The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease
Abstract
:1. Introduction
2. Methods
- (1)
- The first one concerns the neuroanatomy of ENS. In detail, a historical background was provided from the recognition of an autonomous nervous network in the GI tract up to the employment of a vast number of classification methods based on different criteria (morphological, electrophysiological, biochemical, retrograde tracing, transcriptome analysis, etc.), leading to the identification of several cytotypes;
- (2)
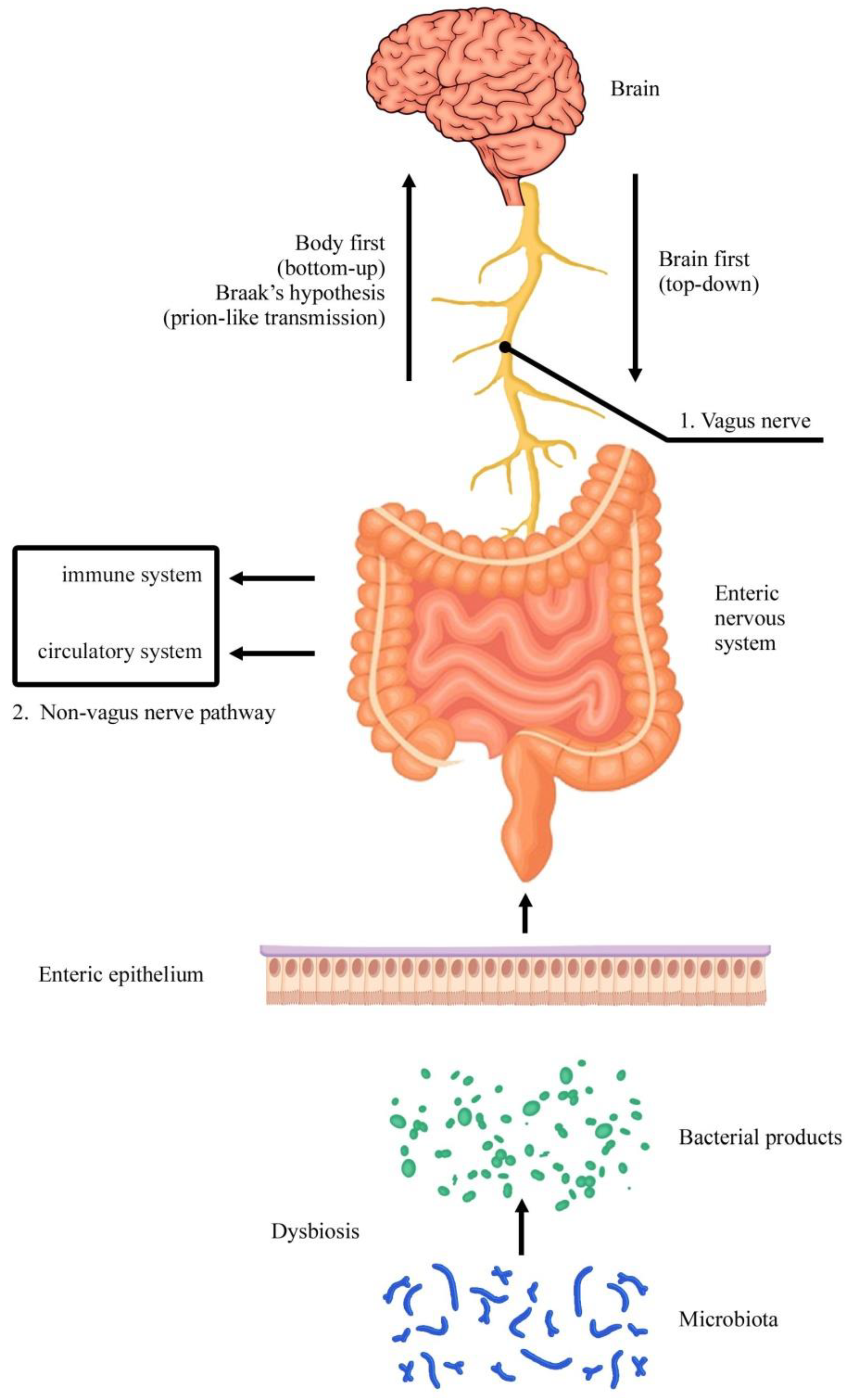
- The second part focuses the attention on the ENS cell population to examine which cytotypes are particularly involved in Parkinson’s disease. Original articles were chronologically examined. The involvement of the GI tract in this neurodegenerative pathology was reported in humans as well as in animals. In particular, different models of experimental Parkinsonism were examined and discussed. Vagal and extra-vagal mechanisms were described. The recent involvement of microbiota was considered.
3. Organization of the ENS
3.1. ENS Cell Types
- (1)
- Five subsets of Chat+Tac1+ putative excitatory motor neurons.
- (2)
- Seven subsets of Nos1+ putative inhibitory motor neurons (four subsets are Nos1+Vip+), which together coordinate muscle contraction and relaxation.
- (3)
- Four subsets of calcitonin gene-related peptide (CGRP+) producing putative sensory neurons, which sense and respond to chemical and mechanical stimuli. These neurons can be largely distinguished by the expression of distinct sensory and effector genes. For example, a subset expressed cholecystokinin (Cck) and vasoactive intestinal polypeptide (Vip), markers of intestinofugal neurons, or brain-derived neurotrophic factor (Bdnf), which is increased in patients affected by irritable bowel syndrome, or Piezo2, a mechanosensitive ion channel involved in the regulation of smooth muscle tone. A second subset was strongly enriched for somatostatin (Sst). Another subset expressed Noggin (Nog), a bone morphogenetic protein antagonist important to maintain the intestinal stem cell niche, and Neuromedin U (Nmu), which activates innate lymphoid cells.
- (4)
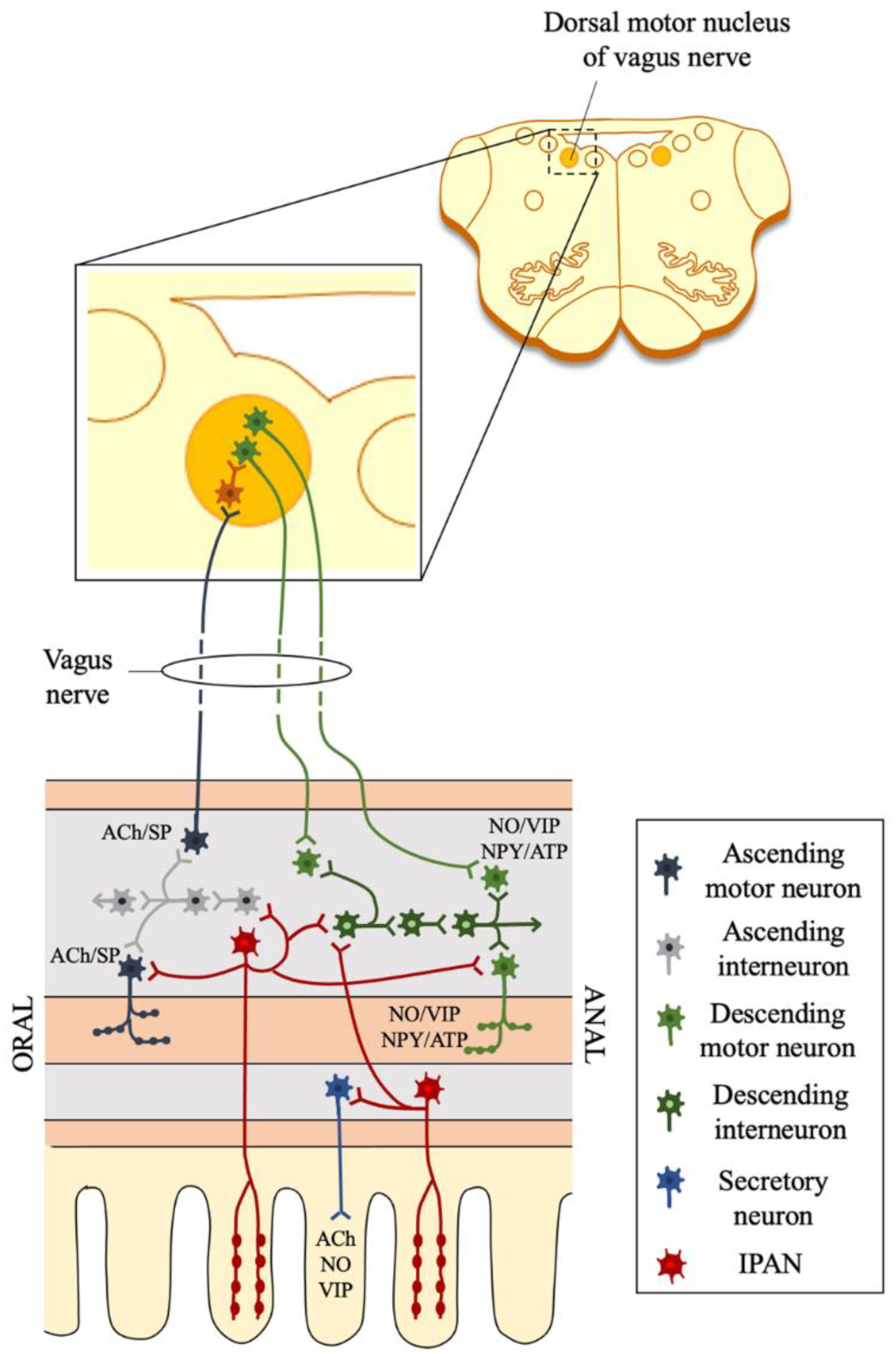
- Three subsets of Penk+ putative interneurons, which relay signals between neurons. Furness [36] listed six subtypes of interneurons: (a) descending interneurons that signal via ACh, serotonin, and ATP; (b) descending Nos1+Vip+Grp+Chat- interneurons; (c) descending Vip+Chat+Nos1+ interneurons with ATP signaling; (d) descending Chat+Sst+ interneurons; (e) descending Penk+ interneurons (responsive to Sst); (f) ascending Chat+Penk+ interneurons with ATP signaling.
- (5)
- Two subsets of Glp2r+ putative secretomotor/vasodilator neurons, including Vip+ non-cholinergic and Chat+ cholinergic subsets, which trigger secretions and fluid movement in other cell types. The latter also expressed galanin (Gal), as in neurons that innervate the epithelium and arterioles, neuropeptide Y, as in secretomotor neurons, and glutamate decarboxylase 2 (Gad2), possibly forming cholinergic/GABAergic neurons [46].
3.2. ENS Circuits and Functions
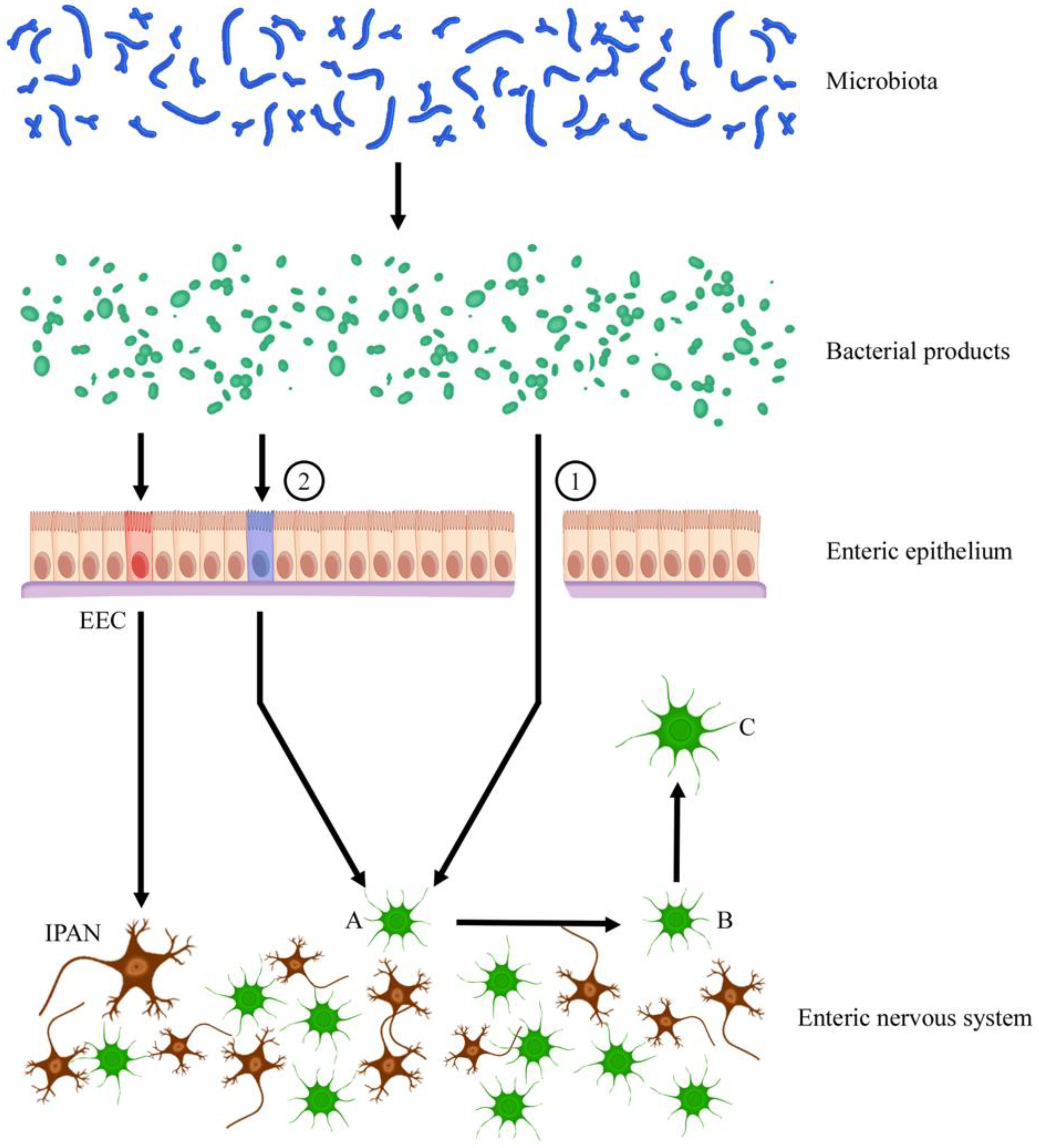
4. ENS and Parkinson’s Disease
5. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Furness, J.B. The Enteric Nervous System; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Sasselli, V.; Pachnis, V.; Burns, A.J. The enteric nervous system. Dev. Biol. 2012, 366, 64–73. [Google Scholar] [CrossRef] [Green Version]
- Natale, G.; Ryskalin, L.; Busceti, C.L.; Biagioni, F.; Fornai, F. The nature of catecholamine-containing neurons in the enteric nervous system in relationship with organogenesis, normal human anatomy and neurodegeneration. Arch. Ital. Biol. 2017, 155, 118–130. [Google Scholar]
- Gershon, M.D. The enteric nervous system: A second brain. Hosp. Pract. 1999, 34, 31–32, 35–38, 41–42 passim. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Schicho, R.; Holzer-Petsche, U.; Lippe, I.T. The gut as a neurological organ. Wien Klin. Wochenschr. 2001, 113, 647–660. [Google Scholar] [PubMed]
- Furness, J.B.; Stebbing, M.J. The first brain: Species comparisons and evolutionary implications for the enteric and central nervous systems. Neurogastroenterol. Motil. 2018, 30, e13234. [Google Scholar] [CrossRef]
- Endres, K.; Schäfer, K.H. Influence of Commensal Microbiota on the Enteric Nervous System and Its Role in Neurodegenerative Diseases. J. Innate Immun. 2018, 10, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; O’Riordan, K.J.; Cowan, C.S.M.; Sandhu, K.V.; Bastiaanssen, T.F.S.; Boehme, M.; Codagnone, M.G.; Cussotto, S.; Fulling, C.; Golubeva, A.V.; et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019, 99, 1877–2013. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Rüb, U.; Gai, W.P.; Del Tredici, K. Idiopathic Parkinson’s disease: Possible routes by which vulnerable neuronal types may be subject to neuroinvasion by an unknown pathogen. J. Neural. Transm. 2003, 110, 517–536. [Google Scholar] [CrossRef]
- Natale, G.; Pasquali, L.; Ruggieri, S.; Paparelli, A.; Fornai, F. Parkinson’s disease and the gut: A well known clinical association in need of an effective cure and explanation. Neurogastroenterol. Motil. 2008, 20, 741–749. [Google Scholar] [CrossRef]
- Natale, G.; Kastsiushenka, O.; Fulceri, F.; Ruggieri, S.; Paparelli, A.; Fornai, F. MPTP-induced parkinsonism extends to a subclass of TH-positive neurons in the gut. Brain Res. 2010, 1355, 195–206. [Google Scholar] [CrossRef]
- Natale, G.; Pasquali, L.; Paparelli, A.; Fornai, F. Parallel manifestations of neuropathologies in the enteric and central nervous systems. Neurogastroenterol. Motil. 2011, 23, 1056–1065. [Google Scholar] [CrossRef]
- Schaeffer, E.; Kluge, A.; Böttner, M.; Zunke, F.; Cossais, F.; Berg, D.; Arnold, P. Alpha Synuclein Connects the Gut-Brain Axis in Parkinson’s Disease Patients—A View on Clinical Aspects, Cellular Pathology and Analytical Methodology. Front. Cell Dev. Biol. 2020, 8, 573696. [Google Scholar] [CrossRef] [PubMed]
- Travagli, R.A.; Browning, K.N.; Camilleri, M. Parkinson disease and the gut: New insights into pathogenesis and clinical relevance. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 673–685. [Google Scholar] [CrossRef]
- Natale, G.; Ferrucci, M.; Lazzeri, G.; Paparelli, A.; Fornai, F. Transmission of prions within the gut and towards the central nervous system. Prion 2011, 5, 142–149. [Google Scholar] [CrossRef] [Green Version]
- Natale, G.; Pompili, E.; Biagioni, F.; Paparelli, S.; Lenzi, P.; Fornai, F. Histochemical approaches to assess cell-to-cell transmission of misfolded proteins in neurodegenerative diseases. Eur. J. Histochem. 2013, 57, e5. [Google Scholar] [CrossRef] [Green Version]
- O’Carroll, A.; Coyle, J.; Gambin, Y. Prions and Prion-like assemblies in neurodegeneration and immunity: The emergence of universal mechanisms across health and disease. Semin. Cell Dev. Biol. 2020, 99, 115–130. [Google Scholar] [CrossRef]
- Zheng, H.; Shi, C.; Luo, H.; Fan, L.; Yang, Z.; Hu, X.; Zhang, Z.; Zhang, S.; Hu, Z.; Fan, Y.; et al. α-Synuclein in Parkinson’s Disease: Does a Prion-Like Mechanism of Propagation from Periphery to the Brain Play a Role? Neuroscientist 2020, 27, 367–387. [Google Scholar] [CrossRef] [PubMed]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Challis, C.; Hori, A.; Sampson, T.R.; Yoo, B.B.; Challis, R.C.; Hamilton, A.M.; Mazmanian, S.K.; Volpicelli-Daley, L.A.; Gradinaru, V. Gut-seeded α-synuclein fibrils promote gut dysfunction and brain pathology specifically in aged mice. Nat. Neurosci. 2020, 23, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Menozzi, E.; Macnaughtan, J.; Schapira, A.H.V. The gut-brain axis and Parkinson disease: Clinical and pathogenetic relevance. Ann. Med. 2021, 53, 611–625. [Google Scholar] [CrossRef] [PubMed]
- Chiang, H.L.; Lin, C.H. Altered Gut Microbiome and Intestinal Pathology in Parkinson’s Disease. J. Mov. Disord. 2019, 12, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Arotcarena, M.L.; Dovero, S.; Prigent, A.; Bourdenx, M.; Camus, S.; Porras, G.; Thiolat, M.L.; Tasselli, M.; Aubert, P.; Kruse, N.; et al. Bidirectional gut-to-brain and brain-to-gut propagation of synucleinopathy in non-human primates. Brain 2020, 143, 1462–1475. [Google Scholar] [CrossRef]
- Horsager, J.; Andersen, K.B.; Knudsen, K.; Skjærbæk, C.; Fedorova, T.D.; Okkels, N.; Schaeffer, E.; Bonkat, S.K.; Geday, J.; Otto, M.; et al. Brain-first versus body-first Parkinson’s disease: A multimodal imaging case-control study. Brain 2020, 143. [Google Scholar] [CrossRef] [PubMed]
- Leclair-Visonneau, L.; Neunlist, M.; Derkinderen, P.; Lebouvier, T. The gut in Parkinson’s disease: Bottom-up, top-down, or neither? Neurogastroenterol. Motil. 2020, 32, e13777. [Google Scholar] [CrossRef] [PubMed]
- O’Donovan, S.M.; Crowley, E.K.; Brown, J.R.; O’Sullivan, O.; O’Leary, O.F.; Timmons, S.; Nolan, Y.M.; Clarke, D.J.; Hyland, N.P.; Joyce, S.A.; et al. Nigral overexpression of α-synuclein in a rat Parkinson’s disease model indicates alterations in the enteric nervous system and the gut microbiome. Neurogastroenterol. Motil. 2020, 32, e13726. [Google Scholar] [CrossRef]
- Chen, Z.; Li, G.; Liu, J. Autonomic dysfunction in Parkinson’s disease: Implications for pathophysiology, diagnosis, and treatment. Neurobiol. Dis. 2020, 134, 104700. [Google Scholar] [CrossRef]
- Langley, J.N. The Autonomic Nervous System; W. Heffer & Sons: Cambridge, UK, 1921. [Google Scholar]
- Burnstock, G.; Campbell, G.; Bennett, M.R.; Holman, M.E. Innervation of the guinea-pig taenia coli: Are there intrinsic nerves which are distinct from sympathetic nerves? Int. J. Neuropharmacol. 1964, 3, 163–166. [Google Scholar] [CrossRef]
- Brierley, S.; Costa, M. (Eds.) The Enteric Nervous System: 30 Years Later. In Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2016; Volume 891. [Google Scholar]
- Huang, Z.; Liao, L.; Wang, Z.; Lu, Y.; Yan, W.; Cao, H.; Tan, B. An efficient approach for wholemount preparation of the myenteric plexus of rat colon. J. Neurosci. Methods 2021, 348, 109012. [Google Scholar] [CrossRef]
- Furness, J.B.; Costa, M. The Enteric Nervous System; Churchill Livingstone: Edinburgh, UK, 1987. [Google Scholar]
- Brehmer, A. Structure of enteric neurons. Adv. Anat. Embryol. Cell Biol. 2006, 186, 1–91. [Google Scholar]
- Sukhanov, A.V.; Vakulin, G.M. Modification of the Bilshovsky method for studies of nerve tissue paraffin sections. Klin. Lab. Diagn. 2002, 2, 42–43. [Google Scholar]
- Gershon, M.D.; Kirchgessner, A.L.; Wade, P.R. Functional anatomy of the enteric nervous system. In Physiology of the Gastrointestinal Tract; Johnson, L.R., Alpers, D.H., Christensen, J., Jacobson, E.D., Walsh, J.H., Eds.; Raven Press: New York, NY, USA, 1994; pp. 381–422. [Google Scholar]
- Furness, J.B. The enteric nervous system and neurogastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2012, 9, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B. Types of neurons in the enteric nervous system. J. Auton. Nerv. Syst. 2000, 81, 87–96. [Google Scholar] [CrossRef]
- Benarroch, E.E. Enteric nervous system: Functional organization and neurologic implications. Neurology 2007, 69, 1953–1957. [Google Scholar] [CrossRef] [PubMed]
- Fung, C.; Vanden Berghe, P. Functional circuits and signal processing in the enteric nervous system. Cell. Mol. Life Sci. 2020, 77, 4505–4522. [Google Scholar] [CrossRef] [PubMed]
- May-Zhang, A.A.; Tycksen, E.; Southard-Smith, A.N.; Deal, K.K.; Benthal, J.T.; Buehler, D.P.; Adam, M.; Simmons, A.J.; Monaghan, J.R.; Matlock, B.K.; et al. Combinatorial Transcriptional Profiling of Mouse and Human Enteric Neurons Identifies Shared and Disparate Subtypes In Situ. Gastroenterology 2021, 160, 755–770.e26. [Google Scholar] [CrossRef]
- Spencer, N.J.; Smith, T.K. Mechanosensory S-neurons rather than AH-neurons appear to generate a rhythmic motor pattern in guinea-pig distal colon. J. Physiol. 2004, 558 Pt 2, 577–596. [Google Scholar] [CrossRef]
- Spencer, N.J.; Hu, H. Enteric nervous system: Sensory transduction, neural circuits and gastrointestinal motility. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 338–351. [Google Scholar] [CrossRef]
- Carbone, S.E.; Jovanovska, V.; Nurgali, K.; Brookes, S.J. Human enteric neurons: Morphological, electrophysiological, and neurochemical identification. Neurogastroenterol. Motil. 2014, 26, 1812–1816. [Google Scholar] [CrossRef] [Green Version]
- Bon-Frauches, A.C.; Boesmans, W. The enteric nervous system: The hub in a star network. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 717–718. [Google Scholar] [CrossRef]
- Wright, C.M.; Schneider, S.; Smith-Edwards, K.M.; Mafra, F.; Leembruggen, A.J.L.; Gonzalez, M.V.; Kothakapa, D.R.; Anderson, J.B.; Maguire, B.A.; Gao, T.; et al. scRNA-Seq Reveals New Enteric Nervous System Roles for GDNF, NRTN, and TBX3. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1548–1592.e1, S2352-345X(20)30213-7. [Google Scholar] [CrossRef]
- Drokhlyansky, E.; Smillie, C.S.; Van Wittenberghe, N.; Ericsson, M.; Griffin, G.K.; Eraslan, G.; Dionne, D.; Cuoco, M.S.; Goder-Reiser, M.N.; Sharova, T.; et al. The Human and Mouse Enteric Nervous System at Single-Cell Resolution. Cell 2020, 182, 1606–1622. [Google Scholar] [CrossRef] [PubMed]
- Morarach, K.; Mikhailova, A.; Knoflach, V.; Memic, F.; Kumar, R.; Li, W.; Ernfors, P.; Marklund, U. Diversification of molecularly defined myenteric neuron classes revealed by single-cell RNA sequencing. Nat. Neurosci. 2021, 24, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Humenick, A.; Chen, B.N.; Wattchow, D.A.; Zagorodnyuk, V.P.; Dinning, P.G.; Spencer, N.J.; Costa, M.; Brookes, S.J.H. Characterization of putative interneurons in the myenteric plexus of human colon. Neurogastroenterol. Motil. 2021, 33, e13964. [Google Scholar] [CrossRef] [PubMed]
- Neuhuber, W.; Wörl, J. Monoamines in the enteric nervous system. Histochem. Cell Biol. 2018, 150, 703–709. [Google Scholar] [CrossRef]
- Boesmans, W.; Lasrado, R.; Vanden Berghe, P.; Pachnis, V. Heterogeneity and phenotypic plasticity of glial cells in the mammalian enteric nervous system. Glia 2015, 63, 229–241. [Google Scholar] [CrossRef] [Green Version]
- Kabouridis, P.S.; Lasrado, R.; McCallum, S.; Chng, S.H.; Snippert, H.J.; Clevers, H.; Pettersson, S.; Pachnis, V. Microbiota controls the homeostasis of glial cells in the gut lamina propria. Neuron 2015, 85, 289–295. [Google Scholar] [CrossRef] [Green Version]
- Veress, B.; Ohlsson, B. Spatial relationship between telocytes, interstitial cells of Cajal and the enteric nervous system in the human ileum and colon. J. Cell. Mol. Med. 2020, 24, 3399–3406. [Google Scholar] [CrossRef] [Green Version]
- Popescu, L.M.; Faussone-Pellegrini, M.S. TELOCYTES—A case of serendipity: The winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J. Cell. Mol. Med. 2010, 14, 729–740. [Google Scholar] [CrossRef] [Green Version]
- Clairembault, T.; Kamphuis, W.; Leclair-Visonneau, L.; Rolli-Derkinderen, M.; Coron, E.; Neunlist, M.; Hol, E.M.; Derkinderen, P. Enteric GFAP expression and phosphorylation in Parkinson’s disease. J. Neurochem. 2014, 130, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Clairembault, T.; Leclair-Visonneau, L.; Neunlist, M.; Derkinderen, P. Enteric glial cells: New players in Parkinson’s disease? Mov. Disord. 2015, 30, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Ibiza, S.; García-Cassani, B.; Ribeiro, H.; Carvalho, T.; Almeida, L.; Marques, R.; Misic, A.M.; Bartow-McKenney, C.; Larson, D.M.; Pavan, W.J.; et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature 2016, 535, 440–443. [Google Scholar] [CrossRef] [Green Version]
- Saffrey, M.J. Cellular changes in the enteric nervous system during ageing. Dev. Biol. 2013, 382, 344–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, K.A.; Pfeiffer, R.F. Gastrointestinal dysfunction in the synucleinopathies. Clin. Auton. Res. 2021, 31, 77–99. [Google Scholar] [CrossRef]
- Qualman, S.J.; Haupt, H.M.; Yang, P.; Hamilton, S.R. Esophageal Lewy bodies associated with ganglion cell loss in achalasia. Similarity to Parkinson’s disease. Gastroenterology 1984, 87, 848–856. [Google Scholar] [CrossRef]
- Singaram, C.; Ashraf, W.; Gaumnitz, E.A.; Torbey, C.; Sengupta, A.; Pfeiffer, R.; Quigley, E.M. Dopaminergic defect of enteric nervous system in Parkinson’s disease patients with chronic constipation. Lancet 1995, 346, 861–864. [Google Scholar] [CrossRef]
- Wakabayashi, K.; Takahashi, H.; Ohama, E.; Ikuta, F. Parkinson’s disease: An immunohistochemical study of Lewy body-containing neurons in the enteric nervous system. Acta Neuropathol. 1990, 79, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Lebouvier, T.; Chaumette, T.; Damier, P.; Coron, E.; Touchefeu, Y.; Vrignaud, S.; Naveilhan, P.; Galmiche, J.P.; Bruley des Varannes, S.; Derkinderen, P.; et al. Pathological lesions in colonic biopsies during Parkinson’s disease. Gut 2008, 57, 1741–1743. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohlsson, B.; Englund, E. Atrophic Myenteric and Submucosal Neurons Are Observed in Parkinson’s Disease. Parkinsons Dis. 2019, 2019, 7935820. [Google Scholar] [CrossRef] [Green Version]
- Fenyi, A.; Duyckaerts, C.; Bousset, L.; Braak, H.; Del Tredici, K.; Melki, R.; On Behalf Of The Brainbank Neuro-Ceb Neuropathology Network. Seeding Propensity and Characteristics of Pathogenic αSyn Assemblies in Formalin-Fixed Human Tissue from the Enteric Nervous System, Olfactory Bulb, and Brainstem in Cases Staged for Parkinson’s Disease. Cells 2021, 10, 139. [Google Scholar] [CrossRef]
- Killinger, B.; Labrie, V. The Appendix in Parkinson’s Disease: From Vestigial Remnant to Vital Organ? J. Parkinsons Dis. 2019, 9, S345–S358. [Google Scholar] [CrossRef]
- Corbillé, A.G.; Coron, E.; Neunlist, M.; Derkinderen, P.; Lebouvier, T. Appraisal of the dopaminergic and noradrenergic innervation of the submucosal plexus in PD. J. Parkinsons Dis. 2014, 4, 571–576. [Google Scholar] [CrossRef] [PubMed]
- Annerino, D.M.; Arshad, S.; Taylor, G.M.; Adler, C.H.; Beach, T.G.; Greene, J.G. Parkinson’s disease is not associated with gastrointestinal myenteric ganglion neuron loss. Acta Neuropathol. 2012, 124, 665–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellegrini, C.; Antonioli, L.; Colucci, R.; Ballabeni, V.; Barocelli, E.; Bernardini, N.; Blandizzi, C.; Fornai, M. Gastric motor dysfunctions in Parkinson’s disease: Current pre-clinical evidence. Parkinsonism Relat. Disord. 2015, 21, 1407–1414. [Google Scholar] [CrossRef]
- Pellegrini, C.; Colucci, R.; Antonioli, L.; Barocelli, E.; Ballabeni, V.; Bernardini, N.; Blandizzi, C.; de Jonge, W.J.; Fornai, M. Intestinal dysfunction in Parkinson’s disease: Lessons learned from translational studies and experimental models. Neurogastroenterol. Motil. 2016, 28, 1781–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsanyiova, J.; Buday, T.; Kralova Trancikova, A. Parkinson’s Disease and the Gut: Future Perspectives for Early Diagnosis. Front. Neurosci. 2020, 14, 626. [Google Scholar] [CrossRef]
- Anderson, G.; Noorian, A.R.; Taylor, G.; Anitha, M.; Bernhard, D.; Srinivasan, S.; Greene, J.G. Loss of enteric dopaminergic neurons and associated changes in colon motility in an MPTP mouse model of Parkinson’s disease. Exp. Neurol. 2007, 207, 4–12. [Google Scholar] [CrossRef] [Green Version]
- Drolet, R.E.; Cannon, J.R.; Montero, L.; Greenamyre, J.T. Chronic rotenone exposure reproduces Parkinson’s disease gastrointestinal neuropathology. Neurobiol. Dis. 2009, 36, 96–102. [Google Scholar] [CrossRef]
- Greene, J.G.; Noorian, A.R.; Srinivasan, S. Delayed gastric emptying and enteric nervous system dysfunction in the rotenone model of Parkinson’s disease. Exp. Neurol. 2009, 218, 154–161. [Google Scholar] [CrossRef] [Green Version]
- Johnson, M.E.; Stringer, A.; Bobrovskaya, L. Rotenone induces gastrointestinal pathology and microbiota alterations in a rat model of Parkinson’s disease. Neurotoxicology 2018, 65, 174–185. [Google Scholar] [CrossRef]
- Miyazaki, I.; Isooka, N.; Imafuku, F.; Sun, J.; Kikuoka, R.; Furukawa, C.; Asanuma, M. Chronic Systemic Exposure to Low-Dose Rotenone Induced Central and Peripheral Neuropathology and Motor Deficits in Mice: Reproducible Animal Model of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 3254. [Google Scholar] [CrossRef]
- Fornai, M.; Pellegrini, C.; Antonioli, L.; Segnani, C.; Ippolito, C.; Barocelli, E.; Ballabeni, V.; Vegezzi, G.; Al Harraq, Z.; Blandini, F.; et al. Enteric Dysfunctions in Experimental Parkinson’s Disease: Alterations of Excitatory Cholinergic Neurotransmission Regulating Colonic Motility in Rats. J. Pharmacol. Exp. Ther. 2016, 356, 434–444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Magen, I.; Yuan, P.Q.; Subramaniam, S.R.; Richter, F.; Chesselet, M.F.; Taché, Y. Mice overexpressing wild-type human alpha-synuclein display alterations in colonic myenteric ganglia and defecation. Neurogastroenterol. Motil. 2012, 24, e425–e436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, R.J.; Walter, G.C.; Ringer, B.E.; Higgs, K.M.; Powley, T.L. Alpha-synuclein immunopositive aggregates in the myenteric plexus of the aging Fischer 344 rat. Exp. Neurol. 2009, 220, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Mulak, A.; Bonaz, B. Brain-gut-microbiota axis in Parkinson’s disease. World J. Gastroenterol. 2015, 21, 10609–10620. [Google Scholar] [CrossRef]
- Rajput, C.; Sarkar, A.; Sachan, N.; Rawat, N.; Singh, M.P. Is Gut Dysbiosis an Epicenter of Parkinson’s Disease? Neurochem. Res. 2021, 46, 425–438. [Google Scholar] [CrossRef]
- Benvenuti, L.; D’Antongiovanni, V.; Pellegrini, C.; Antonioli, L.; Bernardini, N.; Blandizzi, C.; Fornai, M. Enteric Glia at the Crossroads between Intestinal Immune System and Epithelial Barrier: Implications for Parkinson Disease. Int. J. Mol. Sci. 2020, 21, 9199. [Google Scholar] [CrossRef] [PubMed]
- Natale, G.; Biagioni, F.; Busceti, C.L.; Gambardella, S.; Limanaqi, F.; Fornai, F. TREM Receptors Connecting Bowel Inflammation to Neurodegenerative Disorders. Cells 2019, 8, 1124. [Google Scholar] [CrossRef] [Green Version]
- Caputi, V.; Giron, M.C. Microbiome-Gut-Brain Axis and Toll-Like Receptors in Parkinson’s Disease. Int. J. Mol. Sci. 2018, 19, 1689. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.C.; Marcobal, A.; Ursell, L.K.; Larauche, M.; Duboc, H.; Earle, K.A.; Sonnenburg, E.D.; Ferreyra, J.A.; Higginbottom, S.K.; Million, M.; et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013, 144, 967–977. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.K.; Verma, S.; Jain, V.; Surapaneni, B.K.; Vinayek, R.; Phillips, L.; Nair, P.P. Parkinson’s Disease: The Emerging Role of Gut Dysbiosis, Antibiotics, Probiotics, and Fecal Microbiota Transplantation. J. Neurogastroenterol. Motil. 2019, 25, 363–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fleming, M.A., 2nd; Ehsan, L.; Moore, S.R.; Levin, D.E. The Enteric Nervous System and Its Emerging Role as a Therapeutic Target. Gastroenterol. Res. Pract. 2020, 2020, 8024171. [Google Scholar] [CrossRef] [PubMed]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Authors (Year) | GI Tract | Species | Parkinson’s Disease Model |
|---|---|---|---|
| Braak et al., 2003 [9] | Distal esophagus, stomach | Human | - |
| Natale et al., 2010 [11] | Duodenum | Mouse | MPTP |
| Clairembault et al., 2015 [55] | Descending colon, sigmoid | Human | - |
| Chung et al., 2021 [58] | Stomach, small intestine | Human | - |
| Lebouvier et al., 2008 [62] | Colon | Human | - |
| Ohlsson et al., 2019 [63] | Colon | Human | - |
| Fenyi et al., 2021 [64] | Stomach | Human | - |
| Killinger et al., 2019 [65] | Appendix | Human | - |
| Annerino et al., 2012 [67] | Stomach, duodenum, ileum, colon | Human | - |
| Anderson et al., 2007 [71] | Colon | Mouse | MPTP |
| Drolet et al., 2009 [72] | Small intestine | Rat | Rotenone |
| Miyazaki et al., 2020 [75] | Intestine | Mouse | Rotenone |
| Fornai et al., 2016 [76] | Colon | Rat | 6-OHDA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Natale, G.; Ryskalin, L.; Morucci, G.; Lazzeri, G.; Frati, A.; Fornai, F. The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease. Life 2021, 11, 732. https://doi.org/10.3390/life11080732
Natale G, Ryskalin L, Morucci G, Lazzeri G, Frati A, Fornai F. The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease. Life. 2021; 11(8):732. https://doi.org/10.3390/life11080732
Chicago/Turabian StyleNatale, Gianfranco, Larisa Ryskalin, Gabriele Morucci, Gloria Lazzeri, Alessandro Frati, and Francesco Fornai. 2021. "The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease" Life 11, no. 8: 732. https://doi.org/10.3390/life11080732
APA StyleNatale, G., Ryskalin, L., Morucci, G., Lazzeri, G., Frati, A., & Fornai, F. (2021). The Baseline Structure of the Enteric Nervous System and Its Role in Parkinson’s Disease. Life, 11(8), 732. https://doi.org/10.3390/life11080732








