Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Literature Search
2.2. Study Selection and Data Extraction
2.3. Statistical Analyses
3. Results
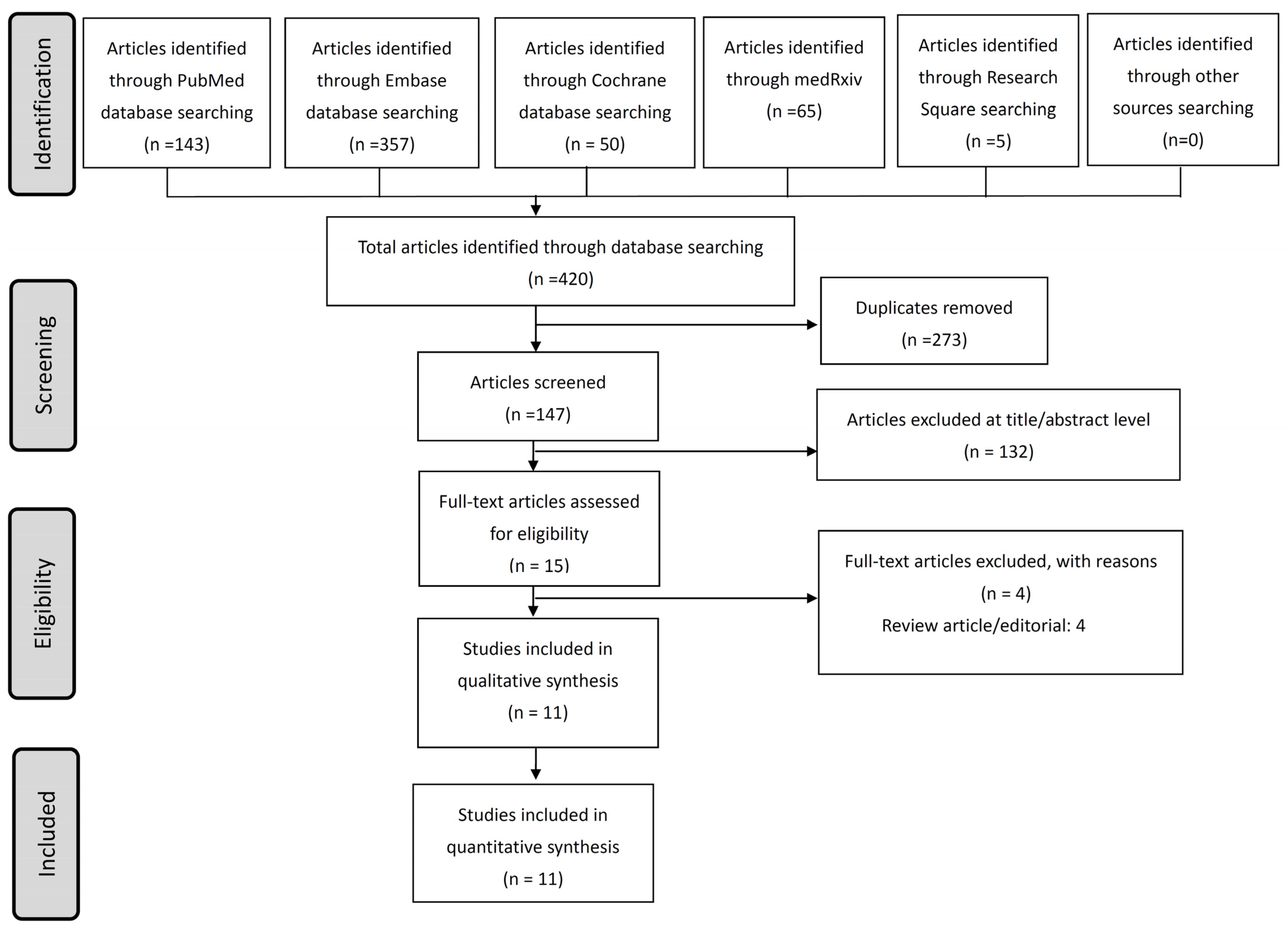
3.1. Enrolled Studies and Demographic Characteristics
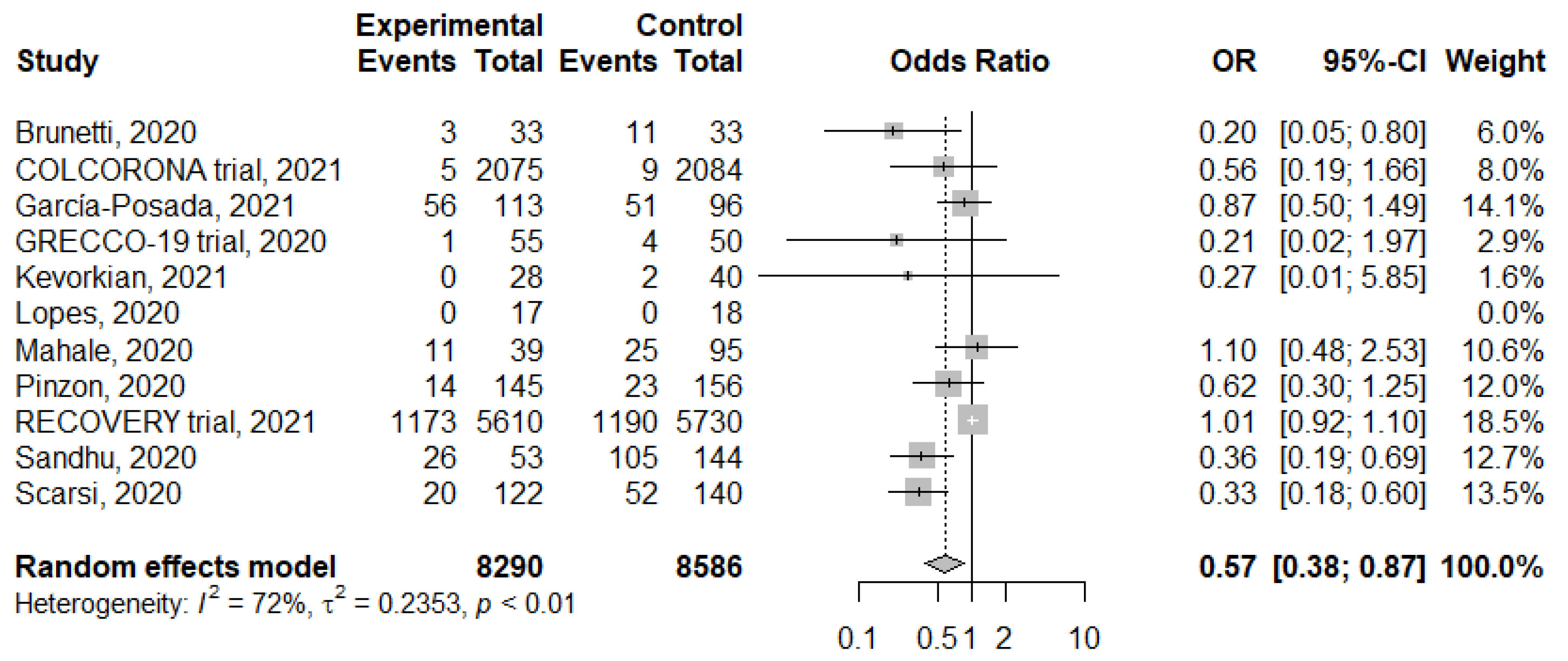
3.2. Meta-Analysis of Colchicine Treatment on Mortality
3.3. Meta-Analysis of Secondary Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Our World in Data. Mortality Risk of COVID-19. Available online: https://ourworldindata.org/mortality-risk-COVID (accessed on 1 June 2021).
- Johns Hopkins University & Medicine. Mortality Analyses. Available online: https://coronavirus.jhu.edu/data/mortality (accessed on 1 June 2021).
- Chen, C.C.; Tseng, C.Y.; Choi, W.M.; Lee, Y.C.; Su, T.H.; Hsieh, C.Y.; Chang, C.M.; Weng, S.L.; Liu, P.H.; Tai, Y.L.; et al. Taiwan government-guided strategies contributed to combating and controlling COVID-19 pandemic. Front. Public Health 2020, 8, 547423. [Google Scholar] [CrossRef]
- Yuki, K.; Fujiogi, M.; Koutsogiannaki, S. COVID-19 pathophysiology: A review. Clin. Immunol. 2020, 215, 108427. [Google Scholar] [CrossRef]
- Fajgenbaum, D.C.; June, C.H. Cytokine storm. N. Engl. J. Med. 2020, 383, 2255–2273. [Google Scholar] [CrossRef] [PubMed]
- Rabaan, A.A.; Al-Ahmed, S.H.; Garout, M.A.; Al-Qaaneh, A.M.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Hasan, A.; Dhawan, M.; et al. Diverse immunological factors influencing pathogenesis in patients with COVID-19: A review on viral dissemination, immunotherapeutic options to counter cytokine storm and inflammatory responses. Pathogens 2021, 10, 565. [Google Scholar] [CrossRef] [PubMed]
- Siemieniuk, R.A.; Bartoszko, J.J.; Ge, L.; Zeraatkar, D.; Izcovich, A.; Kum, E.; Pardo-Hernandez, H.; Rochwerg, B.; Lamontagne, F.; Han, M.A.; et al. Drug treatments for COVID-19: Living systematic review and network meta-analysis. BMJ 2020, 370, m2980. [Google Scholar] [CrossRef]
- Beigel, J.H.; Tomashek, K.M.; Dodd, L.E.; Mehta, A.K.; Zingman, B.S.; Kalil, A.C.; Hohmann, E.; Chu, H.Y.; Luetkemeyer, A.; Kline, S.; et al. Remdesivir for the treatment of COVID-19—Final report. N. Engl. J. Med. 2020, 383, 1813–1826. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.C.; Murthy, S.; Diaz, J.V.; Slutsky, A.S.; Villar, J.; Angus, D.C.; Annane, D.; Azevedo, L.C.P.; Berwanger, O.; Cavalcanti, A.B.; et al. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA 2020, 324, 1330–1341. [Google Scholar]
- Dalbeth, N.; Lauterio, T.J.; Wolfe, H.R. Mechanism of action of colchicine in the treatment of gout. Clin. Ther. 2014, 36, 1465–1479. [Google Scholar] [CrossRef] [Green Version]
- Leung, Y.Y.; Yao Hui, L.L.; Kraus, V.B. Colchicine—Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015, 45, 341–350. [Google Scholar] [CrossRef] [Green Version]
- Stewart, S.; Yang, K.C.K.; Atkins, K.; Dalbeth, N.; Robinson, P.C. Adverse events during oral colchicine use: A systematic review and meta-analysis of randomised controlled trials. Arthritis Res. Ther. 2020, 22, 28. [Google Scholar] [CrossRef] [Green Version]
- Schlesinger, N.; Firestein, B.L.; Brunetti, L. Colchicine in COVID-19: An old drug, new use. Curr. Pharmacol. Rep. 2020, 6, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, J.; Sirois, M.G.; Rhéaume, E.; Nguyen, Q.T.; Clavet-Lanthier, M.-É.; Brand, G.; Mihalache-Avram, T.; Théberge-Julien, G.; Charpentier, D.; Rhainds, D.; et al. Colchicine reduces lung injury in experimental acute respiratory distress syndrome. PLoS ONE 2020, 15, e0242318. [Google Scholar] [CrossRef] [PubMed]
- Hariyanto, T.I.; Halim, D.A.; Jodhinata, C.; Yanto, T.A.; Kurniawan, A. Colchicine treatment can improve outcomes of coronavirus disease 2019 (COVID-19): A systematic review and meta-analysis. Clin. Exp. Pharm. Physiol. 2021, 48, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Horby, P.W.; Campbell, M.; Spata, E.; Emberson, J.R.; Staplin, N.; Pessoa-Amorim, G.; Peto, L.; Wiselka, M.; Wiffen, L.; Tiberi, S.; et al. Colchicine in patients admitted to hospital with COVID-19 (RECOVERY): A randomised, controlled, open-label, platform trial. medRxiv 2021. [Google Scholar] [CrossRef]
- Tardif, J.C.; Bouabdallaoui, N.; L’Allier, P.L.; Gaudet, D.; Shah, B.; Pillinger, M.H.; Lopez-Sendon, J.; da Luz, P.; Verret, L.; Audet, S.; et al. Colchicine for community-treated patients with COVID-19 (COLCORONA): A phase 3, randomised, double-blinded, adaptive, placebo-controlled, multicentre trial. Lancet Respir. Med. 2021, 9, 924–932. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The prisma 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Chi, H.; Chiu, N.C.; Peng, C.C.; Lin, C.H.; Tai, Y.L.; Lee, M.D.; Cheng, Y.J.; Tan, B.F.; Lin, C.Y. One-seventh of patients with COVID-19 had olfactory and gustatory abnormalities as their initial symptoms: A systematic review and meta-analysis. Life 2020, 10, 158. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews Ofinterventions Version 5.1.0 [updated march 2011]. Available online: https://handbook-5-1.cochrane.org/ (accessed on 1 June 2021).
- Wells, G.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (nos) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 1 June 2021).
- Higgins, J.P.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. BMJ 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- Egger, M.; Smith, G.D.; Schneider, M.; Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997, 315, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Brunetti, L.; Diawara, O.; Tsai, A.; Firestein, B.L.; Nahass, R.G.; Poiani, G.; Schlesinger, N. Colchicine to weather the cytokine storm in hospitalized patients with COVID-19. J. Clin. Med. 2020, 9, 2961. [Google Scholar] [CrossRef] [PubMed]
- Deftereos, S.G.; Giannopoulos, G.; Vrachatis, D.A.; Siasos, G.D.; Giotaki, S.G.; Gargalianos, P.; Metallidis, S.; Sianos, G.; Baltagiannis, S.; Panagopoulos, P.; et al. Effect of colchicine vs standard care on cardiac and inflammatory biomarkers and clinical outcomes in patients hospitalized with coronavirus disease 2019: The grecco-19 randomized clinical trial. JAMA Netw. Open 2020, 3, e2013136. [Google Scholar] [CrossRef]
- García-Posada, M.; Aruachan-Vesga, S.; Mestra, D.; Humánez, K.; Serrano-Coll, H.; Cabrales, H.; Faccini, Á.; Mattar, S. Clinical outcomes of patients hospitalized for COVID-19 and evidence-based on the pharmacological management reduce mortality in a region of the colombian caribbean. J. Infect. Public Health 2021, 14, 696–701. [Google Scholar] [CrossRef]
- Kevorkian, J.P.; Lopes, A.; Sène, D.; Riveline, J.P.; Vandiedonck, C.; Féron, F.; Nassarmadji, K.; Mouly, S.; Mauvais-Jarvis, F.; Gautier, J.F.; et al. Oral corticoid, aspirin, anticoagulant, colchicine, and furosemide to improve the outcome of hospitalized COVID-19 patients—The cocaa-cola cohort study. J. Infect. 2021, 82, 276–316. [Google Scholar] [CrossRef] [PubMed]
- Lopes, M.I.; Bonjorno, L.P.; Giannini, M.C.; Amaral, N.B.; Menezes, P.I.; Dib, S.M.; Gigante, S.L.; Benatti, M.N.; Rezek, U.C.; Emrich-Filho, L.L.; et al. Beneficial effects of colchicine for moderate to severe COVID-19: A randomised, double-blinded, placebo-controlled clinical trial. RMD Open 2021, 7, e001455. [Google Scholar] [CrossRef]
- Mahale, N.; Rajhans, P.; Godavarthy, P.; Narasimhan, V.L.; Oak, G.; Marreddy, S.; Bedekar, A.; Dhundi, U.; Pawar, H.S.; Akole, P.; et al. A retrospective observational study of hypoxic COVID-19 patients treated with immunomodulatory drugs in a tertiary care hospital. Indian J. Crit. Care Med. 2020, 24, 1020–1027. [Google Scholar] [PubMed]
- Pinzón, M.A.; Arango, D.C.; Betancur, J.F.; Holguín, H.; Arias, C.A.; Muñoz, B.J.; Amarillo, M.; Llano, J.F.; Montoya, P. Clinical outcome of patients with COVID-19 pneumonia treated with corticosteroids and colchicine in colombia. ResearchSquare 2020. [Google Scholar] [CrossRef]
- Sandhu, T.; Tieng, A.; Chilimuri, S.; Franchin, G. A case control study to evaluate the impact of colchicine on patients admitted to the hospital with moderate to severe COVID-19 infection. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 8865954. [Google Scholar] [CrossRef]
- Scarsi, M.; Piantoni, S.; Colombo, E.; Airó, P.; Richini, D.; Miclini, M.; Bertasi, V.; Bianchi, M.; Bottone, D.; Civelli, P.; et al. Association between treatment with colchicine and improved survival in a single-centre cohort of adult hospitalised patients with COVID-19 pneumonia and acute respiratory distress syndrome. Ann. Rheum. Dis. 2020, 79, 1286–1289. [Google Scholar] [CrossRef]
- Chi, H.; Chiu, N.C.; Tai, Y.L.; Chang, H.Y.; Lin, C.H.; Sung, Y.H.; Tseng, C.Y.; Liu, L.Y.M.; Lin, C.Y. Clinical features of neonates born to mothers with coronavirus disease-2019: A ssytematic review of 105 neonates. J. Microbiol. Immunol. Infect. 2021, 54, 69–76. [Google Scholar] [CrossRef]
- de Rivero Vaccari, J.C.; Dietrich, W.D.; Keane, R.W.; de Rivero Vaccari, J.P. The inflammasome in times of COVID-19. Front. Immunol. 2020, 11, 2474. [Google Scholar] [CrossRef]
- Kowalewski, M.; Fina, D.; Słomka, A.; Raffa, G.M.; Martucci, G.; Lo Coco, V.; De Piero, M.E.; Ranucci, M.; Suwalski, P.; Lorusso, R. Covid-19 and ecmo: The interplay between coagulation and inflammation—A narrative review. Crit. Care 2020, 24, 205. [Google Scholar] [CrossRef]
- Freeman, T.L.; Swartz, T.H. Targeting the nlrp3 inflammasome in severe COVID-19. Front. Immunol. 2020, 11, 1518. [Google Scholar] [CrossRef]
- Shah, A. Novel coronavirus-induced nlrp3 inflammasome activation: A potential drug target in the treatment of COVID-19. Front. Immunol. 2020, 11, 1021. [Google Scholar] [CrossRef] [PubMed]
- van den Berg, D.F.; te Velde, A.A. Severe COVID-19: Nlrp3 inflammasome dysregulated. Front. Immunol. 2020, 11, 1580. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.J.; Robertson, S.; Barraclough, J.; Xia, Q.; Mallat, Z.; Bursill, C.; Celermajer, D.S.; Patel, S. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J. Am. Heart Assoc. 2015, 4, e002128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The nlrp3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Martínez, G.J.; Celermajer, D.S.; Patel, S. The nlrp3 inflammasome and the emerging role of colchicine to inhibit atherosclerosis-associated inflammation. Atherosclerosis 2018, 269, 262–271. [Google Scholar] [CrossRef] [PubMed]




| Study, Year [Ref] | Country | Participants, N | Male, N (%) | Median Age (Years Old) | Severity | Mortality (Overall/Colchicine/Control, %) | Dose/Duration | Study Design | Concomitant Medication | Primary Outcomes | Secondary Outcomes | Author Conclusion * |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Brunetti, 2020 [24] | USA | 66 | 43 (65) | 61.7 | Hospitalized | 21.2/9.1/33.3 | 1.2 mg followed by 0.6 mg 1 h later | Propensity-matched study | HCQ, AZI, tocilizumab, REM | 28-day mortality | Clinical improvement, oxygen weaning, discharge | Y |
| COLCORONA trial, 2021 [17] | Brazil, Canada, Greece, South Africa, Spain, and the USA | 4488 | 2069 (46.1) | 53 | Non-hospitalized | 0.3/0.2/0.4 | 0.5 mg twice per day for 3 days and then once per day for 27 days thereafter | Phase 3 randomized, double-blinded trial | HCQ, anticoagulant, aspirin, other platelet agents | Mortality or hospital admission for COVID-19 30 days after randomization | Mechanical ventilation, pneumonias, adverse events | N (for all cases); Y (for PCR-confirmed cases) |
| García-Posada, 2021 [26] | Colombia | 209 | 127 (61) | 60 | Hospitalized (100 in ICU) | 51.2/49.6/53.1 | 20 days if no intolerance or hypersensitivity | Descriptive observational study | Antibiotics, low molecular weight heparin, corticosteroids, tocilizumab | Mortality | Clinical manifestations | Y |
| GRECCO-19 trial, 2020 [25] | Greece | 105 | 61 (58.1) | 63 | Hospitalized | 4.8/1.8/8 | 1.5 mg loading dose followed by 0.5 mg after 60 min and maintenance doses of 0.5 mg twice daily, 3 weeks | Prospective, open-label, randomized clinical trial | HCQ, AZI, Lopinavir or ritonavir, tocilizumab, anticoagulation | Maximum high-sensitivity cardiac troponin level; time for C-reactive protein increase and clinical deterioration | Mechanical ventilation; all-cause mortality; adverse events | Y (narrow margin of clinical significance) |
| Kevorkian, 2021 [27] | France | 68 | 53 (77.9) | 68 | Hospitalized | 2.9/0/5 | 1 mg followed by 0.5 mg 1 h later, then 0.5 mg q8 h for total 8 mg | Observational cohort study | Prednisolone, furosemide, salicylate, direct anti-Xa inhibitor | Oxygen use; mechanical ventilation; 28-day mortality | Adverse events | Y |
| Lopes, 2020 [28] | Brazil | 35 | 14 (40) | 48 | Hospitalized (moderate to severe cases) | 0/0/0 | 0.5 mg twice daily for 5 days, then 0.5 mg twice daily for 5 days | Randomized, double-blinded, placebo-controlled clinical trial | HCQ, AZI, heparin, methylprednisolone | Oxygen use; time of hospitalization; intensive care unit; death rate; and causes of mortality | Laboratory tests; adverse events, etc. | Y |
| Mahale, 2020 [29] | India | 134 | 91 (68) | 55.6 | Hospitalized patients with oxygen therapy | 26.9/28.2/26.3 | 0.5 mg/day for 1 week | Retrospective observational study | HCQ, AZI, methylprednisolone, etoricoxib, tocilizumab, Abx | In-hospital mortality | Clinical manifestations | ND |
| Pinzón, 2020 [30] | Colombia | 301 | 178 (59.1) | 56.8 | Hospitalized for COVID-19 pneumonia | 12.3/9.7/14.7 | 0.5 mg every 12 h for 7 to 14 days | Observational study | HCQ, AZI, corticosteroid, lopinavir/ritonavir, Abx | Mortality | Cormobidities, clinical manifestations | Y |
| RECOVERY trial, 2021 [16] | U.K. (Indonesia, Nepal) | 11,340 | 7908 (69.7) | 63.4 | Hospitalized | 20.8/20.9/20.8 | 1 mg followed by 0.5 mg 12 h later and then 0.5 mg twice for 10 days | Randomized, controlled, open-label trial | Dexamethasone, HCQ, AZI, lopinavir-ritonavir, tocilizumab, and convalescent plasma | 28-day all-cause mortality | Discharge; mechanical ventilation | N |
| Sandhu, 2020 [31] | USA | 197 | 114 (57.9) | 70 | Hospitalized (moderate to severe) | 66.5/49.1/72.9 | 0.6 mg twice a day for three days and then 0.6 mg once a day (total 12 days) | Prospective comparative cohort study (case control) | HCQ, steroid, enoxaparin, heparin, etc. | Mortality, mechanical ventilation | Inflammatory markers | Y |
| Scarsi, 2020 [32] | Italy | 262 | 167 (63.7) | 69.3 | Hospitalized, with pneumonia | 27.5/16.4/37.1 | 1 mg/day | Prospective cohort study | HCQ, dexamethasone, lopinavir/ritonavir | Mortality | Clinical manifestations | Y |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lien, C.-H.; Lee, M.-D.; Weng, S.-L.; Lin, C.-H.; Liu, L.Y.-M.; Tai, Y.-L.; Lei, W.-T.; Liu, J.-M.; Huang, Y.-N.; Chi, H.; et al. Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis. Life 2021, 11, 864. https://doi.org/10.3390/life11080864
Lien C-H, Lee M-D, Weng S-L, Lin C-H, Liu LY-M, Tai Y-L, Lei W-T, Liu J-M, Huang Y-N, Chi H, et al. Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis. Life. 2021; 11(8):864. https://doi.org/10.3390/life11080864
Chicago/Turabian StyleLien, Chi-Hone, Ming-Dar Lee, Shun-Long Weng, Chao-Hsu Lin, Lawrence Yu-Min Liu, Yu-Lin Tai, Wei-Te Lei, Jui-Ming Liu, Ya-Ning Huang, Hsin Chi, and et al. 2021. "Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis" Life 11, no. 8: 864. https://doi.org/10.3390/life11080864
APA StyleLien, C.-H., Lee, M.-D., Weng, S.-L., Lin, C.-H., Liu, L. Y.-M., Tai, Y.-L., Lei, W.-T., Liu, J.-M., Huang, Y.-N., Chi, H., Chiu, N.-C., & Lin, C.-Y. (2021). Repurposing Colchicine in Treating Patients with COVID-19: A Systematic Review and Meta-Analysis. Life, 11(8), 864. https://doi.org/10.3390/life11080864








