TCA Cycle Replenishing Pathways in Photosynthetic Purple Non-Sulfur Bacteria Growing with Acetate
Abstract
1. Introduction
2. Part 1: TCA Cycle Replenishing Pathways
3. Part 2: Integration of TCA Cycle Replenishing Pathways. Separation of Them on the Basis of Main Metabolites
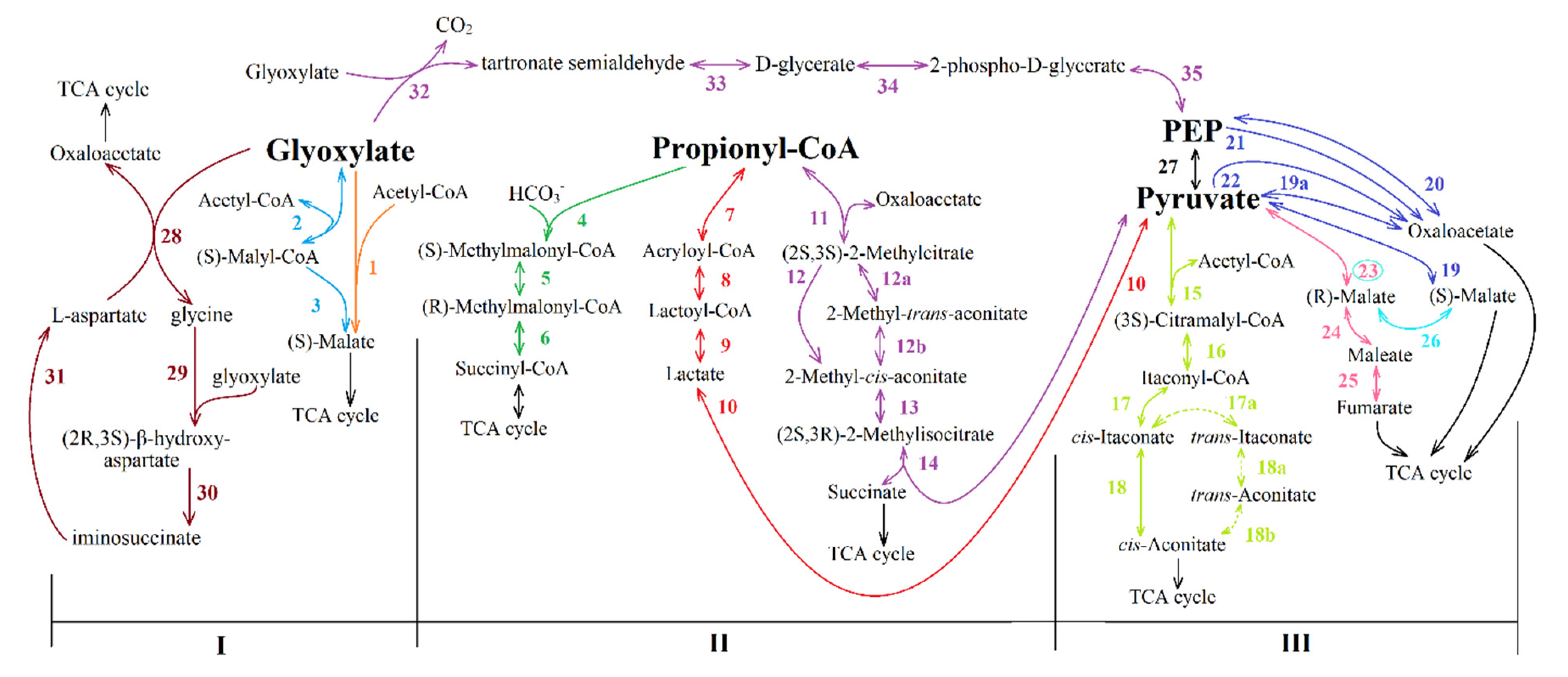
3.1. Four Groups of Pathways
- I.
- Pathways of glyoxylate conversion: the conversion into malate (by two malate synthases different in stability and activators (EC: 2.3.3.9); or malyl-CoA/(S)-citramalyl-CoA-lyase (EC: 4.1.3.24/4.1.3.25) and (3S)-malyl-CoA-thioesterase (EC: 3.1.2.30)); the glycerate pathway for PEP formation from glyoxylate; and the pathways of glyoxylate conversion into OAA through the β-hydroxyaspartate cycle.
- II.
- Pathways of propionyl-CoA conversion into succinyl-CoA or PA: the methylmalonyl-CoA pathway; the methylcitrate pathway; and the pathway of propionyl-CoA oxidation to PA via lactate.
- III.
- Pathways of PA/PEP conversion into TCA cycle intermediates: PA/PEP carboxylating enzymes; the pathway of fumarate or (S)-malate formation from PA; and cis-aconitate formation from PA and acetyl-CoA.
3.1.1. Enzymes Participating in Group I Reactions
3.1.2. Enzymes Participating in Group II Reactions
3.1.3. Enzymes Participating in Group III Reactions
3.1.4. Enzymes Participating in Group IV Reactions
 CO2 + H2. The ‘forward’ reaction (CO2 and H2 production from formate) under physiological fermentative conditions is observed [123,124]. The expression of active formate hydrogenlyase is repressed at a low formate concentration accompanied by a relatively high pH. However, theoretical arguments and experimental data indicate that this enzyme exists under certain conditions [125,126]. An evolutionary progenitor of formate hydrogenlyase on the early Earth could be responsible for a hydrogen-dependent CO2 fixation [127].
CO2 + H2. The ‘forward’ reaction (CO2 and H2 production from formate) under physiological fermentative conditions is observed [123,124]. The expression of active formate hydrogenlyase is repressed at a low formate concentration accompanied by a relatively high pH. However, theoretical arguments and experimental data indicate that this enzyme exists under certain conditions [125,126]. An evolutionary progenitor of formate hydrogenlyase on the early Earth could be responsible for a hydrogen-dependent CO2 fixation [127].3.2. Further Conversion of Synthesized Glyoxylate, Propionyl-CoA and PA/PEP into TCA Cycle Intermediates
3.2.1. Glyoxylate Conversion
3.2.2. Pathways of Propionyl-CoA Conversion into Succinyl-CoA or PA
3.2.3. Pathways of TCA Cycle Intermediate Formation from PA/PEP
4. Part 3: Alternative Functions of Anaplerotic Pathways
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| OAA | oxaloacetic acid |
| CBB cycle | Calvin–Benson–Bassham cycle |
| ICL | isocitrate lyase |
| ILV synthesis pathway | isoleucine and valine synthesis pathway |
| PA | pyruvate |
| PNSB | purple non-sulfur bacteria |
| Rubisco | ribulose bisphosphate carboxylase/oxygenase |
| rTCA cycle | reductive citric acid cycle |
| TCA cycle | tricarboxylic acid cycle (or the citric acid cycle) |
| PGA | phosphoglycerate |
| PFL | pyruvate formate lyase |
References
- Sauer, U.; Eikmanns, B.J. The PEP-pyruvate-oxaloacetate node as the switch point for carbon flux distribution in bacteria. FEMS Microbiol. Rev. 2005, 29, 765–794. [Google Scholar]
- Fuchs, G. Biosynthesis of building blocks. In Biology of the Prokaryotes; Lengeler, J.W., Drews, G., Schlegel, H.G., Eds.; Georg-Thieme Verlag: Stuttgart, Germany, 1999; pp. 110–157. [Google Scholar]
- McKinlay, J.B.; Harwood, C.S. Carbon dioxide fixation as a central redox cofactor recycling mechanism in bacteria. Proc. Natl. Acad. Sci. USA 2010, 107, 11669–11675. [Google Scholar] [CrossRef]
- Eidels, L.; Preiss, J. Carbohydrate metabolism in Rhodopseudomonas capsulata: Enzyme titers, glucose metabolism, and polyglucose polymer synthesis. Arch. Biochem. Biophys. 1970, 140, 75–89. [Google Scholar] [CrossRef]
- Owen, O.E.; Kalhan, S.C.; Hanson, R.W. The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 2002, 277, 30409–30412. [Google Scholar] [CrossRef] [PubMed]
- Zarzycki, J.; Fuchs, G.; Fuchs, G. Coassimilation of Organic Substrates via the Autotrophic 3-Hydroxypropionate Bi-Cycle in Chloroflexus aurantiacus. Appl. Environ. Microbiol. 2011, 77, 6181–6188. [Google Scholar] [CrossRef] [PubMed]
- Leroy, B.; De Meur, Q.; Moulin, C.; Wegria, G.; Wattiez, R. New insight into the photoheterotrophic growth of the isocytrate lyase-lacking purple bacterium Rhodospirillum rubrum on acetate. Microbiology 2015, 161, 1061–1072. [Google Scholar] [CrossRef] [PubMed]
- Tang, K.H.; Tang, Y.J.; Blankenship, R.E. Carbon metabolic pathways in phototrophic bacteria and their broader evolutionary implications. Front. Microbiol. 2011, 2, 1–23. [Google Scholar] [CrossRef]
- Ivanovsky, R.N.; Krasilnikova, E.N.; Berg, I.A. A proposed citramalate cycle for acetate assimilation in the purple non-sulfur bacterium Rhodospirillum rubrum. FEMS Microbiol. Lett. 1997, 153, 399–404. [Google Scholar] [CrossRef]
- Filatova, L.V.; Berg, I.A.; Krasil’nikova, E.N.; Tsygankov, A.A.; Laurinavichene, T.V.; Ivanovsky, R.N. A Study of the Mechanism of Acetate Assimilation in Purple Nonsulfur Bacteria Lacking the Glyoxylate Shunt: Acetate Assimilation in Rhodobacter sphaeroides. Microbiology 2005, 74, 265–269. [Google Scholar] [CrossRef]
- Khomyakova, M.; Bukmez, O.; Thomas, L.K.; Erb, T.J.; Berg, I.A. A Methylaspartate Cycle in Haloarchaea. Science 2011, 331, 334–337. [Google Scholar] [CrossRef] [PubMed]
- Ensign, S.A. Another microbial pathway for acetate assimilation. Science 2011, 331, 294–295. [Google Scholar] [CrossRef] [PubMed]
- Petushkova, E.; Iuzhakov, S.; Tsygankov, A. Differences in possible TCA cycle replenishing pathways in purple non-sulfur bacteria possessing glyoxylate pathway. Photosynth. Res. 2018, 139, 523–537. [Google Scholar] [CrossRef] [PubMed]
- Muller, F.M. On the metabolism of the purple sulfur bacteria in organic media. Arch. Mikrobiol. 1933, 4, 131–166. [Google Scholar] [CrossRef]
- Laguna, R.; Tabita, F.R.; Alber, B.E. Acetate-dependent photoheterotrophic growth and the differential requirement for the Calvin-Benson-Bassham reductive pentose phosphate cycle in Rhodobacter sphaeroides and Rhodopseudomonas palustris. Arch. Microbiol. 2011, 193, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Strauss, G.; Fuchs, G. Enzymes of a novel autotrophic CO2 fixation pathway in the phototrophic bacterium Chloroflexus aurantiacus, the 3-hydroxypropionate cycle. Eur. J. Biochem. 1993, 215, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Herter, S.; Fuchs, G.; Bacher, A.; Eisenreich, W. A bicyclic autotrophic CO2 fixation pathway in Chloroflexus aurantiacus. J. Biol. Chem. 2002, 277, 20277–20283. [Google Scholar] [CrossRef]
- Zarzycki, J.; Brecht, V.; Muller, M.; Fuchs, G. Identifying the missing steps of the autotrophic 3-hydroxypropionate CO2 fixation cycle in Chloroflexus aurantiacus. Proc. Natl. Acad. Sci. USA 2009, 106, 21317–21322. [Google Scholar] [CrossRef] [PubMed]
- Bassham, J.A.; Benson, A.A.; Calvin, M. The path of carbon in photosynthesis. J. Biol. Chem. 1950, 185, 781–787. [Google Scholar] [CrossRef]
- Evans, M.C.W.; Buchanan, B.B.; Arnon, D.I. A new ferredoxin-dependent carbon reduction cycle in a photosynthetic bacterium. Proc. Natl. Acad. Sci. USA 1966, 55, 928–934. [Google Scholar] [PubMed]
- Berg, I.A.; Kockelkorn, D.; Buckel, W.; Fuchs, G. A 3-hydroxypropionate/4-hydroxybutyrate autotrophic carbon dioxide assimilation pathway in archaea. Science 2007, 318, 1782–1786. [Google Scholar] [CrossRef]
- Huber, H.; Gallenberger, M.; Jahn, U.; Eylert, E.; Berg, I.A.; Kockelkorn, D.; Eisenreich, W.; Fuchs, G. A dicarboxylate/4-hydroxybutyrate autotrophic carbon assimilation cycle in the hyperthermophilic Archaeum Ignicoccus hospitalis. Proc. Natl. Acad. Sci. USA 2008, 105, 7851–7856. [Google Scholar] [CrossRef] [PubMed]
- Ljungdahl, L.G. The autotrophic pathway of acetate synthesis in acetogenicbacteria. Annu. Rev. Microbiol. 1986, 40, 415–450. [Google Scholar] [CrossRef]
- Sánchez-Andrea, I.; Guedes, I.A.; Hornung, B.; Boeren, S.; Lawson, C.E.; Sousa, D.Z.; Bar-Even, A.; Claassens, N.J.; Stams, A.J.M. The reductive glycine pathway allows autotrophic growth of Desulfovibrio desulfuricans. Nat. Commun. 2020, 11, 5090. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, H.L.; Krebs, H.A. Synthesis of cell constituents from C2-units by a modified tricarboxylic acid cycle. Nature 1957, 179, 988–991. [Google Scholar] [CrossRef] [PubMed]
- Iuchi, S.; Lin, E.C. arcA (dye), A global regulatory gene in Escherichia coli mediating repression of enzymes in aerobic pathways. Proc. Natl. Acad. Sci. USA 1988, 85, 1888–1892. [Google Scholar] [CrossRef]
- Iuchi, S.; Cameron, D.C.; Lin, E.C. A second global regulator gene (arcB) mediating repression of enzymes in aerobic pathways of Escherichia coli. J. Bacteriol 1989, 171, 868–873. [Google Scholar] [CrossRef] [PubMed]
- Adman, E.T.; Sieker, L.C.; Jensen, L.H. Structure of a bacterial ferredoxin. J. Biol. Chem. 1973, 248, 3987–3996. [Google Scholar] [CrossRef]
- Buchanan, B.B.; Arnon, D.I. Ferredoxins: Chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv. Enzym. Relat. Areas Mol. Biol. 1970, 33, 119–176. [Google Scholar]
- Hordt, A.; Lopez, M.G.; Meier-Kolthoff, J.P.; Schleuning, M.; Weinhold, L.M.; Tindall, B.J.; Gronow, S.; Kyrpides, N.C.; Woyke, T.; Goker, M. Analysis of 1,000+ Type-Strain Genomes Substantially Improves Taxonomic Classification of Alphaproteobacteria. Front. Microbiol. 2020, 11, 468. [Google Scholar] [CrossRef] [PubMed]
- Willems, A.; Gillis, M.; De Ley, J. Transfer of Rhodocyclus gelatinosus to Rubrivivax gelatinosus gen. nov., comb. nov., and phylogenetic relationships with Leptothrix, Sphaerotilus natans, Pseudomonas saccharophila, and Alcaligenes latus. Int. J. Syst. Bacteriol. 1991, 41, 65–73. [Google Scholar] [CrossRef]
- Kornberg, H.L.; Lascelles, J. The formation isocitratase by the athiorhodaceae. J. Gen. Microbiol. 1960, 23, 511–517. [Google Scholar] [CrossRef]
- Albers, H.; Gottschalk, G. Acetate metabolism in Rhodopseudomonas gelatinosa and several other rhodospirillaceae. Arch. Microbiol. 1976, 111, 45–49. [Google Scholar] [CrossRef]
- Nielsen, A.M.; Rampsch, B.J.; Sojka, G.A. Regulation of isocitrate lyase in a mutant of Rhodopseudomonas capsulate. Arch. Microbiol. 1979, 120, 43–46. [Google Scholar] [CrossRef]
- Willison, J.C. Pyruvate and acetate metabolism in the photosynthetic bacterium Rhodobacter Capsulatus. J. Gen. Microbiol. 1988, 134, 2429–2439. [Google Scholar] [CrossRef]
- Blasco, R.; Cardenas, J.; Castillo, F. Regulation of isocitrate lyase in Rhodobacter capsulatus E1F1. Curr. Microbiol. 1991, 22, 73–76. [Google Scholar] [CrossRef]
- Petushkova, E.P.; Tsygankov, A.A. Major factors affecting isocitrate lyase activity in Rhodobacter capsulatus B10 under phototrophic conditions. Microbiology 2011, 80, 619–623. [Google Scholar] [CrossRef]
- Nielsen, A.M.; Rampsch, B.J.; Sojka, G.A. Photoheterotrophic utilization of acetate by the wild type and an acetate-adapted mutant of Rhodopseudomonas capsulate. Arch. Microbiol. 1979, 120, 39–42. [Google Scholar] [CrossRef]
- Petushkova, E.P.; Tsygankov, A.A. Acetate metabolism in purple non-sulphur bacterium Rhodobacter capsulatus. Biochemistry 2017, 82, 786–807. [Google Scholar]
- Petushkova, E.P. Acetate assimilation in purple non-sulfur bacterium Rhodobacter capsulatus B10. Ph.D. Thesis, G.K. Skryabin Institute of Biochemistry and Physiology of Microorganisms, Russian Academy of Sciences, Pushchino, Russia, 2018; pp. 1–132. [Google Scholar]
- Meister, M.; Saum, S.; Alber, B.E.; Fuchs, G. L-Malyl-Coenzyme A/β-Methylmalyl-Coenzyme A Lyase Is Involved in Acetate Assimilation of the Isocitrate Lyase-Negative Bacterium Rhodobacter capsulatus. J. Bacteriol. 2005, 187, 1415–1425. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kremer, K.; van Teeseling, M.C.F.; von Borzyskowski, L.S.; Bernhardsgrutter, I.; van Spanning, R.J.M.; Gates, A.J.; Remus-Emsermann, M.N.P.; Thanbichler, M.; Erb, T.J. Dynamic metabolic rewiring enables efficient acetyl coenzyme A assimilation in Paracoccus denitrificans. Mbio 2019, 10, e00805–e00819. [Google Scholar] [CrossRef]
- Eley, J.H.; Knobloch, K.; Han, T.-W. Effect of growth condition on enzymes of the citric acid cycle and the glyoxylate cycle in the photosynthetic bacterium Rhodopseudomonas palustris. Antonie Van Leeuwenhoek 1979, 45, 521–529. [Google Scholar] [CrossRef]
- Korotkova, N.; Chistoserdova, L.; Kuksa, V.; Lidstrom, M.E. Glyoxylate regeneration pathway in the methylotroph Methylobacterium extorquens AM1. J. Bacteriol. 2002, 184, 1750–1758. [Google Scholar] [CrossRef]
- Peyraud, R.; Kiefer, P.; Christen, P.; Massou, S.; Portais, J.C.; Vorholt, J.A. Demonstration of the ethylmalonyl-CoA pathway by using 13C metabolomics. Proc. Natl. Acad. Sci. USA 2009, 106, 4846–4851. [Google Scholar] [CrossRef]
- Smejkalovб, H.; Erb, T.J.; Fuchs, G. Methanol assimilation in Methylobacterium extorquens AM1: Demonstration of all enzymes and their regulation. PLoS ONE 2010, 5, e13001. [Google Scholar]
- Erb, T.J.; Berg, I.A.; Brecht, V.; Muller, M.; Fuchs, G.; Alber, B.E. Synthesis of C5-dicarboxylic acids from C2-units involving crotonyl-CoA carboxylase/reductase: The ethylmalonyl-CoA pathway. Proc. Natl. Acad. Sci. USA 2007, 104, 10631–10636. [Google Scholar] [CrossRef]
- Alber, B.E.; Spanheimer, R.; Ebenau-Jehle, C.; Fuchs, G. Study of an alternate glyoxylate cycle for acetate assimilation by Rhodobacter sphaeroides. Mol. Microbiol. 2006, 61, 297–309. [Google Scholar] [CrossRef]
- Green, P.N.; Ardley, J.K. Review of the genus Methylobacterium and closely related organisms: A proposal that some Methylobacterium species be reclassified into a new genus, Methylorubrum gen. nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 2727–2748. [Google Scholar] [CrossRef] [PubMed]
- Price, M.N.; Wetmore, K.M.; Waters, R.J.; Callaghan, M.; Ray, J.; Liu, H.; Kuehl, J.V.; Melnyk, R.A.; Lamson, J.S.; Suh, Y.; et al. Mutant phenotypes for thousands of bacterial genes of unknown function. Nature 2018, 557, 503–509. [Google Scholar] [CrossRef]
- De Meur, Q.; Deutschbauer, A.; Koch, M.; Wattiez, R.; Leroy, B. Genetic Plasticity and Ethylmalonyl Coenzyme A Pathway during Acetate Assimilation in Rhodospirillum rubrum S1H under Photoheterotrophic Conditions. Appl. Environ. Microbiol. 2018, 84, e02038-17. [Google Scholar] [CrossRef] [PubMed]
- Ueda, S.; Sato, K.; Shimizu, S. Glyoxylate formation from mesaconyl-CoA and its related reactions in a methanol-utilising bacterium, Protaminobacter Rubber. Agric. Biol. Chem. 1981, 45, 823–830. [Google Scholar]
- Shimizu, S.; Ueda, S.; Sato, K. Physiological role of vitamin B12 in a methanol utilising bacterium, Protaminobacter rubber. In Microbial Growth on C1 Compounds; Crawford, R.L., Hanson, R.S., Eds.; American Society for Microbiology: Washington, DC, USA, 1984; pp. 113–117. [Google Scholar]
- Borjian, F.; Han, J.; Hou, J.; Xiang, H.; Berg, I.A. The methylaspartate cycle in haloarchaea and its possible role in carbon metabolism. ISME J. 2016, 10, 546–557. [Google Scholar] [CrossRef] [PubMed]
- Buckel, W.; Miller, S.L. Equilibrium constants of several reactions involved in the fermentation of glutamate. Eur. J. Biochem. 1987, 164, 565–569. [Google Scholar] [CrossRef] [PubMed]
- Filatova, L.V.; Berg, I.A.; Krasil’nikova, E.N.; Ivanovsky, R.N. A Study of the Mechanism of Acetate Assimilation in Purple Nonsulfur Bacteria Lacking the Glyoxylate Shunt: Enzymes of the Citramalate Cycle in Rhodobacter sphaeroides. Microbiology 2005, 74, 270–278. [Google Scholar] [CrossRef]
- Bayon-Vicente, G.; Wattiez, R.; Leroy, B. Global Proteomic Analysis Reveals High Light Intensity Adaptation Strategies and Polyhydroxyalkanoate Production in Rhodospirillum rubrum Cultivated With Acetate as Carbon Source. Front. Microbiol. 2020, 11, 464. [Google Scholar] [CrossRef]
- McCully, A.L.; Onyeziri, M.C.; LaSarre, B.; Gliessman, J.R.; McKinlay, J.B. Reductive tricarboxylic acid cycle enzymes and reductive amino acid synthesis pathways contribute to electron balance in a Rhodospirillum rubrum Calvin-cycle mutant. Microbiol. 2020, 166, 199–211. [Google Scholar] [CrossRef] [PubMed]
- De Meur, Q.; Deutschbauer, A.; Koch, M.; Bayon-Vicente, G.; Segura, P.C.; Wattiez, R.; Leroy, B. New perspectives on butyrate assimilation in Rhodospirillum rubrum S1H under photoheterotrophic conditions. BMC Microbiol. 2020, 20, 126. [Google Scholar] [CrossRef]
- Thauer, R.K. Citric-acid cycle, 50 years on: Modifications and an alternative pathway in anaerobic-bacteria. Eur. J. Biochem. 1988, 176, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Tang, K.H.; Blankenship, R.E.; Tang, Y.J. Metabolic flux analysis of the mixotrophic metabolisms in the green sulfur bacterium Chlorobaculum tepidum. J. Biol. Chem. 2010, 285, 39544–39550. [Google Scholar] [CrossRef]
- Tang, K.H.; Blankenship, R.E. Both forward and reverse TCA cycles operate in green sulfur bacteria. J. Biol. Chem. 2010, 285, 35848–35854. [Google Scholar] [CrossRef]
- Furdui, C.; Ragsdale, S.W. The role of pyruvate ferredoxin oxidoreductase in pyruvate synthesis during autotrophic growth by the Wood-Ljungdahl pathway. J. Biol. Chem. 2000, 275, 28494–28499. [Google Scholar] [CrossRef]
- McFadden, B.A.; Shively, L.M. Bacterial assimilation of carbon dioxide by the Calvin cycle. In Variations in Autotrophic Life; Shively, L.M., Barton, L.L., Eds.; Acad Press: London, UK, 1991; pp. 25–49. [Google Scholar]
- Tabita, F.R.; Hanson, T.E.; Li, H.; Satagopan, S.; Singh, J.; Chan, S. Function, Structure, and Evolution of the RubisCO-Like Proteins and Their RubisCO Homologs. Microbiol. Mol. Biol. Rev. 2007, 71, 576–599. [Google Scholar] [CrossRef]
- Tabita, F.R. Molecular and cellular regulation of autotrophic carbon dioxide fixation in microorganisms. Microbiol. Rev. 1988, 52, 155–189. [Google Scholar] [CrossRef]
- Gibson, J.L.; Tabita, F.R. Isolation and preliminary characterization of two forms of ribulose-1,5-bisphosphate carboxylase from Rhodopseudomonas capsulata. J. Bacteriol. 1977, 132, 818–823. [Google Scholar] [CrossRef]
- Joshi, G.S.; Romagnoli, S.; VerBerkmoes, N.C.; Hettich, R.L.; Pelletier, D.; Tabita, F.R. Differential Accumulation of Form I RubisCO in Rhodopseudomonas palustris CGA010 under Photoheterotrophic Growth Conditions with Reduced Carbon Sources. J. Bacteriol. 2009, 191, 4243–4250. [Google Scholar] [CrossRef]
- Kusian, B.; Bowien, B. Organization and regulation of cbb CO2 assimilation genes in autotrophic bacteria. FEMS Microbiol. Rev. 1997, 21, 135–155. [Google Scholar] [CrossRef] [PubMed]
- Singh, J.; Tabita, F.R. Roles of RubisCO and the RubisCO-like protein in 5-methylthioadenosine metabolism in the Nonsulfur purple bacterium Rhodospirillum rubrum. J. Bacteriol. 2010, 192, 1324–1331. [Google Scholar] [CrossRef][Green Version]
- Dey, S.; North, J.A.; Sriram, J.; Evans, B.S.; Tabita, F.R. In Vivo Studies in Rhodospirillum rubrum Indicate That Ribulose-1,5-bisphosphate Carboxylase/Oxygenase (Rubisco) Catalyzes Two Obligatorily Required and Physiologically Significant Reactions for Distinct Carbon and Sulfur Metabolic Pathways. J. Biol. Chem. 2015, 290, 30658–30668. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.H.; Gibson, J.L.; McCue, L.A.; Tabita, F.R. Identification, expression, and deduced primary structure of transketolase and other enzymes encoded within the form II CO2 fixation operon of Rhodobacter sphaeroides. J. Biol. Chem. 1991, 266, 20447–20452. [Google Scholar] [CrossRef]
- Falcone, D.L.; Quivey, R.G.; Tabita, F.R. Transposon mutagenesis and physiological analysis of strains containing inactivated form I and form II ribulose bisphosphate carboxylase/oxygenase genes in Rhodobacter sphaeroides. J. Bacteriol. 1988, 170, 5–11. [Google Scholar] [CrossRef] [PubMed]
- Gibson, J.L.; Tabita, F.R. Nucleotide sequence and functional analysis of CbbR, a positive regulator of the Calvin cycle operons of Rhodobacter sphaeroides. J. Bacteriol. 1993, 1755, 778–5784. [Google Scholar] [CrossRef]
- Romagnoli, S.; Tabita, F.R. Anovelthree-proteintwo-component system provides a regulatory twist on an established circuit to modulate expression of the cbbI region of Rhodopseudomonas palustris CGA010. J. Bacteriol. 2006, 188, 2780–2791. [Google Scholar] [CrossRef] [PubMed]
- Vichivanives, P.; Bird, T.H.; Bauer, C.E.; Tabita, F.R. Multiple regulators and their interactions in vivo and in vitro with the cbb regulons of Rhodobacter capsulatus. J. Mol. Biol. 2000, 300, 1079–1099. [Google Scholar] [CrossRef] [PubMed]
- Paoli, G.C.; Vichivanives, P.; Tabita, F.R. Physiological control and regulation of the Rhodobacter capsulatus cbb operons. J. Bacteriol. 1998, 180, 4258–4269. [Google Scholar] [CrossRef] [PubMed]
- Dangel, A.W.; Gibson, J.L.; Janssen, A.P.; Tabita, F.R. Residues that influence in vivo and in vitro CbbR function in Rhodobacter sphaeroides and identification of a specific region critical for co-inducer recognition. Mol. Microbiol. 2005, 57, 1397–1414. [Google Scholar] [CrossRef]
- Dubbs, P.; Dubbs, J.M.; Tabita, F.R. Effector-mediated interaction of CbbRI and CbbRII regulators with target sequences in Rhodobacter capsulatus. J. Bacteriol. 2004, 186, 8026–8035. [Google Scholar] [CrossRef]
- Smith, S.A.; Tabita, F.R. Up-regulated expression of the cbbI and cbbII operons during photoheterotrophic growth of a ribulose 1,5-bisphosphate carboxylase-oxygenase deletion mutant of Rhodobacter sphaeroides. J. Bacteriol. 2002, 184, 6721–6724. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tichi, M.A.; Tabita, F.R. Metabolic signals that lead to control of CBB gene expression in Rhodobacter capsulatus. J. Bacteriol. 2002, 184, 1905–1915. [Google Scholar] [CrossRef]
- Du, S.; Bird, T.H.; Bauer, C.E. DNA binding characteristics of RegA*. A constitutively active anaerobic activator of photosynthesis gene expression in Rhodobacter capsulatus. J. Biol. Chem. 1998, 273, 18509–18513. [Google Scholar] [CrossRef]
- Dubbs, J.M.; Tabita, F.R. Regulators of nonsulfur purple phototrophic bacteria and the interactive control of CO2 assimilation, nitrogen fixation, hydrogen metabolism and energy generation. FEMS Microbiol. Rev. 2004, 28, 353–376. [Google Scholar] [CrossRef]
- Elsen, S.; Dischert, W.; Colbeau, A.; Bauer, C.E. Expression of uptake hydrogenase and molybdenum nitrogenase in Rhodobacter capsulatus is coregulated by the RegB-RegA two-component regulatory system. J. Bacteriol. 2000, 182, 2831–2837. [Google Scholar] [CrossRef]
- Elsen, S.; Swem, L.R.; Swem, D.L.; Bauer, C.E. RegB/RegA, a highly conserved redox-responding global two-component regulatory system. Microbiol. Mol. Biol. Rev. 2004, 68, 263–279. [Google Scholar] [CrossRef] [PubMed]
- Joshi, H.M.; Tabita, F.R. A global two component signal transduction system that integrates the control of photosynthesis, carbon dioxide assimilation, and nitrogen fixation. Proc. Natl. Acad. Sci. USA 1996, 93, 14515–14520. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Tabita, F.R. A global signal transduction system regulates aerobic and anaerobic CO2 fixation in Rhodobacter sphaeroides. J. Bacteriol. 1996, 178, 12–18. [Google Scholar] [CrossRef][Green Version]
- Sganga, M.W.; Bauer, C.E. Regulatory factors controlling photosynthetic reaction center and light-harvesting gene expression in Rhodobacter capsulatus. Cell 1992, 68, 945–954. [Google Scholar] [CrossRef]
- Paoli, G.C.; Morgan, N.S.; Tabita, F.R.; Shively, J.M. Expression of the cbbLcbbS and cbbM genes and distinct organization of the cbb Calvin cycle structural genes of Rhodobacter capsulatus. Arch. Microbiol. 1995, 164, 396–405. [Google Scholar] [CrossRef]
- Shively, J.M.; Van Keulen, G.; Meijer, W.G. Something from almost nothing: Carbon dioxide fixation in chemoautotrophs. Annu Rev. Microbiol. 1998, 52, 191–230. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, Y.; Pohlmann, E.L.; Li, J.; Roberts, G.P. The poor growth of Rhodospirillum rubrum mutants lacking RubisCO is due to the accumulation of ribulose-1,5-bisphosphate. J. Bacteriol. 2011, 193, 3293–3303. [Google Scholar] [CrossRef][Green Version]
- Wang, X.; Modak, H.V.; Tabita, F.R. Photolithoautotrophic growth and control of CO2 fixation in Rhodobacter sphaeroides and Rhodospirillum rubrum in the absence of ribulose bisphosphate carboxylase-oxygenase. J. Bacteriol. 1993, 175, 7109–7114. [Google Scholar] [CrossRef][Green Version]
- Wang, D.; Zhang, Y.; Welch, E.; Li, J.; Roberts, G.P. Elimination of Rubisco alters the regulation of nitrogenase activity and increases hydrogen productionin Rhodospirillum rubrum. Int. J. Hydrogen Energy 2010, 35, 7377–7385. [Google Scholar] [CrossRef]
- Bowes, G.; Ogren, W.L.; Hagerman, R.H. Phosphoglycolate production catalyzed by ribulose diphosphate carboxylase. Biochem. Biophys. Res. Commun. 1971, 45, 716–722. [Google Scholar] [CrossRef]
- Sarles, L.S.; Tabita, F.R. Derepression of the synthesis of d-ribulose 1,5-bisphosphate carboxylase/oxygenase from Rhodospirillum rubrum. J. Bacteriol. 1983, 153, 458–464. [Google Scholar] [CrossRef]
- Anderson, L.; Fuller, R. C Photosynthesis in Rhodospirillum rubrum. 3. Metabolic control of reductive pentose phosphate and tricarboxylic acid cycle enzymes. Plant. Physiol. 1967, 42, 497–502. [Google Scholar] [CrossRef]
- Kondrat’eva, E.N.; Maksimova, I.V.; Samuilov, V.D. Fototrofnye Mikroorganizmy; Izd-vo MGU: Moskva, Rossiya, 1989; p. s. 376. (In Russian) [Google Scholar]
- Weaver, K.E.; Tabita, F.R. Isolation and partial charcterization of Rhodopseudomonas sphaeroides mutants defective in the regulation of ribulose bisphosphate carboxylase/oxygenase. J. Bacteriol. 1983, 156, 507–515. [Google Scholar] [CrossRef]
- Shively, J.M.; Davidson, E.; Marrs, B.L. Depression of the synthesis of the intermediate and large forms of ribulose-l,5-bisphosphate carboxylase/oxy- genase in Rhodopseudomonas capsulata. Arch. Microbiol. 1984, 138, 233–236. [Google Scholar] [CrossRef] [PubMed]
- Gordon, G.C.; McKinlay, J.B. Calvin cycle mutants of photoheterotrophic purple nonsulfur bacteria fail to grow due to an electron imbalance rather than toxic metabolite accumulation. J. Bacteriol. 2014, 196, 1231–1237. [Google Scholar] [CrossRef]
- Tsygankov, A.A.; Laurinaviciene, T.; Krasil’nikova, E.N.; Gogotov, I.N. The hydrogen effect in Rhodospirillum rubrum and Rhodospirillum fulvum. Mikrobiologiya 1992, 61, 571–576. [Google Scholar]
- Shiba, H.; Kawasumi, T.; Igarashi, Y.; Kodama, T.; Minoda, Y. The CO2 assimilation via the reductive tricarboxylic acid cycle in an obligately autotrophic, aerobic hydrogen-oxidizing bacterium, Hydrogenobacter thermophilus. Arch. Microbiol. 1985, 141, 198–203. [Google Scholar] [CrossRef]
- Shih, P.M.; Wardc, L.M.; Fischer, W.W. Evolution of the 3-hydroxypropionate bicycle and recent transfer of anoxygenic photosynthesis into the Chloroflexi. Proc. Natl. Acad. Sci. USA 2017, 114, 10749–10754. [Google Scholar] [CrossRef]
- Eprintsev, A.T.; Klimova, M.A.; Falaleeva, M.I.; Kompantseva, E.I. Regulation of Carbon Flows in the Tricarboxylic Acid Cycle– Glyoxylate Bypass System in Rhodopseudomonas palustris under Different Growth Conditions. Microbiology 2008, 77, 132–136. [Google Scholar] [CrossRef]
- Claassens, N.J.; Scarincia, G.; Fischera, A.; Flamholzb, A.I.; Newella, W.; Frielingsdorfc, S.; Lenzc, O.; Bar-Even, A. Phosphoglycolate salvage in a chemolithoautotroph using the Calvin cycle. Proc. Natl. Acad. Sci. USA 2020, 117, 22452–22461. [Google Scholar] [CrossRef] [PubMed]
- Edvards Dzh Uoker, D. Fotosintez S3 i S4 Rastenij: Mekhanizmy i Regulyaciya; Mir.: Moskva Rossiya, 1986; p. s. 590. [Google Scholar]
- Ort, D.R.; Merchant, S.S.; Alric, J.; Barkan, A.; Blankenship, R.E.; Bock, R.; Croce, R.; Hanson, M.R.; Hibberd, J.M.; Long, S.P.; et al. Redesigning photosynthesis to sustainably meet global food and bioenergy demand. Proc. Natl. Acad. Sci. USA 2015, 112, 8529–8536. [Google Scholar] [CrossRef] [PubMed]
- Hendry, J.I.; Gopalakrishnan, S.; Ungerer, J.; Pakrasi, H.B.; Tang, Y.J.; Maranas, C.D. Genome-scale fluxome of Synechococcus elongatus UTEX 2973 using transient 13C-labeling data. Plant. Physiol. 2019, 179, 761–769. [Google Scholar] [CrossRef]
- Hagemann, M.; Bauwe, H. Photorespiration and the potential to improve photosynthesis. Curr. Opin. Chem. Biol. 2016, 35, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Strnad, H.; Lapidus, A.; Paces, J.; Ulbrich, P.; Vlcek, C.; Paces, V.; Haselkorn, R. Complete Genome Sequence of the Photosynthetic Purple Nonsulfur Bacterium Rhodobacter capsulatus SB 1003. J. Bacteriol. 2010, 192, 3545–3546. [Google Scholar] [CrossRef] [PubMed]
- Erb, T.J.; Frerichs-Revermann, L.; Fuchs, G.; Alber, B.E. The apparent malate synthase activity of Rhodobacter sphaeroides is due to two paralogous enzymes, (3S)-Malyl-coenzyme A (CoA)/β-methylmalyl-CoA lyase and (3S)-Malyl-CoA thioesterase. J. Bacteriol. 2010, 192, 1249–1258. [Google Scholar] [CrossRef]
- Borjian, F.; Johnsen, U.; Schonheit, P.; Berg, I.A. Succinyl-CoA:Mesaconate CoA-Transferase and Mesaconyl-CoA Hydratase, Enzymes of the Methylaspartate Cycle in Haloarcula hispanica. Front. Microbiol. 2017, 8, 1683. [Google Scholar] [CrossRef] [PubMed]
- Risso, C.; van Dien, J.S.; Orloff, A.; Lovley, R.D.; Coppi, V.M. Elucidation of an alternate isoleucine biosynthesis pathway in Geobacter sulfurreducens. J. Bacteriol. 2008, 190, 2266–2274. [Google Scholar] [CrossRef]
- Feng, X.; Bandyopadhyay, A.; Berla, B.; Page, L.; Wu, B.; Pakrasi, H.B.; Tang, Y.J. The citramalate pathway in isoleucine biosynthesis. Microbiology 2010, 156, 596–602. [Google Scholar]
- Kato, Y.; Asano, Y. 3-Methylaspartate ammonia-lyase as a marker enzyme of the mesaconate pathway for (S)-glutamate fermentation in Enterobacteriaceae. Arch. Microbiol. 1997, 168, 457–463. [Google Scholar] [CrossRef]
- Kronen, M.; Berg, I.A. Mesaconase/Fumarase FumD in Escherichia coli O157:H7 and Promiscuity of Escherichia coli Class I Fumarases FumA and FumB. PLoS ONE 2015, 10, e0145098. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, S.; Osumi, T.; Katsuki, H. Properties and metabolic role of mesaconate hydratase of an aerobic bacterium. J. Biochem. 1977, 81, 1917–1925. [Google Scholar] [CrossRef]
- Takamura, Y.; Kitayama, Y. 1981 Purification and some properties of malonate decarboxylase from Pseudomonas ovalis: An oligomeric enzyme with bifunctional properties. Biochem. Int. 1981, 3, 483–491. [Google Scholar]
- Nakamura, K.; Bernheim, F. Studies on malonic semialdehyde dehydrogenase from Pseudomonas aeruginosa. Biochim. Biophys. Acta 1961, 50, 147–152. [Google Scholar] [CrossRef]
- Yakunin, A.F.; Hallenbeck, P.C. Purification and characterization of pyruvate oxidoreductase from the photosynthetic bacterium Rhodobacter capsulatus. Biochim. Et Biophys. Acta (BBA) Bioenerg. 1998, 1409, 39–49. [Google Scholar] [CrossRef]
- Stairs, C.W.; Roger, A.J.; Hampl, V. Eukaryotic pyruvate formate lyase and its activating enzyme were acquired laterally from a Firmicute. Mol. Biol. Evol. 2011, 28, 2087–2099. [Google Scholar] [CrossRef]
- Sauter, M.; Bohm, R.; Bock, A. Mutational analysis of the operon (hyc) determining hydrogenase 3 formation in Escherichia coli. Mol. Microbiol. 1992, 6, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, M.; Stickland, L.H. Hydrogenlyases: Bacterial enzymes liberating molecular hydrogen. Biochem. J. 1932, 26, 712–724. [Google Scholar] [CrossRef]
- Sawers, R.G.; Ballantine, S.; Boxer, D. Differential expression of hydrogenase isoenzymes in Escherichia coli K-12: Evidence for a third isoenzyme. J. Bacteriol. 1985, 164, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Roger, M.; Brown, F.; Gabrielli, W.; Sargent, F. Efficient Hydrogen-Dependent Carbon Dioxide Reduction by Escherichia coli. Curr. Biol. 2018, 28, 140–145. [Google Scholar] [CrossRef] [PubMed]
- Pinske, C.; Sargent, F. Exploring the directionality of Escherichia coli formate hydrogenlyase: A membrane-bound enzyme capable of fixing carbon dioxide to organic acid. Microbiol. Open 2016, 5, 721–737. [Google Scholar] [CrossRef]
- Nitschke, W.; Russell, M.J. Hydrothermal focusing of chemical and chemiosmotic energy, supported by delivery of catalytic Fe, Ni, Mo/W, Co, S and Se, forced life to emerge. J. Mol. Evol. 2009, 69, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Zelcbuch, L.; Lindner, S.N.; Zegman, Y.; Slutskin, V.I.; Antonovsky, N.; Gleizer, S.; Milo, R.; Bar-Even, A. Pyruvate formate-lyase enables efficient growth of Escherichia coli on acetate and formate. Biochemistry 2016, 55, 2423–2426. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, T.; Leimkuhler, S. The oxygen-tolerant and NAD+-dependent formate dehydrogenase from Rhodobacter capsulatus is able to catalyze the reduction of CO2 to formate. FEBS J. 2013, 280, 6083–6096. [Google Scholar] [CrossRef] [PubMed]
- Duffus, B.R.; Schrapers, P.; Schuth, N.; Mebs, S.; Dau, H.; Leimkuhler, S.; Haumann, M. Anion Binding and Oxidative Modification at the Molybdenum Cofactor of Formate Dehydrogenase from Rhodobacter capsulatus Studied by X-ray Absorption Spectroscopy. Inorg. Chem. 2020, 59, 214–225. [Google Scholar] [CrossRef]
- Hartmann, T.; Schrapers, P.; Utesch, T.; Nimtz, M.; Rippers, Y.; Dau, H.; Mroginski, M.-A.; Haumann, M.; Leimkuhler, S. The molybdenum active site of formate dehydrogenase is capable of catalyzing C-H bond cleavage and oxygen atom transfer reactions. Biochemistry 2016, 55, 2381–2389. [Google Scholar] [CrossRef]
- Willison, J.C. Biochemical genetics revisited: The use of mutants to study carbon and nitrogen metabolism in the photosynthetic bacteria. FEMS Microbiol. Rev. 1993, 104, 1–38. [Google Scholar] [CrossRef][Green Version]
- Tsygankov, A.A.; Laurinavichene, T.V. Influence of the degree and mode of light limitation on growth characteristics of the Rhodobacter capsulatus continuous cultures. Biotechnol. Bioeng. 1996, 51, 605–612. [Google Scholar] [CrossRef]
- Molina, I.; Pellicer, M.T.; Badia, J.; Aguilar, J.; Baldoma, L. Molecular characterization of Escherichia coli malate synthase G: Differentiation with the malate synthase A isoenzyme. Eur. J. Biochem. 1994, 224, 541–548. [Google Scholar] [CrossRef]
- Vanderwinkel, E.; De Vlieghere, M. Physiologie et g´en´etique de l’isocitritase et des malate synthases chez Escherichia coli. Eur. J. Biochem. 1968, 5, 81–90. [Google Scholar] [CrossRef]
- Kornberg, H.L.; Sadler, J.R. Microbial oxidation of glycollate via a dicarboxylic acid cycle. Nature 1960, 185, 153–155. [Google Scholar] [CrossRef]
- Ornston, L.N.; Ornston, M.K. Regulation of glyoxylate metabolism in Escherichia coli K-12. J. Bacteriol. 1969, 98, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.W.; Hayashi, J.A. Glycolate metabolism in Escherichia coli. J. Bacteriol. 1962, 83, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Krakow, G.; Barkulis, S.S. Conversion of glyoxylate to hydroxypyruvate by extracts of Escherichia coli. Biochim. Biophys. Acta. 1956, 21, 593–594. [Google Scholar] [CrossRef]
- Kornberg, H.L.; Morris, J.G. Beta-hydroxyaspartate pathway: A new route for biosyntheses from glyoxylate. Nature 1963, 197, 456–457. [Google Scholar] [CrossRef]
- Schada von Borzyskowski, L.; Severi, F.; Kruger, K.; Hermann, L.; Gilardet, A.; Sippel, F.; Pommerenke, B.; Claus, P.; Cortina, N.S.; Glatter, T.; et al. Marine Proteobacteria metabolize glycolate via the beta-hydroxyaspartate cycle. Nature 2019, 575, 500–504. [Google Scholar] [CrossRef]
- Zhang, Y.; Rodionov, D.A.; Gelfand, M.S.; Gladyshev, V.N. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genom. 2009, 10, 78. [Google Scholar] [CrossRef] [PubMed]
- Brock, M.; Maerker, C.; Schutz, A.; Volker, U.; Buckel, W. Oxidation of propionate to pyruvate in Escherichia coli. Involvement of methylcitrate dehydratase and aconitase. Eur. J. Biochem. 2002, 269, 6184–6194. [Google Scholar] [CrossRef]
- Gould, T.A.; Langemheen HVan De Muñoz-Elías, E.J.; McKinney, J.D.; Sacchettini, J.C. Dual role of isocitrate lyase 1 in the glyoxylate and methylcitrate cycles in Mycobacterium Tuberculosis. Mol. Microbiol. 2006, 61, 940–947. [Google Scholar] [CrossRef]
- Horswill, A.R.; Escalante-Semerena, J. In vitro conversion of propionate to pyruvate by Salmonella enterica enzymes: 2-Methylcitrate dehydratase (PrpD) and aconitase enzymes catalyze the conversion of 2-methylcitrate to 2-methylisocitrate. Biochem. 2001, 40, 4703–4713. [Google Scholar] [CrossRef]
- Nuiry, I.I.; Cook, P.F. The pH dependence of the reductive carboxylation of pyruvate by malic enzyme. Biochim. Biophys. Acta. 1985, 829, 295–298. [Google Scholar] [CrossRef]
- Bricker, T.M.; Zhang, S.; Laborde, S.M.; Mayer, P.R.; Frankel, L.K.; Moroney, J.V. The malic enzyme is required for optimal photoautotrophic growth of Synechocystis sp. strain PCC 6803 under continuous light but not under a diurnal light regimen. J. Bacteriol. 2004, 186, 8144–8148. [Google Scholar] [CrossRef][Green Version]
- Schobert, P.; Bowien, B. Unusual C3 and C4 metabolism in the chemoautotroph Alcaligenes eutrophus. J. Bacteriol. 1984, 159, 167–172. [Google Scholar] [CrossRef]
- Bramer, C.O.; Steinbuchel, A. The malate dehydrogenase of Ralstonia eutropha and functionality of the C3/C4 metabolism in a Tn5-induced mdh mutant. FEMS Microbiol. Lett. 2002, 212, 159–164. [Google Scholar] [CrossRef]
- Drevland, R.M.; Waheed, A.; Graham, D.E. Enzymology and evolution of the pyruvate pathway to 2-oxobutyrate in Methanocaldococcus jannaschii. J. Bacteriol. 2007, 189, 4391–4400. [Google Scholar] [CrossRef] [PubMed]
- Martнnez-Luque, M.; Castillo, F.; Blasco, R. Assimilation of d-malate by Rhodobacter capsulatus E1F1. Curr. Microbiol. 2001, 43, 154–157. [Google Scholar] [CrossRef] [PubMed]
- Gray, C.T.; Kornberg, H.L. Enzymic formation of citramalate from acetyl-coenzyme A and pyruvate in Pseudomonas ovalis Chester, catalysed by “pyruvate transacetase”. Biochim. Biophys. Acta. 1960, 42, 371–372. [Google Scholar] [CrossRef]
- Steiger, M.G.; Blumhoff, M.L.; Mattanovich, D.; Sauer, M. Biochemistry of microbial itaconic acid production. Front. Microbiol. 2013, 4, 23. [Google Scholar] [CrossRef]
- Geiser, E.; Przybilla, S.K.; Friedrich, A.; Buckel, W.; Wierckx, N.; Blank, L.M.; Bolker, M. Ustilago maydis produces itaconic acid via the unusual intermediate trans-aconitate. Microb. Biotechnol. 2016, 9, 116–126. [Google Scholar] [CrossRef] [PubMed]
- The UniProt Consortium UniProt: A hub for protein information. Nucleic. Acids. Res. 2015, 43, D204–D212. [CrossRef]
- Pinhal, S.; Ropers, D.; Geiselmann, J.; De Jong, H. Acetate Metabolism and the Inhibition of Bacterial Growth by Acetate. J. Bacteriol. 2019, 201, e00147-19. [Google Scholar] [CrossRef] [PubMed]
- Patrusheva, E.V.; Fedorov, A.S.; Belera, V.V.; Minkevich, I.G.; Tsygankov, A.A. Synthesis of Bacteriochlorophyll a by the Purple Nonsulfur Bacterium Rhodobacter capsulatus. Appl. Biochem. Microbiol. 2007, 43, 187–192. [Google Scholar] [CrossRef]
- Grime, J.M.; Edwards, M.A.; Rudd, N.C.; Unwin, P.R. Quantitative visualization of passive transport across bilayer lipid membranes. Proc. Natl. Acad. Sci. USA 2008, 105, 14277–14282. [Google Scholar] [CrossRef] [PubMed]
- Roe, A.J.; McLaggan, D.; Davidson, I.; O’Byrne, C.; Booth, I.R. Perturbation of anion balance during inhibition of growth of Escherichia coli by weak acids. J. Bacteriol. 1998, 180, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Russell, J.B. Another explanation for the toxicity of fermentation acids at low pH: Anion accumulation versus uncoupling. J. Appl. Bacteriol. 1992, 73, 363–370. [Google Scholar] [CrossRef]
- Castaсo-Cerezo, S.; Bernal, V.; Post, H.; Fuhrer, T.; Cappadona, S.; Sбnchez-Dнaz, N.C.; Sauer, U.; Heck, A.J.R.; Altelaar, A.F.M.; Cбnovas, M. Protein acetylation affects acetate metabolism, motility and acid stress response in Escherichia coli. Mol. Syst. Biol. 2014, 10, 762. [Google Scholar] [CrossRef]
- Kuhn, M.; Zemaitaitis, B.; Hu, L.; Sahu, A.; Sorensen, D.; Minasov, G.; Lima, B.; Scholle, M.; Mrksich, M.; Anderson, W.; et al. Structural, kinetic and proteomic characterization of acetyl phosphate-dependent bacterial protein acetylation. PLoS ONE 2014, 9, e94816. [Google Scholar] [CrossRef]
- Weinert, B.T.; Iesmantavicius, V.; Wagner, S.A.; Scholz, C.; Gummesson, B.; Beli, P.; Nystrom, T.; Choudhary, C. Acetyl-phosphate is a critical determinant of lysine acetylation in E. coli. Mol. Cell 2013, 51, 265–272. [Google Scholar] [CrossRef]
- Erb, T.J. Carboxylases in Natural and Synthetic Microbial Pathways. Appl. Env. Microbiol. 2011, 77, 8466–8477. [Google Scholar] [CrossRef] [PubMed]
- Eidles, L. Regulation of carbohydrate metabolism in Rhodopseudomonas capsulata. Ph.D. Thesis, University of California, Davis, CA, USA, 1969; p. 398.
- Herter, S.M.; Kortluke, C.M.; Drews, G. Complex I of Rhodobacter capsulatus and its role in reverted electron transport. Arch. Microbiol. 1998, 169, 98–105. [Google Scholar] [CrossRef]
- Golomysova, A.; Gomelsky, M.; Ivanov, P.S. Flux balance analysis of photoheterotrophic growth of purple nonsulfur bacteria relevant to biohydrogen production. Int. J. Hydrogen Energy 2010, 35, 12751–12760. [Google Scholar] [CrossRef]
- Yilmaz, L.S.; Kontur, W.S.; Sanders, A.P.; Sohmen, U.; Donohue, T.J.; Noguera, D.R. Electron Partitioning During Light- and Nutrient-Powered Hydrogen Production by Rhodobacter sphaeroides. BioEnergy Res. 2010, 3, 55–66. [Google Scholar] [CrossRef]
- Hauf, W.; Schlebusch, M.; Huge, J.; Kopka, J.; Hagemann, M.; Forchhammer, K. Metabolic changes in Synechocystis PCC6803 upon nitrogen-starvation: Excess NADPH sustains polyhydroxybutyrate accumulation. Metabolites 2013, 3, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Kondrat’eva ENKrasil’nikova, E.N. Fototrofnye bakterii kak producenty poli-β-gidroksibutirata. Prikl. Biohim. I Mikrobiol. 1989, 25, 785–789. [Google Scholar]
- Mas, J.; Van Gemerden, H. Storage products in purple and green sulfur bacteria. In Anoxygenic Photosynthetic Bacteria; Blankenship, R.E., Madigan, M.T., Bauer, C.E., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; pp. 973–990. [Google Scholar]
- Schada von Borzyskowski, L.; Bernhardsgrutter, I.; Erb, T.J. Biochemical unity revisited: Microbial central carbon metabolism holds new discoveries, multi-tasking pathways, and redundancies with a reason. Biol. Chem. 2020, 401, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
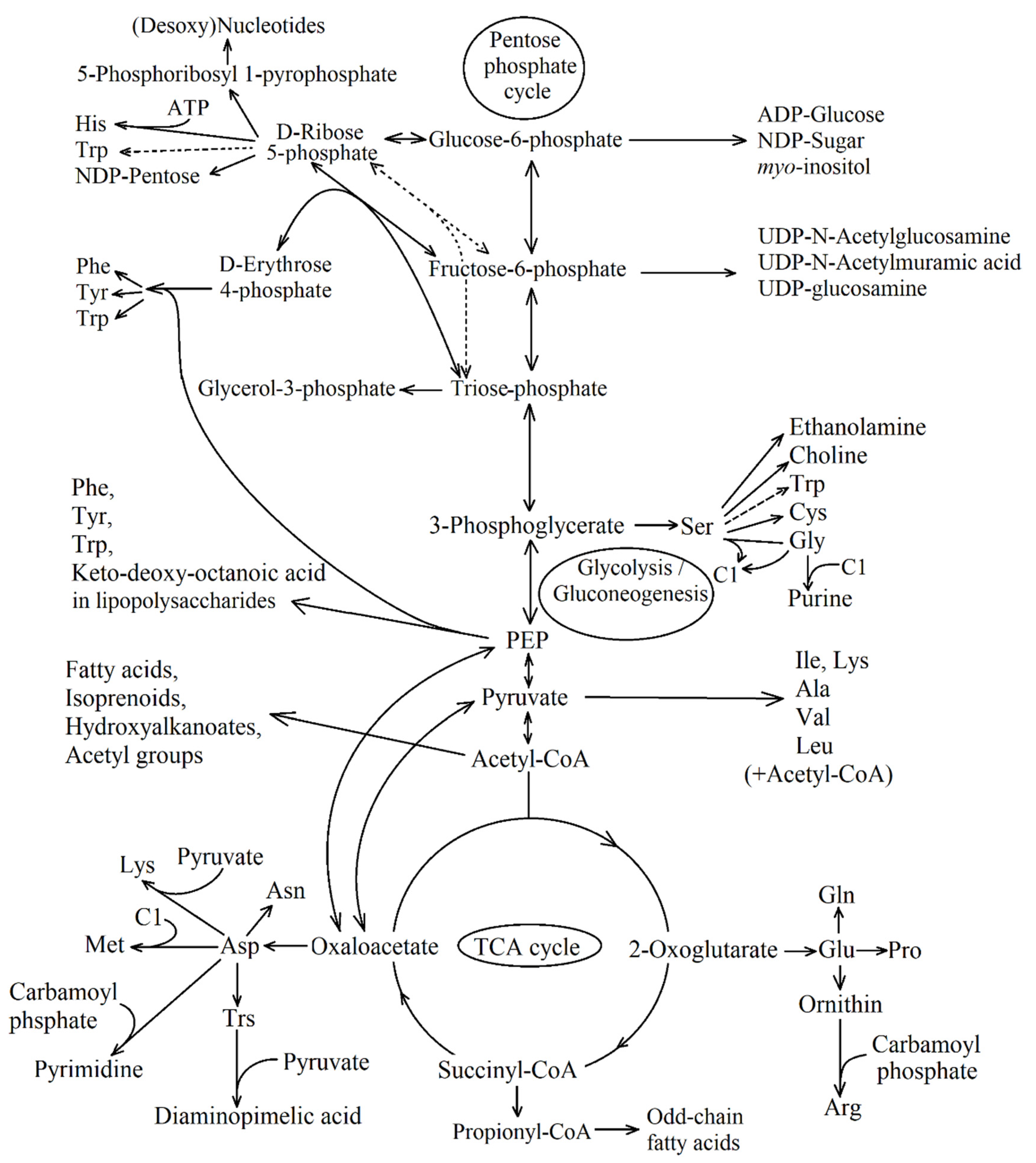
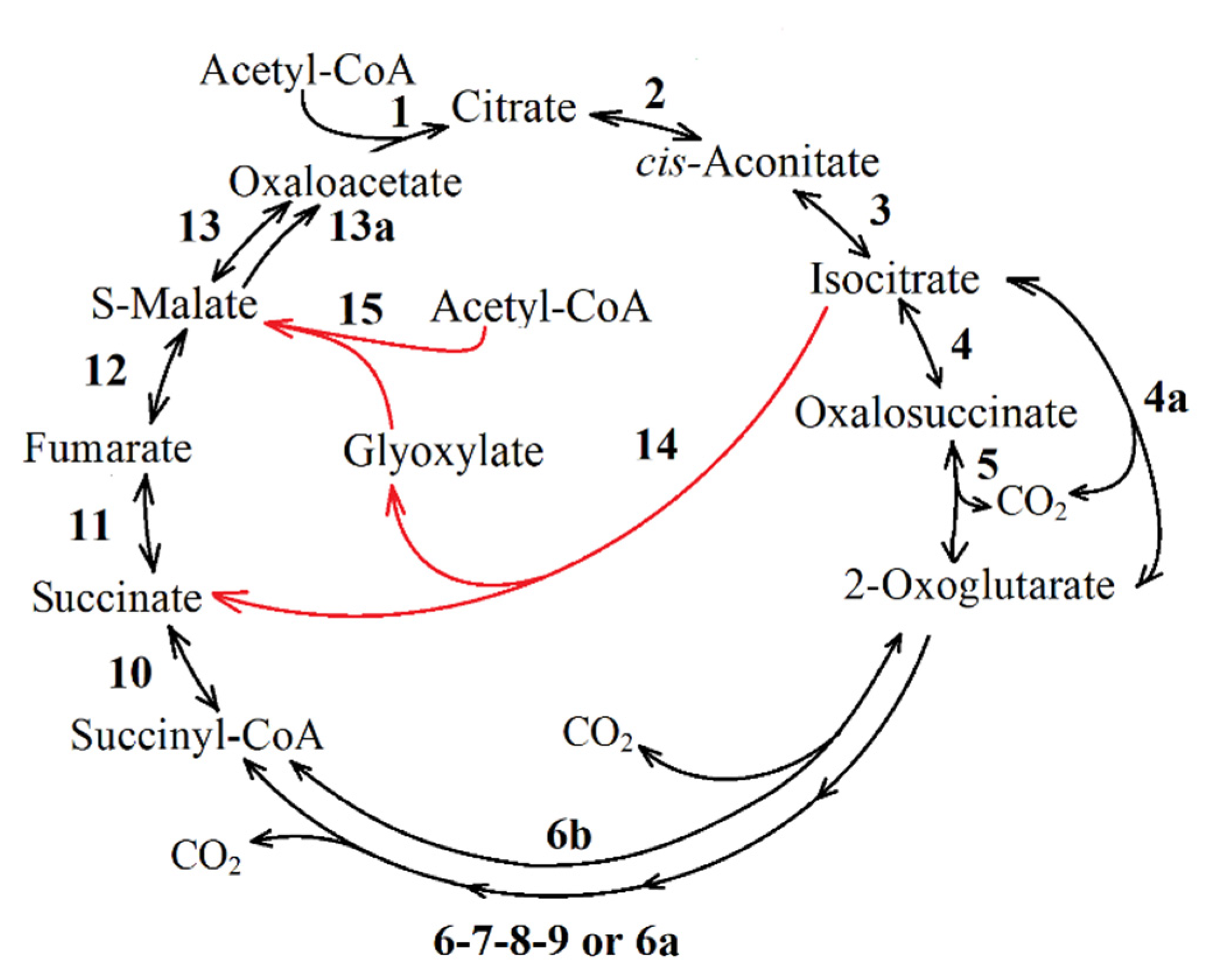
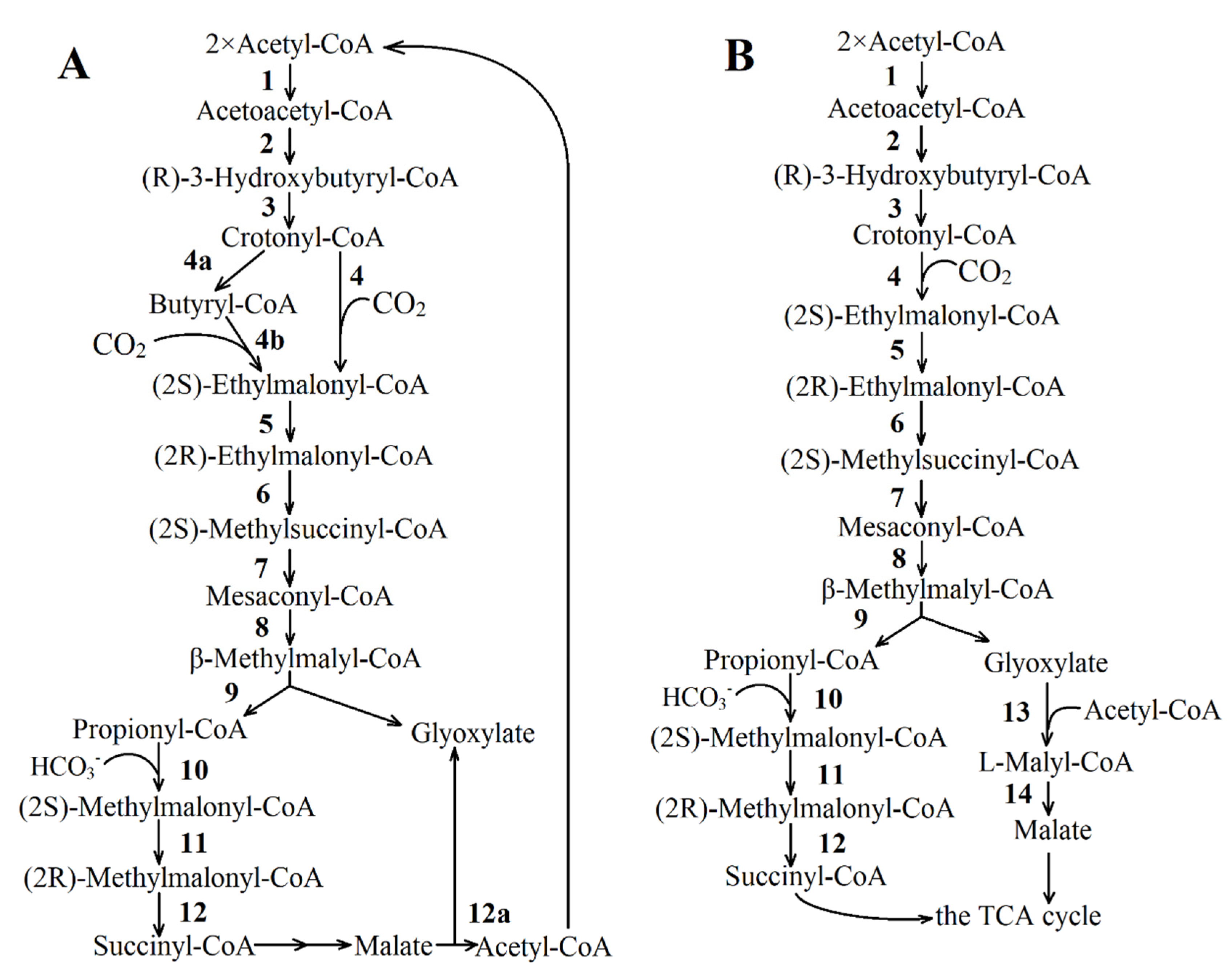
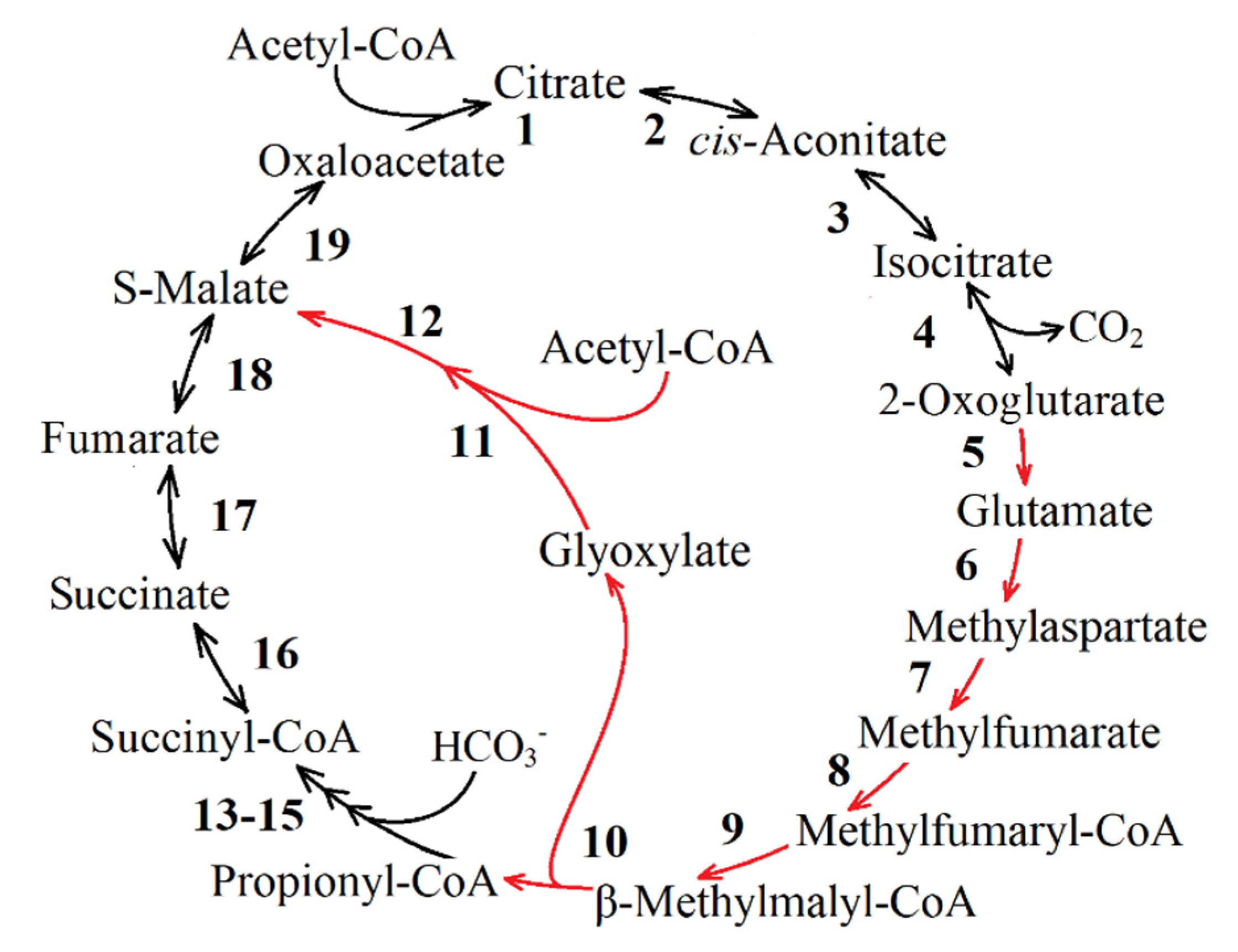
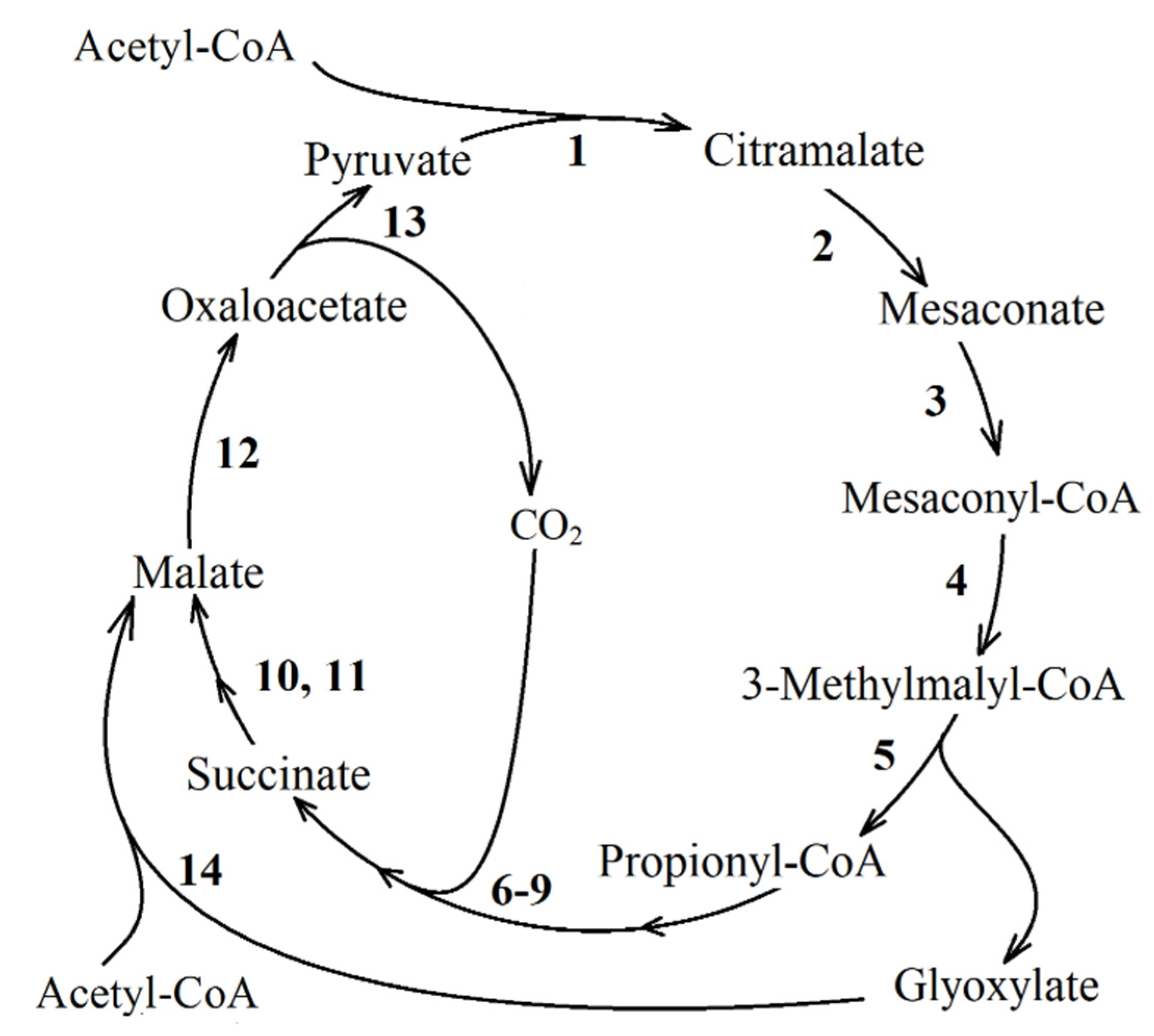
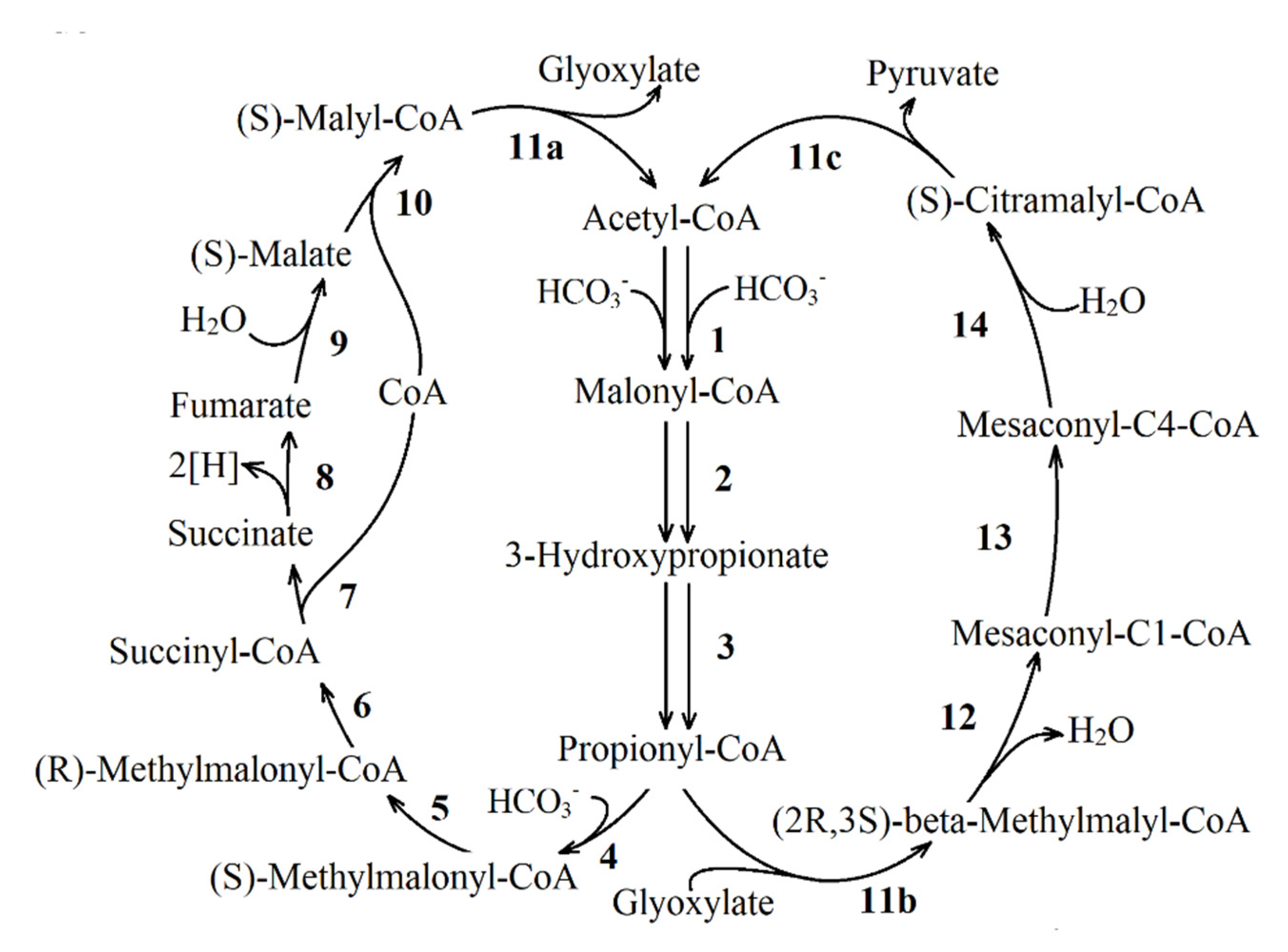
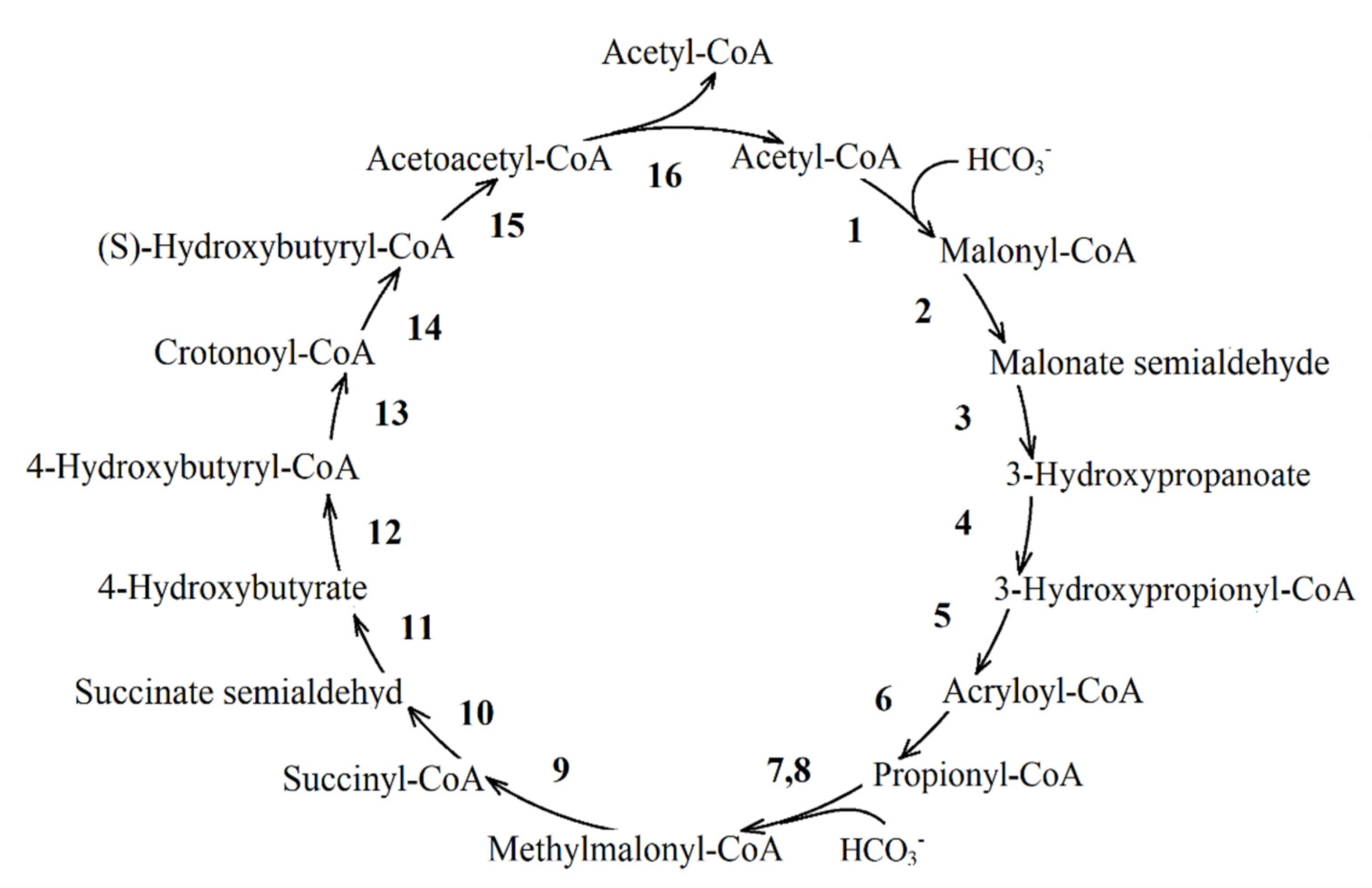
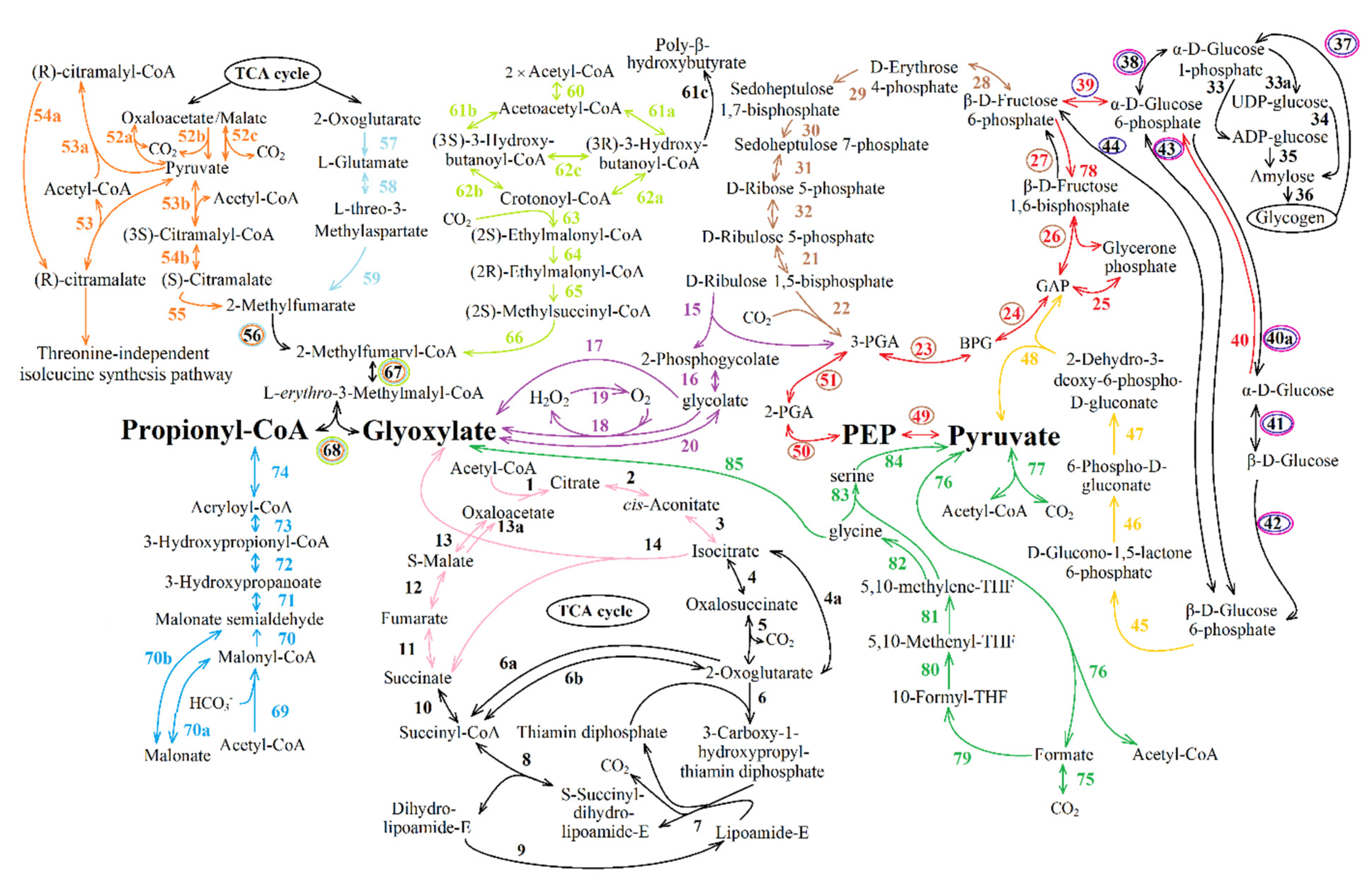
| Reaction Number in Figure 8 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 1 | K01647 citrate synthase [EC:2.3.3.1] |
| 2, 3 | K01681 aconitate hydratase [EC:4.2.1.3] |
| 4, 5 | K00031 isocitrate dehydrogenase [EC:1.1.1.42] |
| 4, 5/1 | K00906 isocitrate dehydrogenase kinase/phosphatase [EC:2.7.11.5 3.1.3.-] |
| 4a | K00030 isocitrate dehydrogenase (NAD+) [EC:1.1.1.41] |
| 4a | K17753 isocitrate—homoisocitrate dehydrogenase [EC:1.1.1.286] |
| 6, 7 | K00164 2-oxoglutarate dehydrogenase E1 component [EC:1.2.4.2] |
| 8 | K00658 2-oxoglutarate dehydrogenase E2 component (dihydrolipoamide succinyltransferase) [EC:2.3.1.61] |
| 9 | K00382 dihydrolipoamide dehydrogenase [EC:1.8.1.4] |
| 6a | K00174 2-oxoglutarate/2-oxoacid ferredoxin oxidoreductase subunit alpha [EC:1.2.7.3 1.2.7.11] |
| 6b | K00175 2-oxoglutarate/2-oxoacid ferredoxin oxidoreductase subunit beta [EC:1.2.7.3 1.2.7.11] |
| 10 | K01902 succinyl-CoA synthetase alpha subunit [EC:6.2.1.5] |
| K01903 succinyl-CoA synthetase beta subunit [EC:6.2.1.5] | |
| 10 | K18118 succinyl-CoA:acetate CoA-transferase [EC:2.8.3.18] |
| 10 | K01899 succinyl-CoA synthetase alpha subunit [EC:6.2.1.4 6.2.1.5] |
| K01900 succinyl-CoA synthetase beta subunit [EC:6.2.1.4 6.2.1.5] | |
| 11 | K00239 succinate dehydrogenase/fumarate reductase, flavoprotein subunit [EC:1.3.5.1 1.3.5.4] |
| K00240 succinate dehydrogenase/fumarate reductase, iron-sulfur subunit [EC:1.3.5.1 1.3.5.4] | |
| K00242 succinate dehydrogenase/fumarate reductase, membrane anchor subunit | |
| K00241 succinate dehydrogenase/fumarate reductase, cytochrome b subunit | |
| 12 | K01679 fumarate hydratase, class II [EC:4.2.1.2] |
| 12 | K01676 fumarate hydratase, class I [EC:4.2.1.2] |
| 13 * | K00024 malate dehydrogenase [EC:1.1.1.37] |
| 13 * | K00116 malate dehydrogenase (quinone) [EC:1.1.5.4] |
| 14 | K01637 isocitrate lyase [EC:4.1.3.1] |
| Reaction Number in Figure 8 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 16 | K01091 phosphoglycolate phosphatase [EC:3.1.3.18] |
| 17 | No function number in KEGG; glycolate dehydrogenase [1.1.99.14] |
| 18 | K11473 glycolate oxidase iron-sulfur subunit |
| K11472 glycolate oxidase FAD binding subunit | |
| K00104 glycolate oxidase [EC:1.1.3.15] | |
| 19 | K03782 catalase-peroxidase [EC:1.11.1.21] |
| 19 | K03781 catalase [EC:1.11.1.6] |
| 20 | K00015 glyoxylate reductase [EC:1.1.1.26] |
| 20 | K12972 glyoxylate/hydroxypyruvate reductase [EC:1.1.1.79; 1.1.1.81] |
| Reaction Number in Figure 8 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 21 | K00855 phosphoribulokinase [EC: 2.7.1.19] |
| 15, 22 | K01601 ribulose-bisphosphate carboxylase large chain [EC: 4.1.1.39] |
| K01602 ribulose-bisphosphate carboxylase small chain [EC: 4.1.1.39] | |
| 23 | K00927 phosphoglycerate kinase [EC: 2.7.2.3] |
| 24 | K00134 glyceraldehyde 3-phosphate dehydrogenase [EC: 1.2.1.12] |
| 25 | K01803 triosephosphate isomerase (TIM) [EC: 5.3.1.1] |
| 26, 29 | K01623 fructose-bisphosphate aldolase, class I [EC: 4.1.2.13] |
| 26,29 | K01624 fructose-bisphosphate aldolase, class II [EC: 4.1.2.13] |
| 27 | K03841 fructose-1,6-bisphosphatase I [EC: 3.1.3.11] |
| 27, 30 | K11532 fructose-1,6-bisphosphatase II/sedoheptulose-1,7-bisphosphatase [EC: 3.1.3.11 3.1.3.37] |
| 28, 31 | K00615 transketolase [EC: 2.2.1.1] |
| 32 | K01807 ribose 5-phosphate isomerase A [EC: 5.3.1.6] |
| 32 | K01808 ribose 5-phosphate isomerase B [EC: 5.3.1.6] |
| 33 | K00975 glucose-1-phosphate adenylyltransferase [EC: 2.7.7.27] |
| 33a | K00963 UTP—glucose-1-phosphate uridylyltransferase [EC: 2.7.7.9] |
| 34 | K00693/K16150 glycogen synthase [EC: 2.4.1.11] |
| 34 | K00750 glycogenin [EC: 2.4.1.186] |
| 34 | K16153 glycogen phosphorylase/synthase [EC: 2.4.1.1 2.4.1.11] |
| 34 | K13679/K20812 granule-bound starch synthase [EC: 2.4.1.242] |
| 35 | K00703 starch synthase [EC: 2.4.1.21] |
| 35 | K13679 granule-bound starch synthase [EC: 2.4.1.242] |
| 35 | K20812/K13679 glycogen synthase [EC: 2.4.1.242] |
| 36 | K00700 1,4-alpha-glucan branching enzyme [EC: 2.4.1.18 |
| 37 | K00688 glycogen phosphorylase [EC: 2.4.1.1] |
| 37 | K16153 glycogen phosphorylase/synthase [EC: 2.4.1.1 2.4.1.11] |
| 37 | K01196 glycogen debranching enzyme [EC: 2.4.1.25 3.2.1.33] |
| 37 | K02438 glycogen debranching enzyme [EC: 3.2.1.196] |
| 37 | K01200 pullulanase [EC: 3.2.1.41] |
| 38 | K01835 phosphoglucomutase [EC: 5.4.2.2] |
| 38 | K15779 phosphoglucomutase/phosphopentomutase [EC: 5.4.2.2 5.4.2.7] |
| 38 | K15778 phosphomannomutase/phosphoglucomutase [EC: 5.4.2.8 5.4.2.2] |
| 39, 43, 44 | K01810 glucose-6-phosphate isomerase [EC: 5.3.1.9] |
| 39 | K00688 glycogen phosphorylase [EC: 2.4.1.1] |
| 39 | K16153 glycogen phosphorylase/synthase [EC: 2.4.1.1 2.4.1.11] |
| 39 | K01196 glycogen debranching enzyme [EC: 2.4.1.25 3.2.1.33] |
| 40, 42 | K00845 glucokinase [EC: 2.7.1.2] |
| 40, 42 | K12407 glucokinase [EC: 2.7.1.2] |
| 40, 42 | K00844 hexokinase [EC: 2.7.1.1] |
| 40a | K01084 glucose-6-phosphatase [EC: 3.1.3.9] |
| 41 | K01785 aldose 1-epimerase [EC: 5.1.3.3] |
| 45 | K00036 glucose-6-phosphate 1-dehydrogenase [EC: 1.1.1.49 1.1.1.363] |
| 45 | K19243 NAD+ dependent glucose-6-phosphate dehydrogenase [EC: 1.1.1.388] |
| 46 | K01057, K07404 6-phosphogluconolactonase [EC: 3.1.1.31] |
| 47 | K01690 phosphogluconate dehydratase [EC: 4.2.1.12] |
| 48 | K01625 2-dehydro-3-deoxyphosphogluconate aldolase/(4S)-4-hydroxy-2-oxoglutarate aldolase [EC: 4.1.2.14 4.1.3.42] |
| 49 | K00873 pyruvate kinase [EC: 2.7.1.40] |
| 50 | K01689 enolase [EC: 4.2.1.11] |
| 51 | K15633 2,3-bisphosphoglycerate-independent phosphoglycerate mutase [EC: 5.4.2.12] |
| 51 | K01834 2,3-bisphosphoglycerate-dependent phosphoglycerate mutase [EC: 5.4.2.11] |
| 78 | K16370 6-phosphofructokinase 2 [EC: 2.7.1.11] |
| Reaction Number in Figure 8 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 75 | K15022 formate dehydrogenase (NADP+) beta subunit [EC: 1.17.1.10] |
| 75 | K00126 formate dehydrogenase subunit delta [EC: 1.17.1.9] |
| K00123 formate dehydrogenase major subunit [EC: 1.17.1.9] | |
| K00124 formate dehydrogenase iron-sulfur subunit | |
| K00127 formate dehydrogenase subunit gamma | |
| 76 | K00656 formate C-acetyltransferase [EC: 2.3.1.54] |
| 77 | K03737 pyruvate-ferredoxin/flavodoxin oxidoreductase [EC: 1.2.7.1 1.2.7.-] |
| - | K06212 formate transporter |
| 79 | K01938 formate—tetrahydrofolate ligase [EC 6.3.4.3] |
| 80 | K01500 methenyltetrahydrofolate cyclohydrolase [EC: 3.5.4.9] |
| 80,81 | K01491 methylenetetrahydrofolate dehydrogenase (NADP+)/methenyltetrahydrofolate cyclohydrolase [EC: 1.5.1.5 3.5.4.9] |
| 81 | K00300 methylenetetrahydrofolate/methylenetetrahydromethanopterin dehydrogenase (NADP+) [EC: 1.5.1.5 1.5.1.-] |
| - | No function number in KEGG; ammonia importer |
| 82a | K00605 aminomethyltransferase [2.1.2.10] |
| 82b | K00282 glycine dehydrogenase subunit 1 [EC: 1.4.4.2] |
| 82b | K00283 glycine dehydrogenase subunit 2 [EC: 1.4.4.2] |
| 82c | K00382 dihydrolipoamide dehydrogenase [1.8.1.4] |
| 83 | K00600 glycine hydroxymethyltransferase [2.1.2.1] |
| 84 | K17989 L-serine/L-threonine ammonia-lyase [4.3.1.19] |
| 85 | No function number in KEGG; glycine dehydrogenase (cytochrome); (EC: 1.4.2.1) |
| Reaction Number in Figure 8 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 60 | K00626 acetyl-CoA C-acetyltransferase [EC: 2.3.1.9] |
| 61a | K00023 acetoacetyl-CoA reductase [EC: 1.1.1.36] |
| 62a | K17865 3-hydroxybutyryl-CoA dehydratase [EC: 4.2.1.55] |
| 61b/62b | K01782 3-hydroxyacyl-CoA dehydrogenase/enoyl-CoA hydratase/3-hydroxybutyryl-CoA epimerase [EC: 1.1.1.35 4.2.1.17 5.1.2.3] |
| 61b/62b | No function number in KEGG; fatty acid oxidation complex, α-subunit (EC: 1.1.1.35; 4.2.1.17; 5.3.3.8) |
| 61b | No function number in KEGG; 3-hydroxyacyl-CoA dehydrogenase/3-hydroxy-2-methylbutyryl-CoA dehydrogenase (EC: 1.1.1.178; 1.1.1.35) |
| 61b | K00074 3-hydroxybutyryl-CoA dehydrogenase [EC: 1.1.1.157] |
| 62b | K01715 enoyl-CoA hydratase [EC: 4.2.1.17] |
| 61c | K03821 polyhydroxyalkanoate synthase subunit PhaC [EC: 2.3.1.-] |
| 62c | K01782 3-hydroxyacyl-CoA dehydrogenase/enoyl-CoA hydratase/3-hydroxybutyryl-CoA epimerase [EC: 1.1.1.35 4.2.1.17 5.1.2.3] |
| 62c | K01825 3-hydroxyacyl-CoA dehydrogenase/enoyl-CoA hydratase/3-hydroxybutyryl-CoA epimerase/enoyl-CoA isomerase [EC: 1.1.1.35 4.2.1.17 5.1.2.3 5.3.3.8] |
| 63 | K14446 crotonyl-CoA carboxylase/reductase [EC: 1.3.1.85] |
| 64 | K05606 methylmalonyl-CoA/ethylmalonyl-CoA epimerase [EC: 5.1.99.1] |
| 65 | K14447 ethylmalonyl-CoA mutase [EC: 5.4.99.63] |
| 66 | K14448 (2S)-methylsuccinyl-CoA dehydrogenase [EC: 1.3.8.12] |
| 67 | K14449 2-methylfumaryl-CoA hydratase [EC: 4.2.1.148] |
| 68,53 (Figure 8) and 2 (Figure 9) | K08691 malyl-CoA/(S)-citramalyl-CoA lyase [EC: 4.1.3.24 4.1.3.25] * |
| 3 (Figure 9) | K14451 (3S)-malyl-CoA thioesterase [EC: 3.1.2.30] * |
| Reaction Number in Figure 8 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 57 | K00260 glutamate dehydrogenase [EC: 1.4.1.2] |
| 57 | K00261 glutamate dehydrogenase (NAD(P)+) [EC: 1.4.1.3] |
| 57 | K15371 glutamate dehydrogenase [EC: 1.4.1.2] |
| 58 | K19268 methylaspartate mutase epsilon subunit [EC: 5.4.99.1] |
| 59 | K04835 methylaspartate ammonia-lyase [EC: 4.3.1.2] |
| 56 | K19280 succinyl-CoA:mesaconate CoA transferase [EC: 2.8.3.26] |
| 53 | K09011 (R)-citramalate synthase [EC: 2.3.1.182] |
| 53a | K18314 (R)-citramalyl-CoA lyase [EC:4.1.3.46] |
| 54a | K18313 succinyl-CoA—D-citramalate CoA-transferase [EC: 2.8.3.20] |
| 53b | K18292 (S)-citramalyl-CoA lyase [EC: 4.1.3.25] |
| 54b | No function number in KEGG; (S)-citramalate-CoA transferase (EC: 2.8.3.11) |
| 54b | No function number in KEGG; succinyl-CoA:(S)-malate/(S)-citramalate CoA-transferase (EC: 2.8.3.22) |
| 55 | No function number in KEGG; mesaconase (EC: 4.2.1.34) |
| 52a, 52c (Figure 8) and 19a (Figure 9) | K00027 malate dehydrogenase (oxaloacetate-decarboxylating) [EC: 1.1.1.38] |
| 52 b | K01571 oxaloacetate decarboxylase (Na+ extruding) subunit alpha [EC: 7.2.4.2] |
| 52 b | K01003 oxaloacetate decarboxylase [EC: 4.1.1.112] |
| 52c (Figure 8) and 19 (Figure 9) | K00028 malate dehydrogenase (decarboxylating) [EC: 1.1.1.39] |
| Reaction Number in Figure 8 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 69 | K02160 acetyl-CoA carboxylase biotin carboxyl carrier protein |
| K01961 acetyl-CoA carboxylase, biotin carboxylase subunit [EC: 6.4.1.2 6.3.4.14] | |
| K01962 acetyl-CoA carboxylase carboxyl transferase subunit alpha [EC: 6.4.1.2 2.1.3.15] | |
| K01963 acetyl-CoA carboxylase carboxyl transferase subunit beta [EC: 6.4.1.2 2.1.3.15] | |
| 70 | K14468 malonyl-CoA reductase/3-hydroxypropionate dehydrogenase (NADP+) [EC: 1.2.1.75 1.1.1.298] |
| 70 | K14468/K15017 malonyl-CoA reductase (malonate semialdehyde-forming) [EC: 1.2.1.75 1.1.1.298] |
| 70a | No function number in KEGG; malonate CoA-transferase [EC: 2.8.3.3] |
| 70a | K20511 malonyl-S-ACP:biotin-protein carboxyltransferase subunit MadD [EC: 2.1.3.10] |
| K20510 malonyl-S-ACP:biotin-protein carboxyltransferase subunit MadC [EC: 2.1.3.10] | |
| K13931 malonate decarboxylase delta subunit | |
| K13929 malonate decarboxylase alpha subunit [EC: 2.3.1.187] | |
| 70a | K01578 malonyl-CoA decarboxylase [EC: 4.1.1.9] |
| 70b | No function number in KEGG;malonate-semialdehyde dehydrogenase [EC: 1.2.1.15] |
| 71 | No function number in KEGG; 3-hydroxypropionate dehydrogenase [EC: 1.1.1.59] |
| 71 | K18602 malonic semialdehyde reductase [EC: 1.1.1.-] |
| 72, 73, 74 | K14469 acrylyl-CoA reductase (NADPH)/3-hydroxypropionyl-CoA dehydratase/3-hydroxypropionyl-CoA synthetase [EC: 1.3.1.84 4.2.1.116 6.2.1.36] |
| 72 | K18594 3-hydroxypropionyl-CoA synthetase (ADP-forming) [EC: 6.2.1.-] |
| 72 | K05605 3-hydroxyisobutyryl-CoA hydrolase [EC: 3.1.2.4] |
| 73 | K01782 3-hydroxyacyl-CoA dehydrogenase/enoyl-CoA hydratase/3-hydroxybutyryl-CoA epimerase [EC: 1.1.1.35 4.2.1.17 5.1.2.3] |
| 73 | No function number in KEGG; fatty acid oxidation complex, α-subunit [EC: 1.1.1.35; 4.2.1.17; 5.3.3.8] |
| 73 | K01715 enoyl-CoA hydratase [EC: 4.2.1.17] |
| 74 | K00249 acyl-CoA dehydrogenase [EC: 1.3.8.7] |
| 74 | K19745 acrylyl-CoA reductase (NADPH) [EC: 1.3.1.-] |
| Reaction Number in Figure 9 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 1 | K01638 malate synthase [EC: 2.3.3.9] |
| 2 (Figure 9) and 68,53 (Figure 8) | K08691 malyl-CoA/(S)-citramalyl-CoA lyase [EC: 4.1.3.24 4.1.3.25] * |
| 3 (Figure 9) | K14451 (3S)-malyl-CoA thioesterase [EC: 3.1.2.30] * |
| 28 | No function number in KEGG; aspartate-glyoxylate aminotransferase [EC: 2.6.1.35] |
| 29 | K18425 β-hydroxyaspartate aldolase [EC: 4.1.3.41] |
| 30 | No function number in KEGG; β-hydroxyaspartate dehydratase |
| 31 | No function number in KEGG; iminosuccinate reductase (EC: 1.4.1.-) |
| 32 | K01608 tartronate-semialdehyde synthase [EC: 4.1.1.47] |
| 33 | K00042 2-hydroxy-3-oxopropionate reductase [EC: 1.1.1.60] |
| 34 | K00865, K11529 glycerate 2-kinase [EC: 2.7.1.165] |
| 35 | K01689 enolase [EC: 4.2.1.11] |
| Reaction Number in Figure 9 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 19a, 19 and (Figure 9) 52a, 52c (Figure 8) | K00027 malate dehydrogenase (oxaloacetate-decarboxylating) [EC: 1.1.1.38] |
| 19a, 19 | K00029 malate dehydrogenase (oxaloacetate-decarboxylating) (NADP+) [EC: 1.1.1.40] |
| 19 (Figure 9) and 52c (Figure 8) | K00028 malate dehydrogenase (decarboxylating) [EC: 1.1.1.39] |
| 20 | K01610 phosphoenolpyruvate carboxykinase (ATP) [EC: 4.1.1.49] |
| 20 | K01596 phosphoenolpyruvate carboxykinase (GTP) [EC: 4.1.1.32] |
| 20 | K20370 phosphoenolpyruvate carboxykinase (diphosphate) [EC: 4.1.1.38] |
| 21 | K01595 phosphoenolpyruvate carboxylase [EC: 4.1.1.31] |
| 22 | K01958 pyruvate carboxylase [EC: 6.4.1.1] |
| 23 | K07246 tartrate dehydrogenase/decarboxylase/d-malate dehydrogenase [EC: 1.1.1.93 4.1.1.73 1.1.1.83] |
| 24 | No function number in KEGG; maleate hydratase EC 4.2.1.31 |
| 25 | K01799 maleate isomerase [EC: 5.2.1.1] |
| 26 | No function number in KEGG; (R)/(S)-malate racemase |
| 15, 2 (Figure 9) and 68, 53 (Figure 8) | K08691 malyl-CoA/(S)-citramalyl-CoA lyase [EC: 4.1.3.24 4.1.3.25] * |
| 16 | K18290 itaconyl-CoA hydratase [EC: 4.2.1.56] |
| 17 | K01902 succinyl-CoA synthetase alpha subunit [EC: 6.2.1.5] |
| 17 | No function number in KEGG; succinyl-CoA:itaconate CoA-transferase (EC: 2.8.3.-) |
| 18 | K17724 aconitate decarboxylase [EC: 4.1.1.6] |
| Reaction Number in Figure 9 | Function Number, Enzyme Name and Number (KEGG Orthology Database) |
|---|---|
| 4 | K01965 propionyl-CoA carboxylase alpha chain [EC: 6.4.1.3] |
| K01966 propionyl-CoA carboxylase beta chain [EC: 6.4.1.3 2.1.3.15] | |
| 4 | K03416; K17489; K17490 methylmalonyl-CoA carboxytransferase [EC: 2.1.3.1] |
| 5 | K05606 methylmalonyl-CoA/ethylmalonyl-CoA epimerase [EC: 5.1.99.1] |
| 6 | K01847 methylmalonyl-CoA mutase [EC: 5.4.99.2] |
| 7 | K14469, K15020 acrylyl-CoA reductases (NADPH) [EC: 1.3.1.84] |
| 7 | K01039, K01040 glutaconate CoA-transferases (EC: 2.8.3.12) |
| 7 | K20143 acrylyl-CoA reductase (NADH) [EC: 1.3.1.95] |
| 7 | No function number in KEGG; acyl-CoA-dehydrogenase |
| 8 | lactoyl-CoA- dehydratase (EC: 4.2.1.54) |
| 9 | propionate CoA-transferase (EC: 2.8.3.1) |
| 10 | K00016 L-lactate dehydrogenase [EC: 1.1.1.27] |
| 10 | K00101 L-lactate dehydrogenase (cytochrome) [EC: 1.1.2.3] |
| 10 | No function number in KEGG; malate-lactate transhydrogenase (EC: 1.1.99.7) |
| 11 | K01659 2-methylcitrate synthase [EC:2.3.3.5] |
| 12 | K01720 2-methylcitrate dehydratase [EC:4.2.1.79] |
| 12a | K20455 2-methylcitrate dehydratase (2-methyl-trans-aconitate forming) [EC:4.2.1.117] |
| 12b | No function number in KEGG; 2-methylaconitate isomerase (EC: 5.2.1.-) |
| 13 (2, 3 in Figure 8) | K01681 aconitate hydratase [EC:4.2.1.3] |
| 13 | K01682 aconitate hydratase 2/2-methylisocitrate dehydratase [EC: 4.2.1.3/EC: 4.2.1.99] |
| 14 | K03417 methylisocitrate lyase [EC:4.1.3.30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petushkova, E.; Mayorova, E.; Tsygankov, A. TCA Cycle Replenishing Pathways in Photosynthetic Purple Non-Sulfur Bacteria Growing with Acetate. Life 2021, 11, 711. https://doi.org/10.3390/life11070711
Petushkova E, Mayorova E, Tsygankov A. TCA Cycle Replenishing Pathways in Photosynthetic Purple Non-Sulfur Bacteria Growing with Acetate. Life. 2021; 11(7):711. https://doi.org/10.3390/life11070711
Chicago/Turabian StylePetushkova, Ekaterina, Ekaterina Mayorova, and Anatoly Tsygankov. 2021. "TCA Cycle Replenishing Pathways in Photosynthetic Purple Non-Sulfur Bacteria Growing with Acetate" Life 11, no. 7: 711. https://doi.org/10.3390/life11070711
APA StylePetushkova, E., Mayorova, E., & Tsygankov, A. (2021). TCA Cycle Replenishing Pathways in Photosynthetic Purple Non-Sulfur Bacteria Growing with Acetate. Life, 11(7), 711. https://doi.org/10.3390/life11070711





