Fascia Mobility, Proprioception, and Myofascial Pain
Abstract
1. Introduction
2. Fasciae, Proprioception, and Interoception
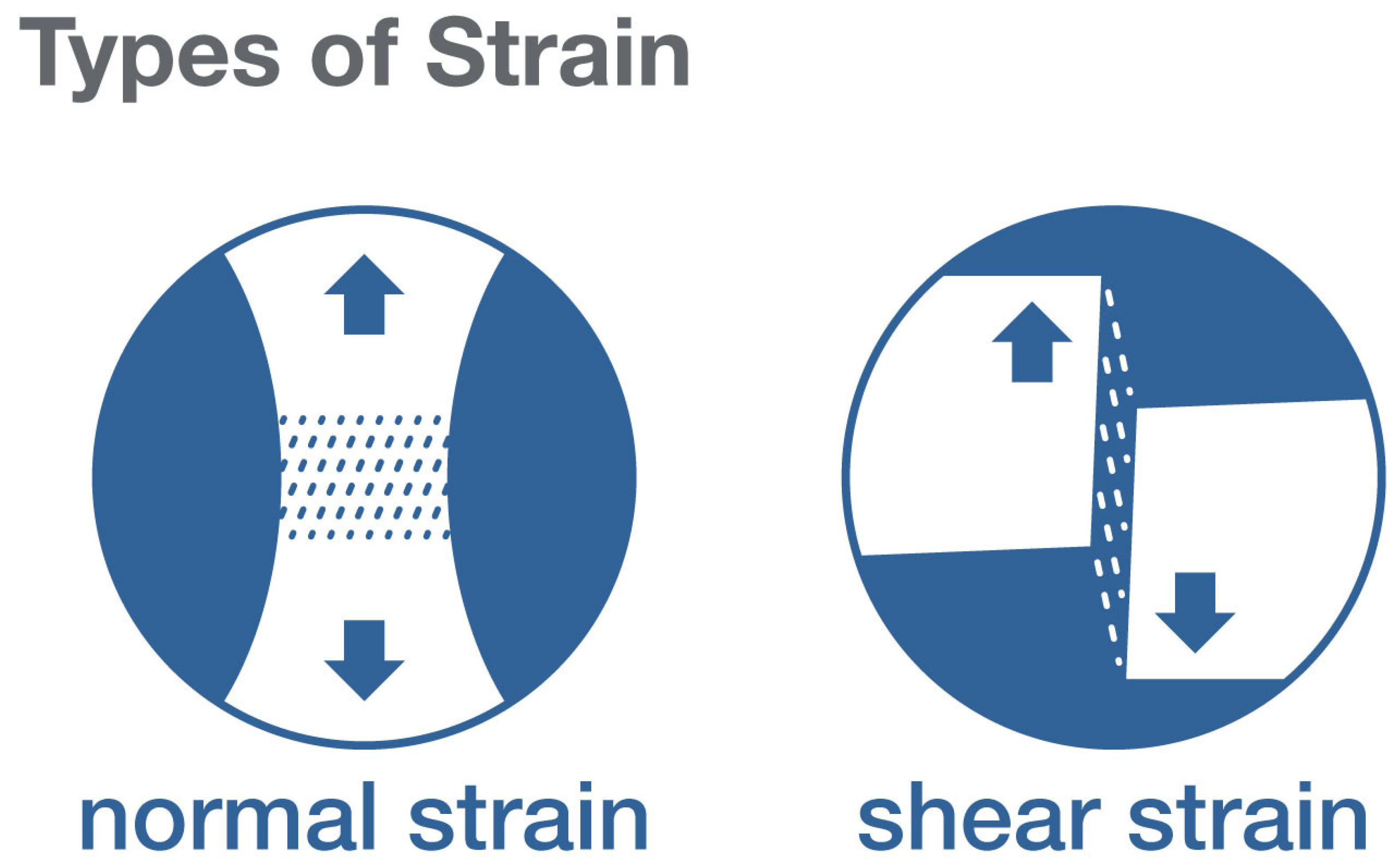
3. What Types of Stresses and Strains May Play a Role in Proprioceptive Signals from Fascia?
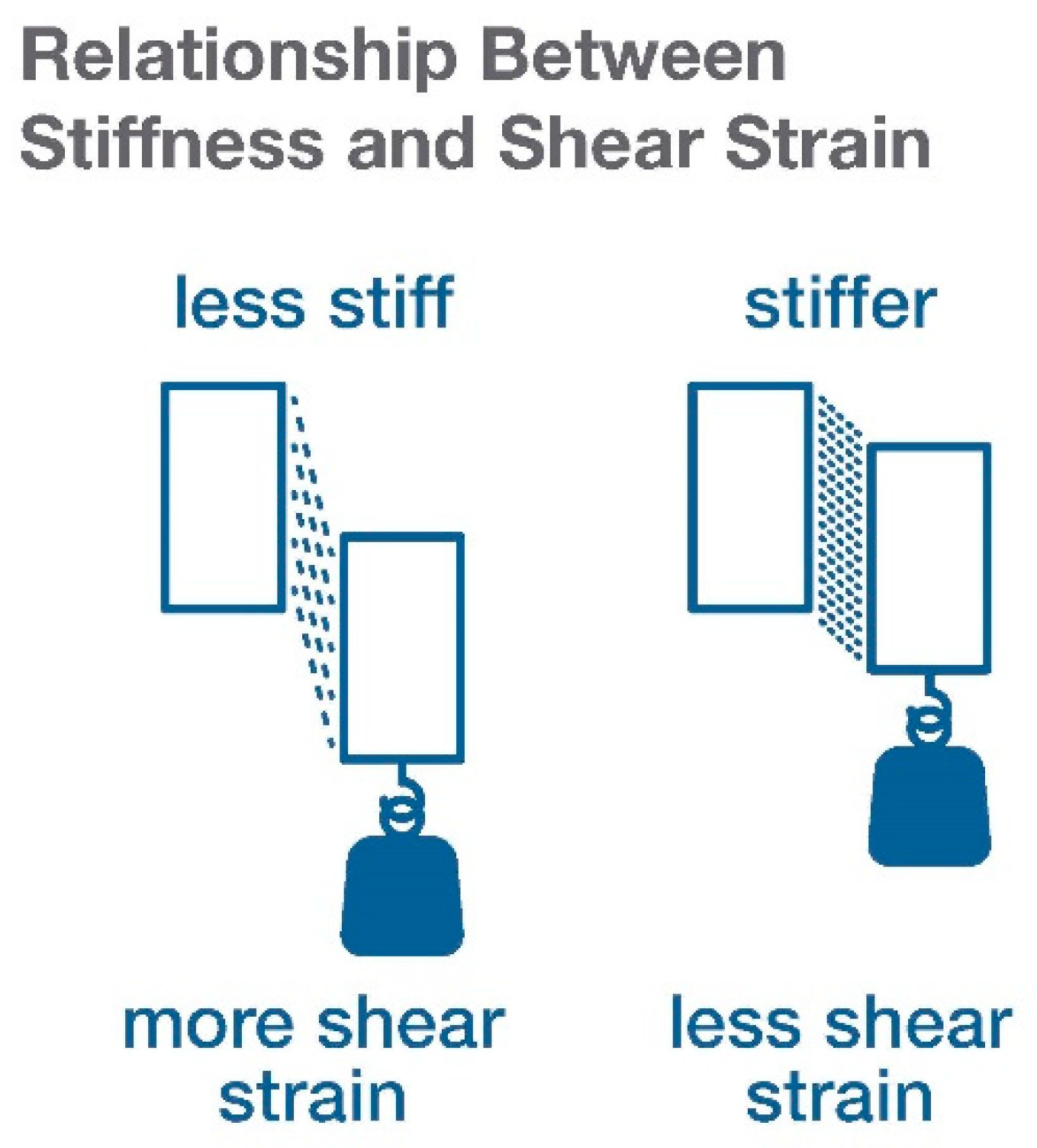
4. Tissue Stress, Strain, and Stiffness Measurement In Vivo
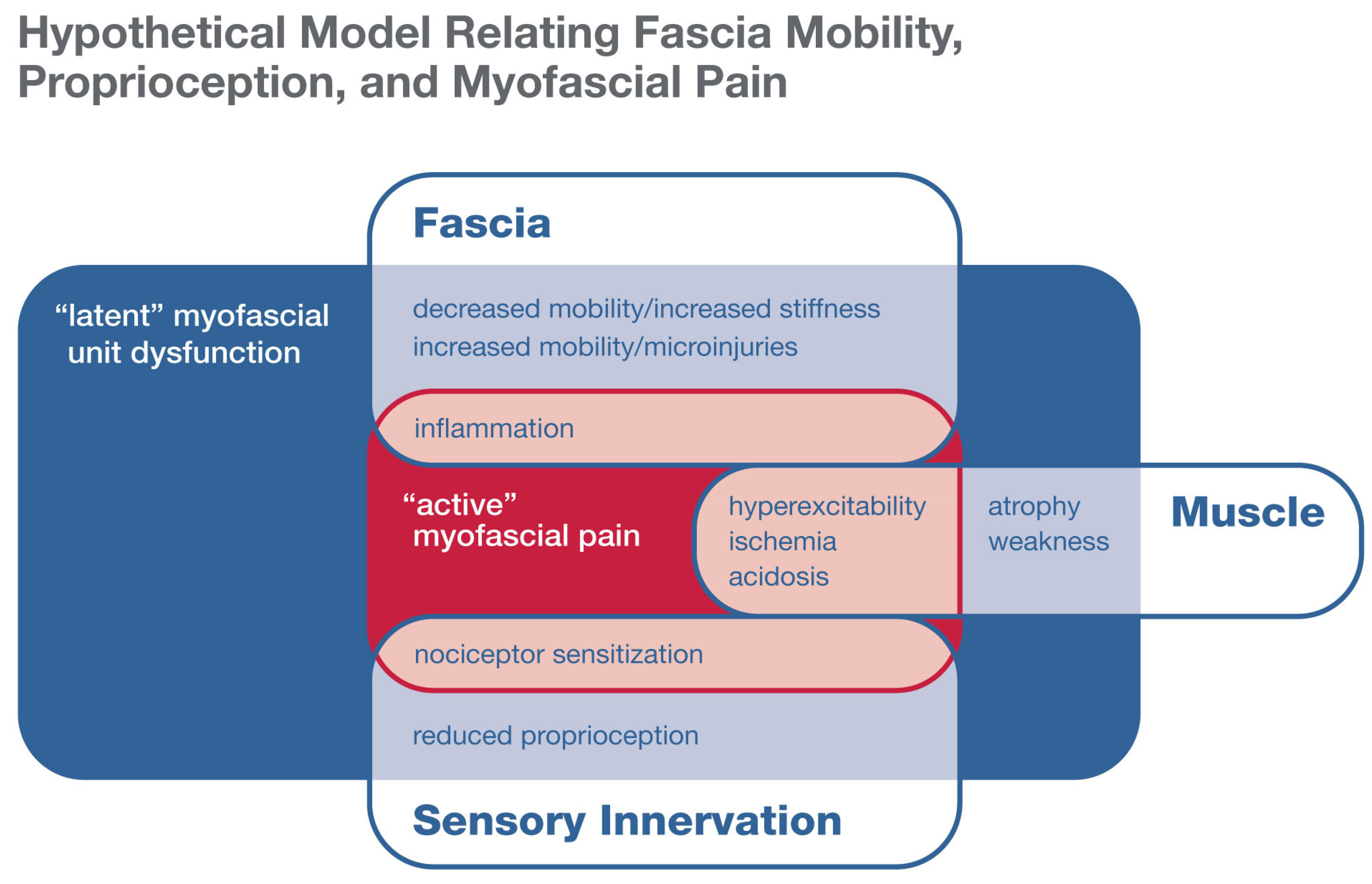
5. Fascia Mobility and Myofascial Pain
6. Does Generalized Hypo- or Hypermobility Predispose One to, or Protect from, Myofascial Pain?
7. What Role Could Proprioception Play in Connective Tissue Hyper- or Hypomobility Syndromes?
8. Implications for Personalized Treatment
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stecco, C.; Porzionato, A.; Lancerotto, L.; Stecco, A.; Macchi, V.; Day, J.A.; De Caro, R. Histological study of the deep fasciae of the limbs. J. Bodyw. Mov. Ther. 2008, 12, 225–230. [Google Scholar] [CrossRef]
- Langevin, H.M.; Huijing, P.A. Communicating about fascia: History, pitfalls, and recommendations. Int. J. Ther. Massage Bodyw. 2009, 2, 3–8. [Google Scholar]
- Langevin, H.M.; Fox, J.R.; Koptiuch, C.; Badger, G.J.; Greenan-Naumann, A.C.; Bouffard, N.A.; Konofagou, E.E.; Lee, W.-N.; Triano, J.J.; Henry, S.M. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet. Disord. 2011, 12, 203. [Google Scholar] [CrossRef]
- Skootsky, S.A.; Jaeger, B.; Oye, R.K. Prevalence of myofascial pain in general internal medicine practice. West. J. Med. 1989, 151, 157–160. [Google Scholar] [PubMed]
- Fricton, J. Myofascial pain: Mechanisms to management. Oral. Maxillofac. Surg. Clin. N. Am. 2016, 28, 289–311. [Google Scholar] [CrossRef]
- National Institutes of Health. NIH HEAL Initiative Workshop on Myofascial Pain. Available online: https://www.nccih.nih.gov/news/events/nih-heal-initiative-workshop-on-myofascial-pain (accessed on 16 September 2020).
- National Institutes of Health. Neurocircuitry of Force-Based Manipulations. Available online: https://www.nccih.nih.gov/news/events/neurocircuitry-of-forcebased-manipulations (accessed on 17 September 2020).
- National Institutes of Health. The Science of Interoception and Its Roles in Nervous System Disorders. Available online: https://www.nccih.nih.gov/news/events/the-science-of-interoception-and-its-roles-in-nervous-system-disorders (accessed on 16 April 2019).
- Hoheisel, U.; Rosner, J.; Mense, S. Innervation changes induced by inflammation of the rat thoracolumbar fascia. Neuroscience 2015, 300, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Mense, S. Innervation of the thoracolumbar fascia. Eur. J. Transl. Myol. 2019, 29, 8297. [Google Scholar] [CrossRef] [PubMed]
- Stecco, C.; Pirri, C.; Fede, C.; Fan, C.; Giordani, F.; Stecco, L.; Foti, C.; De Caro, R. Dermatome and fasciatome. Clin. Anat. 2019, 32, 896–902. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.G.; Schloesser, D.; Arensdorf, A.M.; Simmons, J.M.; Cui, C.; Valentino, R.; Gnadt, J.W.; Nielsen, L.; St Hillaire-Clarke, C.; Spruance, V.; et al. The emerging science of interoception: Sensing, integrating, interpreting, and regulating signals within the self. Trends Neurosci. 2021, 44, 3–16. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Turvey, M.T.; Carello, C. Obtaining information by dynamic (effortful) touching. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2011, 366, 3123–3132. [Google Scholar] [CrossRef]
- Chesler, A.T.; Szczot, M.; Bharucha-Goebel, D.; Cěko, M.; Donkervoort, S.; Laubacher, C.; Hayes, L.H.; Alter, K.; Zampieri, C.; Stanley, C.; et al. The role of PIEZO2 in human mechanosensation. N. Engl. J. Med. 2016, 375, 1355–1364. [Google Scholar] [CrossRef] [PubMed]
- Case, L.K.; Liljencrantz, J.; Madian, N.; Necaise, A.; Tubbs, J.; McCall, M.; Bradson, M.L.; Szczot, M.; Pitcher, M.H.; Ghitani, N.; et al. Innocuous pressure sensation requires A-type afferents but not functional PIEZO2 channels in humans. Nat. Commun. 2021, 12, 657. [Google Scholar] [CrossRef]
- Mehling, W.E.; Gopisetty, V.; Daubenmier, J.; Price, C.J.; Hecht, F.M.; Stewart, A. Body awareness: Construct and self-report measures. PLoS ONE 2009, 4, e5614. [Google Scholar] [CrossRef] [PubMed]
- Langevin, H.M.; Churchill, D.L.; Wu, J.; Badger, G.J.; Yandow, J.A.; Fox, J.R.; Krag, M.H. Evidence of connective tissue involvement in acupuncture. FASEB J. 2002, 16, 872–874. [Google Scholar] [CrossRef]
- Wang, X.; Chan, S.-T.; Fang, J.; Nixon, E.E.; Liu, J.; Kwong, K.K.; Rosen, B.R.; Hui, K.K.S. Neural encoding of acupuncture needling sensations: Evidence from a FMRI study. Evid. Based Complement. Altern. Med. 2013, 2013, 483105. [Google Scholar] [CrossRef]
- Klingberg, F.; Hinz, B.; White, E.S. The myofibroblast matrix: Implications for tissue repair and fibrosis. J. Pathol. 2013, 229, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Elosegui-Artola, A. The extracellular matrix viscoelasticity as a regulator of cell and tissue dynamics. Curr. Opin. Cell Biol. 2021, 72, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [CrossRef]
- Stecco, C.; Pirri, C.; Fede, C.; Yuceosy, C.A.; De Caro, R.; Stecco, A. Fascial or muscle Stretching? A narrative review. Appl. Sci. 2021, 11, 307. [Google Scholar] [CrossRef]
- Fung, Y.-C. Biomechanics: Mechanical Properties of Living Tissues, 2nd ed.; Springer: New York, NY, USA, 1993. [Google Scholar]
- Pavan, P.G.; Stecco, A.; Stern, R.; Stecco, C. Painful connections: Densification versus fibrosis of fascia. Curr. Pain Headache Rep. 2014, 18, 441. [Google Scholar] [CrossRef]
- Pethő, Z.; Najder, K.; Bulk, E.; Schwab, A. Mechanosensitive ion channels push cancer progression. Cell Calcium 2019, 80, 79–90. [Google Scholar] [CrossRef]
- Broders-Bondon, F.; Ho-Bouldoires, T.H.N.; Fernandez-Sanchez, M.-E.; Farge, E. Mechanotransduction in tumor progression: The dark side of the force. J. Cell Biol. 2018, 217, 1571–1587. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.-H.; Aroush, D.R.-B.; Asnacios, A.; Chen, W.-C.; Dokukin, M.E.; Doss, B.L.; Durand-Smet, P.; Ekpenyong, A.; Guck, J.; Guz, N.V.; et al. A comparison of methods to assess cell mechanical properties. Nat. Methods 2018, 15, 491–498. [Google Scholar] [CrossRef]
- Ophir, J.; Alam, S.K.; Garra, B.S.; Kallel, F.; Konofagou, E.E.; Krouskop, T.; Merritt, C.R.B.; Righetti, R.; Souchon, R.; Srinivasan, S.; et al. Elastography: Imaging the elastic properties of soft tissues with ultrasound. J. Med. Ultrason. 2002, 29, 155. [Google Scholar] [CrossRef]
- Ormachea, J.; Parker, K.J. Elastography imaging: The 30-year perspective. Phys. Med. Biol. 2020, 65, 24TR06. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-A.; Chen, Y.-J.; Chang, K.-V.; Wu, W.-T.; Özçakar, L. Reliability of sonoelastography measurement of tongue muscles and its application on obstructive sleep apnea. Front. Physiol. 2021, 12, 394. [Google Scholar] [CrossRef]
- Chiu, Y.-H.; Chang, K.-V.; Chen, I.-J.; Wu, W.-T.; Özçakar, L. Utility of sonoelastography for the evaluation of rotator cuff tendon and pertinent disorders: A systematic review and meta-analysis. Eur. Radiol. 2020, 30, 6663–6672. [Google Scholar] [CrossRef] [PubMed]
- Manduca, A.; Bayly, P.J.; Ehman, R.L.; Kolipaka, A.; Royston, T.J.; Sack, I.; Sinkus, R.; Van Beers, B.E. MR elastrography: Principles, guidelines, and terminology. Magn. Reson. Med. 2021, 85, 2377–2390. [Google Scholar] [CrossRef]
- Bishop, J.H.; Fox, J.R.; Maple, R.; Loretan, C.; Badger, G.J.; Henry, S.M.; Vizzard, M.A.; Langevin, H.M. Ultrasound evaluation of the combined effects of thoracolumbar fascia injury and movement restriction in a porcine model. PLoS ONE 2016, 11, e0147393. [Google Scholar] [CrossRef]
- Yin, Z.; Lu, X.; Cohen, S.C.; Sui, Y.; Manduca, A.; Van Gompel, J.J.; Ehman, R.L.; Huston, J., 3rd. A new method for quantification and 3D visualization of brain tumor adhesion using slip interface imaging in patients with meningiomas. Eur. Radiol. 2021, 2021. [Google Scholar] [CrossRef]
- Menon, R.G.; Oswald, S.F.; Raghavan, P.; Regatte, R.R.; Stecco, A. T1ρ-mapping for musculoskeletal pain diagnosis: Case series of variation of water bound glycosaminoglycans quantification before and after fascial manipulation® in subjects with elbow pain. Int. J. Environ. Res. Public Health 2020, 17, 708. [Google Scholar] [CrossRef] [PubMed]
- Tesarz, J.; Hoheisel, U.; Wiedenhöfer, B.; Mense, S. Sensory innervation of the thoracolumbar fascia in rats and humans. Neuroscience 2011, 194, 302–308. [Google Scholar] [CrossRef]
- Simons, D.G. New views of myofascial trigger points: Etiology and diagnosis. Arch. Phys. Med. Rehabil. 2008, 89, 157–159. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.P.; Thaker, N.; Heimur, J.; Aredo, J.V.; Sikdar, S.; Gerber, L. Myofascial trigger points then and now: A historical and scientific perspective. PM R 2015, 7, 746–761. [Google Scholar]
- Weller, J.L.; Comeau, D.; Otis, J.A.D. Myofascial pain. Semin. Neurol. 2018, 38, 640–643. [Google Scholar]
- Stecco, A.; Gesi, M.; Stecco, C.; Stern, R. Fascial components of the myofascial pain syndrome. Curr. Pain Headache Rep. 2013, 17, 352. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Gallagher, S.; Massicotte, V.S.; Tytell, M.; Popoff, S.N.; Barr-Gillespie, A.E. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet. Disord. 2013, 14, 303. [Google Scholar] [CrossRef]
- Deyo, R.A.; Weinstein, J.N. Low back pain. N. Engl. J. Med. 2001, 344, 363–370. [Google Scholar] [CrossRef]
- Wassenaar, M.; van Rijn, R.M.; van Tulder, M.W.; Verhagen, A.P.; van der Windt, D.A.W.M.; Koes, B.W.; de Boer, M.R.; Ginai, A.Z.; Ostelo, R.W.J.G. Magnetic resonance imaging for diagnosing lumbar spinal pathology in adult patients with low back pain or sciatica: A diagnostic systematic review. Eur. Spine J. 2012, 21, 220–227. [Google Scholar] [CrossRef]
- Langevin, H.M. Reconnecting the brain with the rest of the body in musculoskeletal pain research. J. Pain. 2021, 22, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Mazza, D.F.; Boutin, R.D.; Chaudhari, A.J. Assessment of myofascial trigger points via imaging: A systematic review. Am. J. Phys. Med. Rehabil. 2021. [Google Scholar] [CrossRef]
- Stanley, S.; Balic, Z.; Hubmacher, D. Acromelic dysplasias: How rare musculoskeletal disorders reveal biological functions of extracellular matrix proteins. Ann. N. Y. Acad. Sci. 2021, 1490, 57–76. [Google Scholar] [CrossRef]
- Varjú, C.; Kumánovics, G.; Czirják, L.; Matucci-Cerinic, M.; Minier, T. Sclerodermalike syndromes: Great imitators. Clin. Dermatol. 2020, 38, 235–249. [Google Scholar] [CrossRef]
- Willems, L.M.; Kwakkenbos, L.; Leite, C.C.; Thombs, B.D.; van den Hoogen, F.H.J.; Maia, A.C.; Vlieland, T.P.M.V.; van den Ende, C.H.M. Frequency and impact of disease symptoms experienced by patients with systemic sclerosis from five European countries. Clin. Exp. Rheumatol. 2014, 32, S88–S93. [Google Scholar]
- Xiong, X.; Berrueta, L.; Urso, K.; Olenich, S.; Muskaj, I.; Badger, G.J.; Aliprantis, A.; Lafyatis, R.; Langevin, H.M. Stretching reduces skin thickness and improves subcutaneous tissue mobility in a murine model of systemic sclerosis. Front. Immunol. 2017, 8, 124. [Google Scholar] [CrossRef]
- Song, B.; Yeh, P.; Harrell, J. Systemic manifestations of Ehlers-Danlos syndrome. Proc. Bayl. Univ. Med. Cent. 2020, 34, 49–53. [Google Scholar] [CrossRef] [PubMed]
- Stern, C.M.; Pepin, M.J.; Stoler, J.M.; Kramer, D.E.; Spencer, S.A.; Stein, C.J. Musculoskeletal conditions in a pediatric population with Ehlers-Danlos syndrome. J. Pediatr. 2017, 181, 261–266. [Google Scholar] [CrossRef]
- Yucesoy, C.A.; Koopman, B.H.F.J.M.; Baan, G.C.; Grootenboer, H.J.; Huijing, P.A. Extramuscular myofascial force transmission: Experiments and finite element modeling. Arch. Physiol. Biochem. 2003, 111, 377–388. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wilke, J.; Schleip, R.; Yucesoy, C.A.; Banzer, W. Not merely a protective packing organ? A review of fascia and its force transmission capacity. J. Appl. Physiol. 2018, 124, 234–244. [Google Scholar]
- Smith, T.O.; Jerman, E.; Easton, V.; Bacon, H.; Armon, K.; Poland, F.; Macgregor, A.J. Do people with benign joint hypermobility syndrome (BJHS) have reduced joint proprioception? A systematic review and meta-analysis. Rheumatol. Int. 2013, 33, 2709–2716. [Google Scholar] [CrossRef] [PubMed]
- Haliloglu, G.; Becker, K.; Temucin, C.; Talim, B.; Küçükşahin, N.; Pergande, M.; Motameny, S.; Nürnberg, P.; Aydingoz, U.; Topaloglu, H.; et al. Recessive PIEZO2 stop mutation causes distal arthrogryposis with distal muscle weakness, scoliosis and proprioception defects. J. Hum. Genet. 2017, 62, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Alper, S.L. Genetic diseases of PIEZO1 and PIEZO2 dysfunction. Curr. Top. Membr. 2017, 79, 97–134. [Google Scholar] [PubMed]
- Chou, R.; Qaseem, A.; Snow, V.; Casey, D.; Cross, J.T., Jr.; Shekelle, P.; Owens, D.K.; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: A joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann. Intern. Med. 2007, 147, 478–491. [Google Scholar] [CrossRef]
- Bassel, M.; Hudson, M.; Baron, M.; Taillefer, S.S.; Mouthon, L.; Poiraudeau, S.; Poole, J.L.; Thombs, B.D. Physical and occupational therapy referral and use among systemic sclerosis patients with impaired hand function: Results from a Canadian national survey. Clin. Exp. Rheumatol. 2012, 30, 574–577. [Google Scholar]
- Cramer, H.; Quinker, D.; Schumann, D.; Wardle, J.; Dobos, G.; Lauche, R. Adverse effects of yoga: A national cross-sectional survey. BMC Complement. Altern. Med. 2019, 19, 190. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langevin, H.M. Fascia Mobility, Proprioception, and Myofascial Pain. Life 2021, 11, 668. https://doi.org/10.3390/life11070668
Langevin HM. Fascia Mobility, Proprioception, and Myofascial Pain. Life. 2021; 11(7):668. https://doi.org/10.3390/life11070668
Chicago/Turabian StyleLangevin, Helene M. 2021. "Fascia Mobility, Proprioception, and Myofascial Pain" Life 11, no. 7: 668. https://doi.org/10.3390/life11070668
APA StyleLangevin, H. M. (2021). Fascia Mobility, Proprioception, and Myofascial Pain. Life, 11(7), 668. https://doi.org/10.3390/life11070668




