Kim, H.; Ku, S.-Y.; Kim, S.H.; Moon, S.Y.; Choi, Y.M. The utility of sperm DNA damage assay using toluidine blue and aniline blue staining in routine semen analysis. Clin. Exp. Reprod. Med. 2013, 40, 23–28. [Google Scholar] [CrossRef] [Green Version]
- Cooper, T.G.; Noonan, E.; von Eckardstein, S.; Auger, J.; Baker, H.W.; Behre, H.M.; Haugen, T.B.; Kruger, T.; Wang, C.; Mbizvo, M.T.; et al. World Health Organization reference values for human semen characteristics. Hum. Reprod. Update 2010, 16, 231–245. [Google Scholar] [CrossRef]
- Neto, F.T.; Bach, P.V.; Najari, B.B.; Li, P.S.; Goldstein, M. Spermatogenesis in humans and its affecting factors. Semin. Cell Dev. Biol. 2016, 59, 10–26. [Google Scholar] [CrossRef]
- Cannarella, R.; Condorelli, R.A.; Mongioì, L.M.; La Vignera, S.; Calogero, A.E. Molecular Biology of Spermatogenesis: Novel Targets of Apparently Idiopathic Male Infertility. Int. J. Mol. Sci. 2020, 21, 1728. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Gong, T.-T.; Jiang, Y.-T.; Zhang, S.; Zhao, Y.-H.; Wu, Q.-J. Global, regional, and national prevalence and disability-adjusted life-years for infertility in 195 countries and territories, 1990–2017: Results from a global burden of disease study, 2017. Aging 2019, 11, 10952–10991. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Baskaran, S.; Parekh, N.; Cho, C.-L.; Henkel, R.; Vij, S.; Arafa, M.; Panner Selvam, M.K.; Shah, R. Male infertility. Lancet 2021, 397, 319–333. [Google Scholar] [CrossRef]
- Saleh, R.A.; Agarwal, A.; Nada, E.A.; El-Tonsy, M.H.; Sharma, R.K.; Meyer, A.; Nelson, D.R.; Thomas, A.J. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil. Steril. 2003, 79 (Suppl. S3), 1597–1605. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA fragmentation index as a promising predictive tool for male infertility diagnosis and treatment management—Meta-analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.-C.; Jing, J.; Chen, L.; Ge, Y.-F.; Feng, R.-X.; Liang, Y.-J.; Yao, B. Analysis of human sperm DNA fragmentation index (DFI) related factors: A report of 1010 subfertile men in China. Reprod. Biol. Endocrinol. 2018, 16, 23. [Google Scholar] [CrossRef]
- Giwercman, A.; Richthoff, J.; Hjøllund, H.; Bonde, J.P.; Jepson, K.; Frohm, B.; Spano, M. Correlation between sperm motility and sperm chromatin structure assay parameters. Fertil. Steril. 2003, 80, 1404–1412. [Google Scholar] [CrossRef]
- Boe-Hansen, G.B.; Fedder, J.; Ersbøll, A.K.; Christensen, P. The sperm chromatin structure assay as a diagnostic tool in the human fertility clinic. Hum. Reprod. 2006, 21, 1576–1582. [Google Scholar] [CrossRef] [Green Version]
- McEvoy, A.; Roberts, P.; Yap, K.; Matson, P. Development of a simplified method of human semen storage for the testing of sperm DNA fragmentation using the Halosperm G2 test kit. Fertil. Steril. 2014, 102, 981–988. [Google Scholar] [CrossRef]
- Belloc, S.; Benkhalifa, M.; Cohen-Bacrie, M.; Dalleac, A.; Chahine, H.; Amar, E.; Zini, A. Which isolated sperm abnormality is most related to sperm DNA damage in men presenting for infertility evaluation. J. Assist. Reprod. Genet. 2014, 31, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Le, M.T.; Nguyen, T.A.T.; Nguyen, H.T.T.; Nguyen, T.T.T.; Nguyen, V.T.; Le, D.D.; Nguyen, V.Q.H.; Cao, N.T. Does sperm DNA fragmentation correlate with semen parameters? Reprod. Med. Biol. 2019, 18, 390–396. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; Lin, D.P.-C.; Tsao, H.-M.; Cheng, T.-C.; Liu, C.-H.; Lee, M.-S. Sperm DNA fragmentation negatively correlates with velocity and fertilization rates but might not affect pregnancy rates. Fertil. Steril. 2005, 84, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Larson, K.L.; DeJonge, C.J.; Barnes, A.M.; Jost, L.K.; Evenson, D.P. Sperm chromatin structure assay parameters as predictors of failed pregnancy following assisted reproductive techniques. Hum. Reprod. 2000, 15, 1717–1722. [Google Scholar] [CrossRef] [PubMed]
- Irvine, D.S.; Twigg, J.P.; Gordon, E.L.; Fulton, N.; Milne, P.A.; Aitken, R.J. DNA integrity in human spermatozoa: Relationships with semen quality. J. Androl. 2000, 21, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Majzoub, A.; Esteves, S.C.; Ko, E.; Ramasamy, R.; Zini, A. Clinical utility of sperm DNA fragmentation testing: Practice recommendations based on clinical scenarios. Transl. Androl. Urol. 2016, 5, 935–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samplaski, M.K.; Dimitromanolakis, A.; Lo, K.C.; Grober, E.D.; Mullen, B.; Garbens, A.; Jarvi, K.A. The relationship between sperm viability and DNA fragmentation rates. Reprod. Biol. Endocrinol. 2015, 13, 42. [Google Scholar] [CrossRef] [Green Version]
- Maciel, V.L., Jr.; Tamashiro, L.K.; Bertolla, R.P. Post-translational modifications of seminal proteins and their importance in male fertility potential. Expert Rev. Proteom. 2019, 16, 941–950. [Google Scholar] [CrossRef]
- Schröter, S.; Osterhoff, C.; McArdle, W.; Ivell, R. The glycocalyx of the sperm surface. Hum. Reprod. Update 1999, 5, 302–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, R.; Frenette, G.; Girouard, J. Epididymosomes are involved in the acquisition of new sperm proteins during epididymal transit. Asian J. Androl. 2007, 9, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Tecle, E.; Gagneux, P. Sugar-coated sperm: Unraveling the functions of the mammalian sperm glycocalyx. Mol. Reprod. Dev. 2015, 82, 635–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janiszewska, E.; Kratz, E.M. Could the glycosylation analysis of seminal plasma clusterin become a novel male infertility biomarker? Mol. Reprod. Dev. 2020, 87, 515–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olejnik, B.; Jarzab, A.; Kratz, E.M.; Zimmer, M.; Gamian, A.; Ferens-Sieczkowska, M. Terminal Mannose Residues in Seminal Plasma Glycoproteins of Infertile Men Compared to Fertile Donors. Int. J. Mol. Sci. 2015, 16, 14933–14950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olejnik, B.; Kratz, E.M.; Zimmer, M.; Ferens-Sieczkowska, M. Glycoprotein fucosylation is increased in seminal plasma of subfertile men. Asian J. Androl. 2015, 17, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Kaluza, A.; Nouta, J.; Nicolardi, S.; Ferens-Sieczkowska, M.; Wuhrer, M.; Lageveen-Kammeijer, G.S.M.; de Haan, N. High-throughput glycopeptide profiling of prostate-specific antigen from seminal plasma by MALDI-MS. Talanta 2021, 222, 121495. [Google Scholar] [CrossRef] [PubMed]
- Janiszewska, E.; Kokot, I.; Gilowska, I.; Faundez, R.; Kratz, E.M. The possible association of clusterin fucosylation changes with male fertility disorders. Sci. Rep. 2021, 11, 15674. [Google Scholar] [CrossRef]
- Ka, U.A.A.; Ferens-Sieczkowska, M.A.; Olejnik, B.; Ko Odziejczyk, J.; Zimmer, M.; Kratz, E.M. The content of immunomodulatory glycoepitopes in seminal plasma glycoproteins of fertile and infertile men. Reprod. Fertil. Dev. 2019, 31, 579–589. [Google Scholar] [CrossRef]
- Simon, L.; Liu, L.; Murphy, K.; Ge, S.; Hotaling, J.; Aston, K.I.; Emery, B.; Carrell, D.T. Comparative analysis of three sperm DNA damage assays and sperm nuclear protein content in couples undergoing assisted reproduction treatment. Hum. Reprod. 2014, 29, 904–917. [Google Scholar] [CrossRef]
- Sellami, A.; Chakroun, N.; Ben Zarrouk, S.; Sellami, H.; Kebaili, S.; Rebai, T.; Keskes, L. Assessment of chromatin maturity in human spermatozoa: Useful aniline blue assay for routine diagnosis of male infertility. Adv. Urol. 2013, 2013, 578631. [Google Scholar] [CrossRef] [Green Version]
- Hammadeh, M.E.; Zeginiadov, T.; Rosenbaum, P.; Georg, T.; Schmidt, W.; Strehler, E. Predictive value of sperm chromatin condensation (aniline blue staining) in the assessment of male fertility. Arch. Androl. 2001, 46, 99–104. [Google Scholar] [CrossRef]
- Pourmasumi, S.; Khoradmehr, A.; Rahiminia, T.; Sabeti, P.; Talebi, A.R.; Ghasemzadeh, J. Evaluation of Sperm Chromatin Integrity Using Aniline Blue and Toluidine Blue Staining in Infertile and Normozoospermic Men. J. Reprod. Infertil. 2019, 20, 95–101. [Google Scholar]
- Belloc, S.; Benkhalifa, M.; Junca, A.M.; Dumont, M.; Bacrie, P.C.; Ménézo, Y. Paternal age and sperm DNA decay: Discrepancy between chromomycin and aniline blue staining. Reprod. Biomed. Online 2009, 19, 264–269. [Google Scholar] [CrossRef]
- Mohammadi, Z.; Tavalaee, M.; Gharagozloo, P.; Drevet, J.R.; Nasr-Esfahani, M.H. Could high DNA stainability (HDS) be a valuable indicator of sperm nuclear integrity? Basic Clin. Androl. 2020, 30, 12. [Google Scholar] [CrossRef]
- Rashki Ghaleno, L.; Alizadeh, A.; Drevet, J.R.; Shahverdi, A.; Valojerdi, M.R. Oxidation of Sperm DNA and Male Infertility. Antioxidants 2021, 10, 97. [Google Scholar] [CrossRef] [PubMed]
- Nanassy, L.; Liu, L.; Griffin, J.; Carrell, D.T. The clinical utility of the protamine 1/protamine 2 ratio in sperm. Protein Pept. Lett. 2011, 18, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Bench, G.; Corzett, M.H.; Kramer, C.E.; Grant, P.G.; Balhorn, R. Zinc is sufficiently abundant within mammalian sperm nuclei to bind stoichiometrically with protamine 2. Mol. Reprod. Dev. 2000, 56, 512–519. [Google Scholar] [CrossRef]
- Bjorndahl, L.; Kvist, U. Human sperm chromatin stabilization: A proposed model including zinc bridges. Mol. Hum. Reprod. 2010, 16, 23–29. [Google Scholar] [CrossRef] [Green Version]
- Girouard, J.; Frenette, G.; Sullivan, R. Seminal Plasma Proteins Regulate the Association of Lipids and Proteins Within Detergent-Resistant Membrane Domains of Bovine Spermatozoa1. Biol. Reprod. 2008, 78, 921–931. [Google Scholar] [CrossRef] [Green Version]
- Trbojević-Akmačić, I.; Ugrina, I.; Lauc, G. Comparative Analysis and Validation of Different Steps in Glycomics Studies. Methods Enzymol. 2017, 586, 37–55. [Google Scholar] [CrossRef] [PubMed]


| N (N = 82) | P (N = 84) | p-Value | |||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Age | 34.5 | 6.4 | 36.0 | 6.8 | 0.178 |
| Sperm concentration (106/mL) | 59.1 | 29.0 | 33.4 | 92.9 | <0.001 |
| Total sperm count (106/ejaculate) | 188.3 | 116.1 | 72.4 | 115.4 | <0.001 |
| Volume (mL) | 3.3 | 1.3 | 3.2 | 1.8 | 0.269 |
| DFI (%) | 17.8 | 9.2 | 30.7 | 17.5 | <0.001 |
| Big halo sperm (%) | 58.1 | 18.7 | 42.1 | 20.3 | <0.001 |
| Medium halo sperm (%) | 24.1 | 15.4 | 27.4 | 12.3 | 0.010 |
| Small halo sperm (%) | 3.6 | 2.1 | 5.4 | 5.3 | 0.019 |
| Sperm without halo (%) | 13.6 | 8.4 | 24.7 | 14.2 | <0.001 |
| Degraded sperm (%) | 0.6 | 0.8 | 0.6 | 0.8 | 0.421 |
| Sperm chromatin maturity (%) | 94.5 | 4.8 | 95.4 | 4.1 | 0.197 |
| N (N = 82) | A (N = 29) | O (N = 21) | OA (N = 34) | p-Value | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| Age | 34.5 | 6.4 | 36.0 | 7.7 | 37.0 | 6.7 | 35.4 | 6.0 | 0.516 |
| Sperm concentration (106/mL) | 59.1 | 29.0 | 52.7 | 29.8 | 12.1 | 10.6 | 30.2 | 142.3 | <0.001 |
| Total sperm count (106/ejaculate) | 188.3 | 116.1 | 165.1 | 158.4 | 26.3 | 19.2 | 21.8 | 21.2 | <0.001 |
| Volume (mL) | 3.3 | 1.3 | 3.0 | 1.3 | 2.7 | 1.4 | 3.7 | 2.2 | 0.229 |
| DFI (%) | 17.8 | 9.2 | 27.6 | 15.3 | 21.6 | 7.2 | 38.9 | 20.3 | <0.001 |
| Big halo sperm (%) | 58.1 | 18.7 | 49.3 | 20.4 | 49.6 | 15.8 | 31.3 | 18.3 | <0.001 |
| Medium halo sperm (%) | 24.1 | 15.4 | 23.5 | 10.3 | 28.8 | 12.5 | 29.8 | 13.3 | 0.019 |
| Small halo sperm (%) | 3.6 | 2.1 | 4.9 | 4.6 | 3.6 | 2.0 | 7.1 | 6.6 | 0.005 |
| Sperm without halo (%) | 13.6 | 8.4 | 22.1 | 12.2 | 17.7 | 6.3 | 31.1 | 16.7 | <0.001 |
| Degraded sperm (%) | 0.6 | 0.8 | 0.7 | 0.8 | 0.3 | 0.4 | 0.7 | 1.0 | 0.164 |
| Sperm chromatin maturity (%) | 94.5 | 4.8 | 94.7 | 4.0 | 96.2 | 4.6 | 95.5 | 3.8 | 0.216 |
| Seminal Plasma Glycan Peak (SPGP) | N (N = 82) | P (N = 84) | Nominal p-Value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| SPGP1 | 2.27 | 0.40 | 2.35 | 0.65 | 0.989 |
| SPGP2 | 0.23 | 0.09 | 0.23 | 0.10 | 0.406 |
| SPGP3 | 0.32 | 0.12 | 0.34 | 0.11 | 0.307 |
| SPGP4 | 0.21 | 0.08 | 0.22 | 0.10 | 0.887 |
| SPGP5 | 0.11 | 0.07 | 0.12 | 0.06 | 0.336 |
| SPGP6 | 0.16 | 0.08 | 0.14 | 0.07 | 0.085 |
| SPGP7 | 4.94 | 1.95 | 4.98 | 2.24 | 0.957 |
| SPGP8 | 0.85 | 1.03 | 0.71 | 0.27 | 0.384 |
| SPGP9 | 2.87 | 0.85 | 2.90 | 1.10 | 0.992 |
| SPGP10 | 3.03 | 0.66 | 3.07 | 0.93 | 0.744 |
| SPGP11 | 0.19 | 0.43 | 0.16 | 0.18 | 0.961 |
| SPGP12 | 7.18 | 1.70 | 7.26 | 2.16 | 0.906 |
| SPGP13 | 6.93 | 1.21 | 6.80 | 1.26 | 0.579 |
| SPGP14 * | 3.85 | 0.89 | 3.46 | 1.24 | <0.001 |
| SPGP15 | 11.36 | 7.46 | 12.68 | 7.89 | 0.150 |
| SPGP16 | 3.81 | 1.12 | 3.65 | 1.33 | 0.167 |
| SPGP17 | 2.78 | 1.47 | 2.79 | 1.23 | 0.791 |
| SPGP18 | 9.73 | 3.56 | 9.60 | 4.05 | 0.855 |
| SPGP19 | 5.40 | 2.41 | 5.38 | 2.10 | 0.899 |
| SPGP20 | 7.46 | 4.39 | 7.04 | 3.85 | 0.492 |
| SPGP21 | 1.16 | 0.59 | 1.13 | 0.53 | 0.952 |
| SPGP22 | 2.15 | 1.21 | 2.17 | 1.35 | 0.960 |
| SPGP23 | 3.65 | 1.91 | 3.46 | 2.00 | 0.217 |
| SPGP24 | 2.11 | 1.86 | 2.18 | 1.79 | 0.497 |
| SPGP25 | 1.43 | 0.96 | 1.31 | 0.82 | 0.349 |
| SPGP26 | 1.19 | 0.77 | 1.39 | 1.02 | 0.134 |
| SPGP27 | 1.38 | 0.61 | 1.59 | 0.64 | 0.018 |
| SPGP28 | 1.74 | 0.71 | 1.79 | 0.85 | 0.997 |
| SPGP29 | 2.40 | 1.05 | 2.48 | 1.14 | 0.896 |
| SPGP30 | 1.12 | 0.83 | 1.00 | 0.55 | 0.548 |
| SPGP31 | 2.89 | 1.74 | 2.72 | 1.80 | 0.436 |
| SPGP32 | 1.23 | 0.52 | 1.19 | 0.66 | 0.063 |
| SPGP33 | 0.74 | 0.49 | 0.71 | 0.47 | 0.906 |
| SPGP34 | 0.77 | 0.37 | 0.85 | 0.52 | 0.426 |
| SPGP35 | 0.49 | 0.30 | 0.44 | 0.25 | 0.423 |
| SPGP36 | 0.85 | 0.72 | 0.81 | 0.51 | 0.745 |
| SPGP37 | 1.00 | 0.53 | 0.90 | 0.43 | 0.294 |
| Seminal Plasma Glycan Peak (SPGP) | N (N = 82) | A (N = 29) | O (N = 21) | OA (N = 34) | Nominal p-Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| SPGP1 | 2.27 | 0.40 | 2.30 | 0.52 | 2.35 | 0.89 | 2.40 | 0.60 | 0.676 |
| SPGP2 | 0.23 | 0.09 | 0.22 | 0.10 | 0.25 | 0.09 | 0.22 | 0.10 | 0.183 |
| SPGP3 | 0.32 | 0.12 | 0.35 | 0.14 | 0.36 | 0.09 | 0.31 | 0.09 | 0.112 |
| SPGP4 | 0.21 | 0.08 | 0.23 | 0.13 | 0.25 | 0.10 | 0.19 | 0.07 | 0.117 |
| SPGP5 | 0.11 | 0.07 | 0.10 | 0.05 | 0.14 | 0.04 | 0.11 | 0.08 | 0.042 |
| SPGP6 | 0.16 | 0.08 | 0.16 | 0.09 | 0.14 | 0.05 | 0.13 | 0.06 | 0.168 |
| SPGP7 | 4.94 | 1.95 | 4.31 | 1.95 | 5.34 | 2.53 | 5.33 | 2.23 | 0.135 |
| SPGP8 | 0.85 | 1.03 | 0.64 | 0.21 | 0.72 | 0.28 | 0.75 | 0.30 | 0.314 |
| SPGP9 | 2.87 | 0.85 | 3.00 | 1.22 | 3.04 | 1.43 | 2.73 | 0.72 | 0.851 |
| SPGP10 | 3.03 | 0.66 | 2.95 | 0.64 | 3.37 | 1.52 | 2.99 | 0.61 | 0.386 |
| SPGP11 | 0.19 | 0.43 | 0.13 | 0.08 | 0.22 | 0.33 | 0.16 | 0.07 | 0.244 |
| SPGP12 | 7.18 | 1.70 | 7.68 | 2.36 | 7.31 | 2.62 | 6.87 | 1.60 | 0.670 |
| SPGP13 | 6.93 | 1.21 | 6.70 | 1.37 | 6.82 | 1.14 | 6.87 | 1.26 | 0.655 |
| SPGP14 * | 3.85 | 0.89 | 3.89 | 1.33 | 3.39 | 1.50 | 3.13 | 0.86 | <0.001 |
| SPGP15 | 11.36 | 7.46 | 13.84 | 9.19 | 11.99 | 7.79 | 12.11 | 6.81 | 0.499 |
| SPGP16 | 3.81 | 1.12 | 3.54 | 1.17 | 3.67 | 1.44 | 3.73 | 1.41 | 0.553 |
| SPGP17 | 2.78 | 1.47 | 3.12 | 1.30 | 3.19 | 1.47 | 2.27 | 0.78 | 0.032 |
| SPGP18 | 9.73 | 3.56 | 8.58 | 3.72 | 9.46 | 3.37 | 10.55 | 4.55 | 0.348 |
| SPGP19 | 5.40 | 2.41 | 5.04 | 2.38 | 5.09 | 1.98 | 5.84 | 1.88 | 0.387 |
| SPGP20 | 7.46 | 4.39 | 6.21 | 3.76 | 6.62 | 3.53 | 8.01 | 4.00 | 0.442 |
| SPGP21 | 1.16 | 0.59 | 1.16 | 0.45 | 1.19 | 0.73 | 1.07 | 0.44 | 0.801 |
| SPGP22 | 2.15 | 1.21 | 2.25 | 1.60 | 2.25 | 1.57 | 2.06 | 0.94 | 0.950 |
| SPGP23 | 3.65 | 1.91 | 4.13 | 2.22 | 3.01 | 1.59 | 3.17 | 1.93 | 0.071 |
| SPGP24 | 2.11 | 1.86 | 2.21 | 1.74 | 2.20 | 1.52 | 2.14 | 2.01 | 0.770 |
| SPGP25 | 1.43 | 0.96 | 1.39 | 0.84 | 1.31 | 0.91 | 1.23 | 0.75 | 0.632 |
| SPGP26 | 1.19 | 0.77 | 1.51 | 0.76 | 1.70 | 1.66 | 1.09 | 0.54 | 0.012 |
| SPGP27 | 1.38 | 0.61 | 1.55 | 0.70 | 1.55 | 0.64 | 1.65 | 0.60 | 0.086 |
| SPGP28 | 1.74 | 0.71 | 1.58 | 0.71 | 1.98 | 0.93 | 1.86 | 0.90 | 0.478 |
| SPGP29 | 2.40 | 1.05 | 2.66 | 1.50 | 2.36 | 1.05 | 2.40 | 0.79 | 0.974 |
| SPGP30 | 1.12 | 0.83 | 1.01 | 0.49 | 0.97 | 0.51 | 1.00 | 0.63 | 0.609 |
| SPGP31 | 2.89 | 1.74 | 2.39 | 1.37 | 2.74 | 1.21 | 3.00 | 2.34 | 0.436 |
| SPGP32 | 1.23 | 0.52 | 1.38 | 0.69 | 1.07 | 0.46 | 1.09 | 0.72 | 0.035 |
| SPGP33 | 0.74 | 0.49 | 0.77 | 0.47 | 0.67 | 0.41 | 0.70 | 0.50 | 0.887 |
| SPGP34 | 0.77 | 0.37 | 0.82 | 0.41 | 1.02 | 0.64 | 0.77 | 0.51 | 0.420 |
| SPGP35 | 0.49 | 0.30 | 0.42 | 0.26 | 0.49 | 0.18 | 0.43 | 0.28 | 0.314 |
| SPGP36 | 0.85 | 0.72 | 0.84 | 0.45 | 0.88 | 0.70 | 0.74 | 0.44 | 0.748 |
| SPGP37 | 1.00 | 0.53 | 0.94 | 0.46 | 0.88 | 0.44 | 0.89 | 0.41 | 0.712 |
| Seminal Plasma Glycan Peak (SPGP) | Change (%) in the Mean Relative Area of N-Glycans | ||
|---|---|---|---|
| A | O | OA | |
| SPGP2 | −14% (0.079) | +8% (0.399) | −22% (0.008) |
| SPGP4 | −2% (0.818) | +11% (0.258) | −22% (0.003) |
| SPGP6 | −8% (0.406) | −18% (0.105) | −30% (0.002) |
| SPGP14 * | −5% (0.409) | −19% (0.004) | −26% (<0.001) |
| SPGP18 | −7% (0.433) | +3% (0.769) | +29% (0.012) |
| SPGP26 | +33% (0.009) | +28% (0.043) | 0% (0.978) |
| SPGP35 | −25% (0.035) | +10% (0.524) | −27% (0.033) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maric, T.; Katusic Bojanac, A.; Matijevic, A.; Ceppi, M.; Bruzzone, M.; Evgeni, E.; Petrovic, T.; Wójcik, I.; Trbojevic-Akmacic, I.; Lauc, G.; et al. Seminal Plasma Protein N-Glycan Peaks Are Potential Predictors of Semen Pathology and Sperm Chromatin Maturity in Men. Life 2021, 11, 989. https://doi.org/10.3390/life11090989
Maric T, Katusic Bojanac A, Matijevic A, Ceppi M, Bruzzone M, Evgeni E, Petrovic T, Wójcik I, Trbojevic-Akmacic I, Lauc G, et al. Seminal Plasma Protein N-Glycan Peaks Are Potential Predictors of Semen Pathology and Sperm Chromatin Maturity in Men. Life. 2021; 11(9):989. https://doi.org/10.3390/life11090989
Chicago/Turabian StyleMaric, Tihana, Ana Katusic Bojanac, Ana Matijevic, Marcello Ceppi, Marco Bruzzone, Evangelini Evgeni, Tea Petrovic, Iwona Wójcik, Irena Trbojevic-Akmacic, Gordan Lauc, and et al. 2021. "Seminal Plasma Protein N-Glycan Peaks Are Potential Predictors of Semen Pathology and Sperm Chromatin Maturity in Men" Life 11, no. 9: 989. https://doi.org/10.3390/life11090989
APA StyleMaric, T., Katusic Bojanac, A., Matijevic, A., Ceppi, M., Bruzzone, M., Evgeni, E., Petrovic, T., Wójcik, I., Trbojevic-Akmacic, I., Lauc, G., Jezek, D., & Fucic, A. (2021). Seminal Plasma Protein N-Glycan Peaks Are Potential Predictors of Semen Pathology and Sperm Chromatin Maturity in Men. Life, 11(9), 989. https://doi.org/10.3390/life11090989







