Combination Treatment of Arazyme and Soy Leaf Extract Attenuates Hyperglycemia and Hepatic Steatosis in High-Fat Diet-Fed C57BL/6J Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of ESLs
2.2. Animals and Diets
2.3. Measurement of Biochemical Parameters
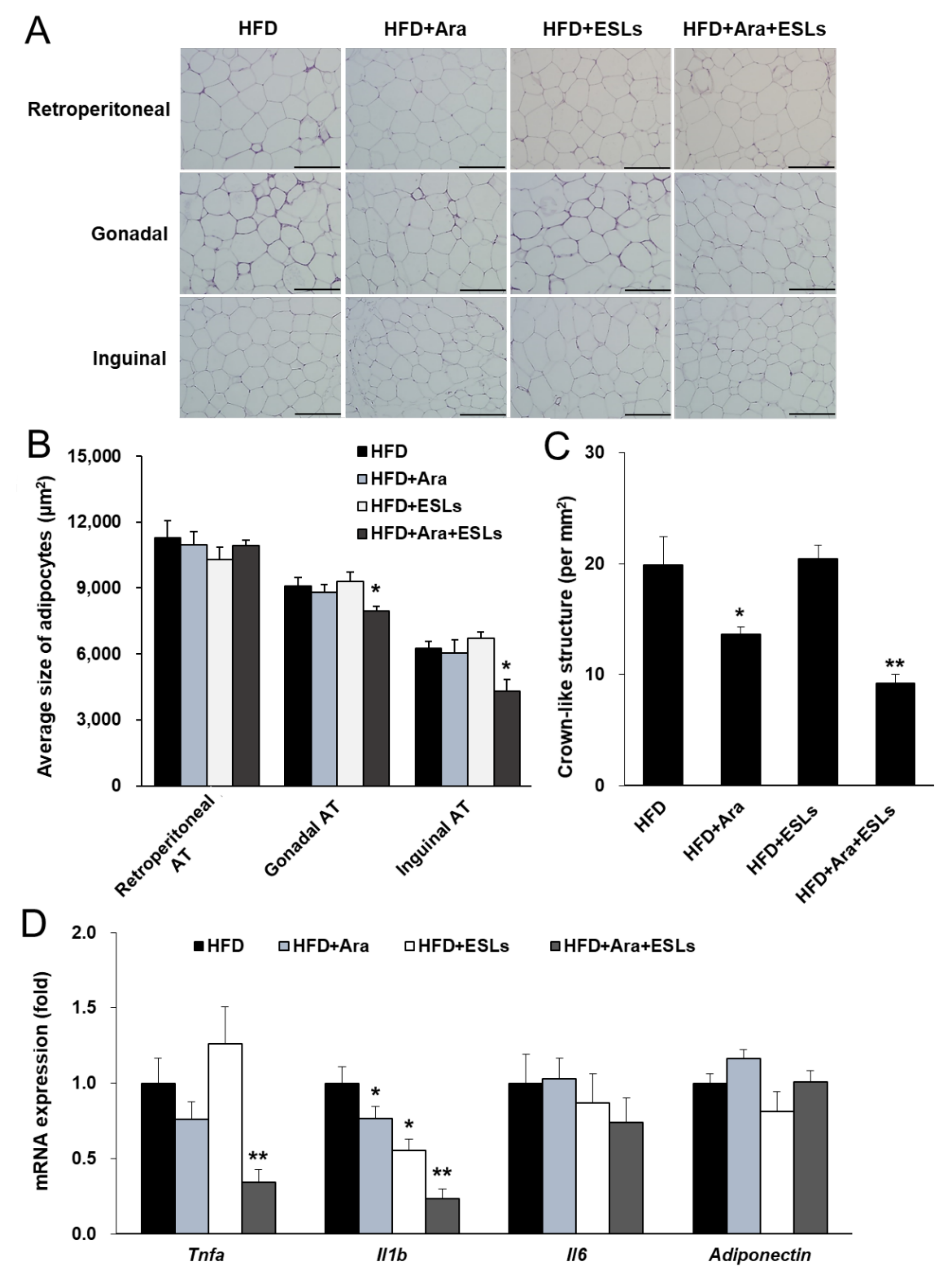
2.4. Histological Analysis of the White Adipose Tissue, Liver, and Pancreas
2.5. RNA Analysis
2.6. Western Blot Analysis
2.7. Data Analysis
3. Results
3.1. Combination of Arazyme and ESLs Reduced Body Weight and Adipocyte Hypertrophy
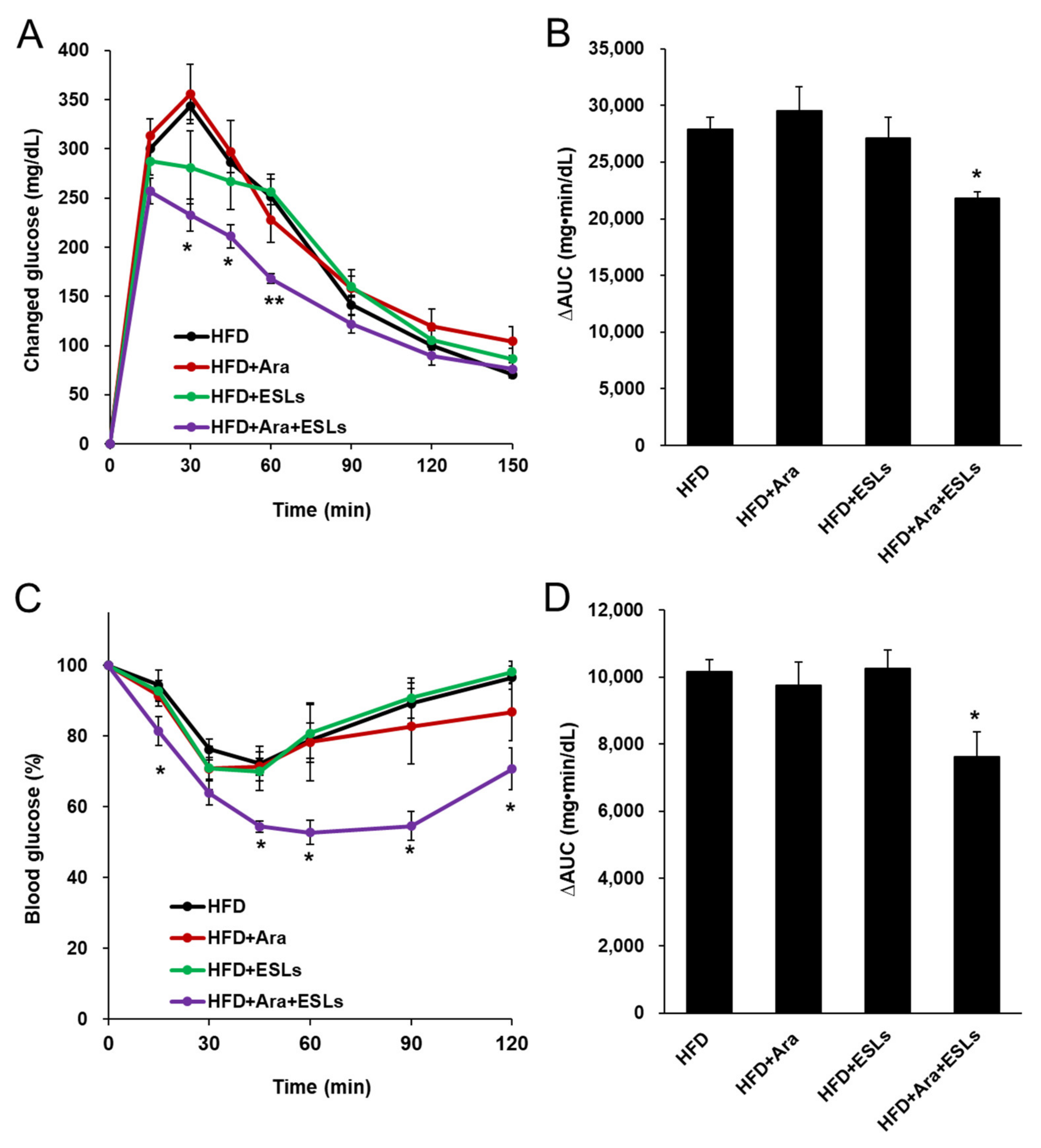
3.2. Arazyme and ESLs Supplementation Improved Plasma Glucose and Lipid Homeostasis
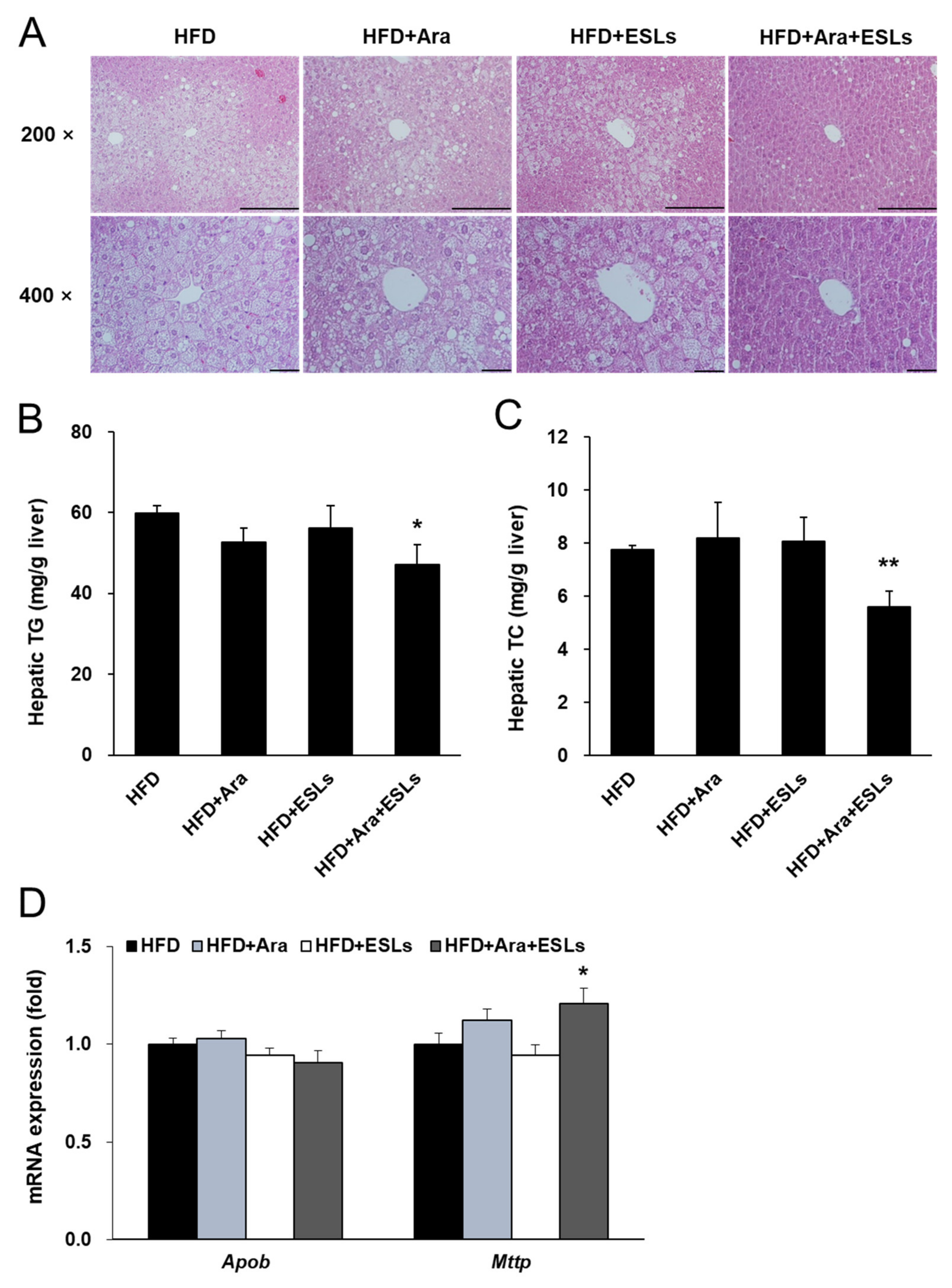
3.3. Arazyme and ESLs Supplementation Reduced HFD-Induced Hepatic Steatosis
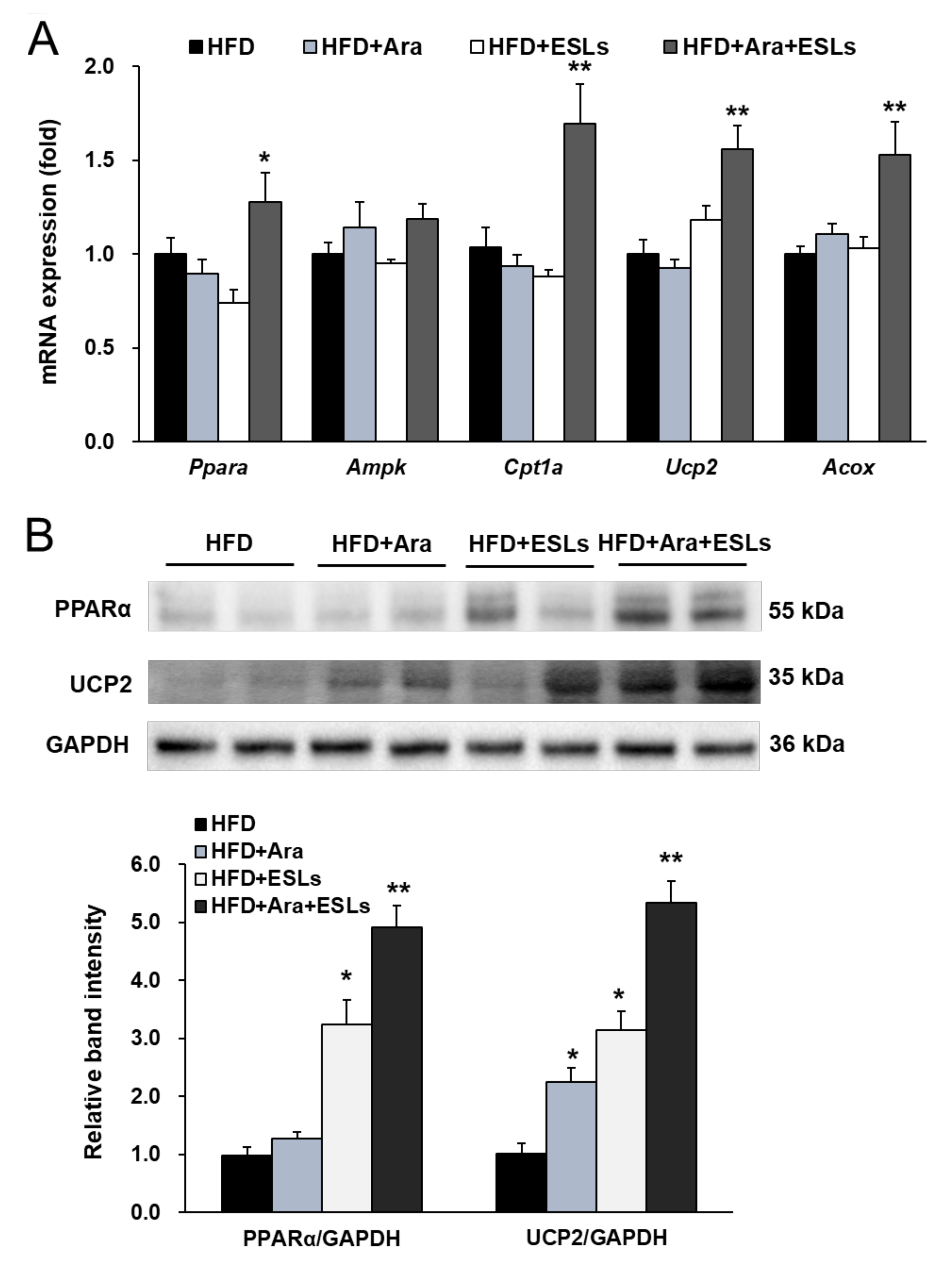
3.4. Arazyme and ESLs Supplementation Enhanced Hepatic β-Oxidation
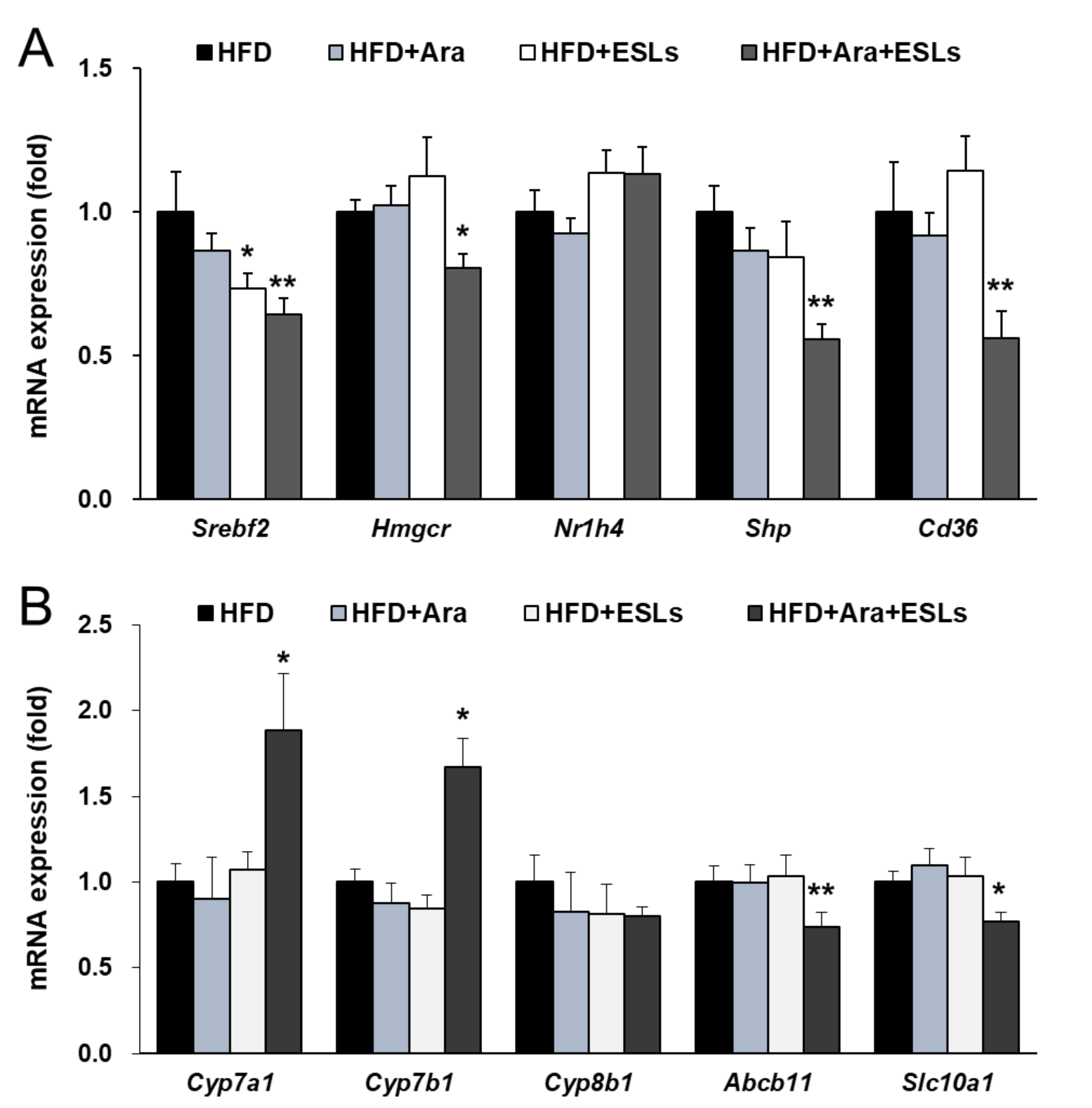
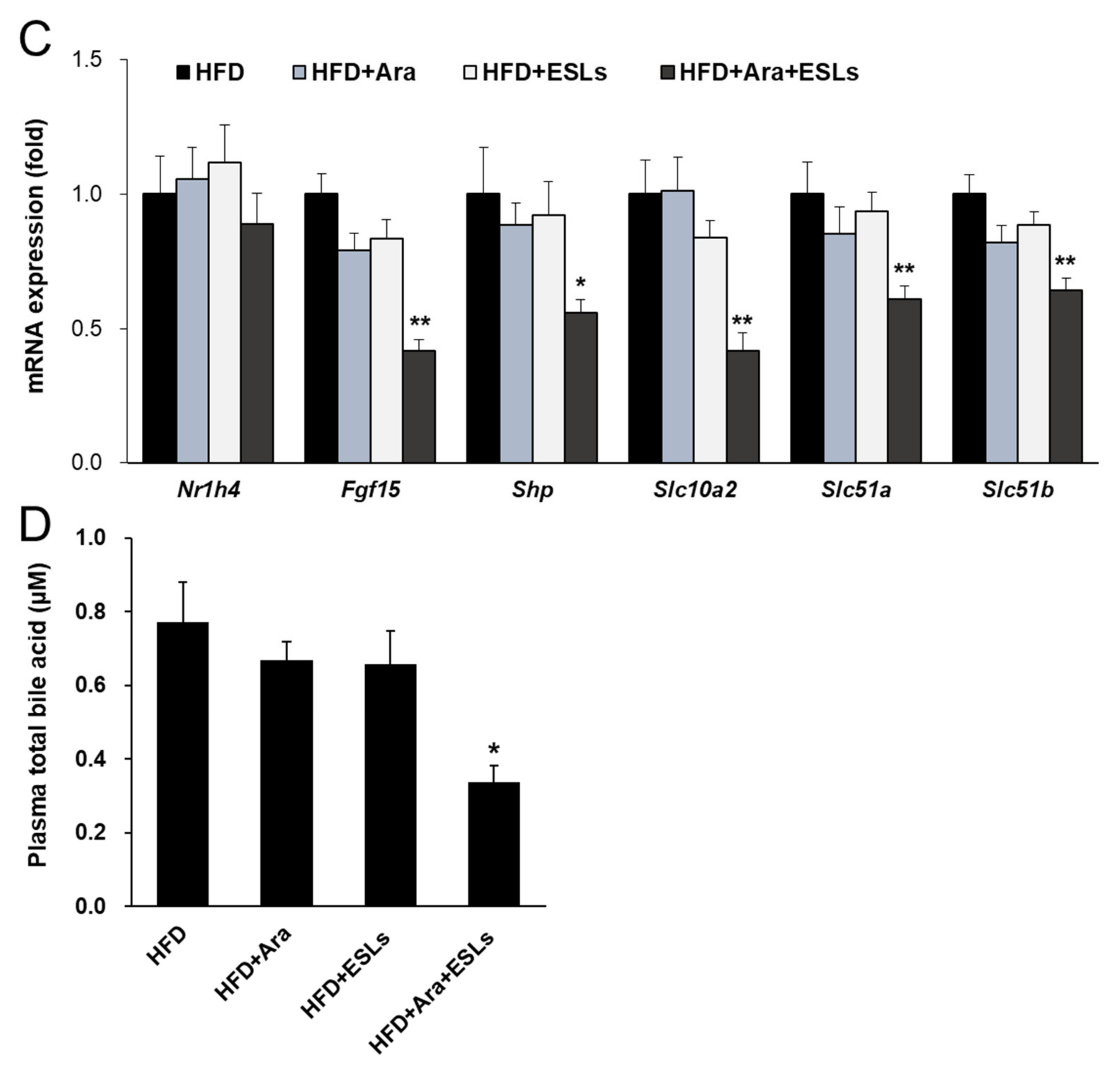
3.5. Arazyme and ESLs Supplementation Regulated Cholesterol and Bile Acid Metabolism
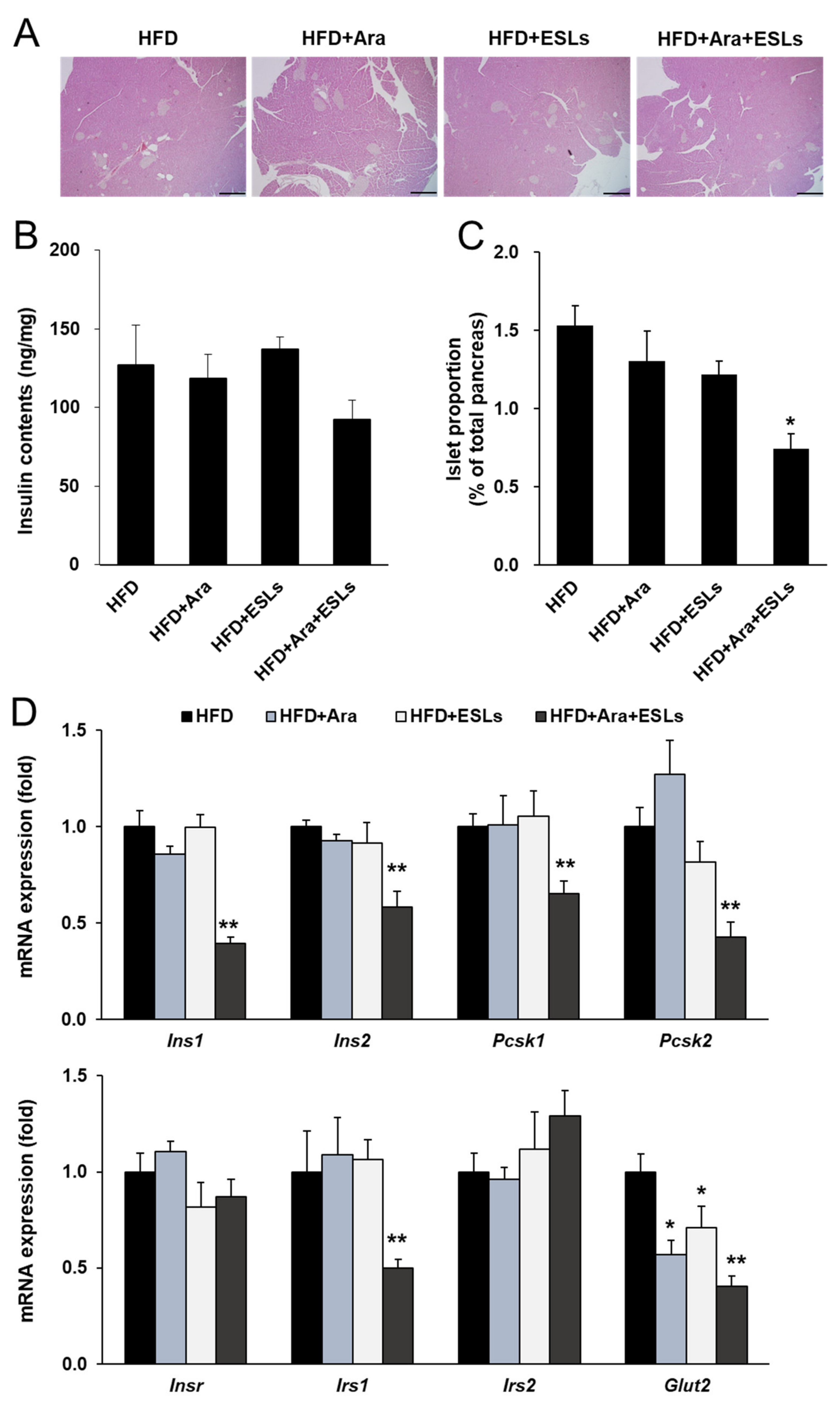
3.6. Arazyme and ESLs Supplementation Prevented HFD-Induced Islet Compensation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Loomba, R.; Sanyal, A.J. The global NAFLD epidemic. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 686–690. [Google Scholar] [CrossRef] [PubMed]
- Meldrum, D.R.; Morris, M.A.; Gambone, J.C. Obesity pandemic: Causes, consequences, and solutions-but do we have the will? Fertil. Steril. 2017, 107, 833–839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Divella, R.; Mazzocca, A.; Daniele, A.; Sabba, C.; Paradiso, A. Obesity, Nonalcoholic Fatty Liver Disease and Adipocytokines Network in Promotion of Cancer. Int. J. Biol. Sci. 2019, 15, 610–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Softic, S.; Cohen, D.E.; Kahn, C.R. Role of dietary fructose and hepatic de novo lipogenesis in fatty liver disease. Dig. Dis. Sci. 2016, 61, 1282–1293. [Google Scholar] [CrossRef] [Green Version]
- Shimano, H.; Sato, R. SREBP-regulated lipid metabolism: Convergent physiology—Divergent pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Ortega-Prieto, P.; Postic, C. Carbohydrate sensing through the transcription factor ChREBP. Front. Genet. 2019, 10, 472. [Google Scholar] [CrossRef] [Green Version]
- Krycer, J.R.; Sharpe, L.J.; Luu, W.; Brown, A.J. The Akt-SREBP nexus: Cell signaling meets lipid metabolism. Trends Endocrinol. Metab. 2010, 21, 268–276. [Google Scholar] [CrossRef]
- Jump, D.B.; Tripathy, S.; Depner, C.M. Fatty acid-regulated transcription factors in the liver. Annu. Rev. Nutr. 2013, 33, 249–269. [Google Scholar] [CrossRef] [Green Version]
- Hannah, W.N., Jr.; Harrison, S.A. Effect of weight loss, diet, exercise, and bariatric surgery on nonalcoholic fatty liver disease. Clin. Liver Dis. 2016, 20, 339–350. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, M.A.; Salas-Salvado, J.; Estruch, R.; Corella, D.; Fito, M.; Ros, E.; Predimed, I. Benefits of the mediterranean diet: Insights from the PREDIMED study. Prog. Cardiovasc. Dis. 2015, 58, 50–60. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Perez, C.; Segura-Carretero, A.; Contreras, M.D.M. Phenolic compounds as natural and multifunctional anti-obesity agents: A review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1212–1229. [Google Scholar] [CrossRef] [PubMed]
- Durazzo, A.; Lucarini, M.; Souto, E.B.; Cicala, C.; Caiazzo, E.; Izzo, A.A.; Novellino, E.; Santini, A. Polyphenols: A concise overview on the chemistry, occurrence, and human health. Phytother. Res. 2019, 33, 2221–2243. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Moustaid-Moussa, N.; Chen, L.; Mo, H.; Shastri, A.; Su, R.; Bapat, P.; Kwun, I.; Shen, C.L. Novel insights of dietary polyphenols and obesity. J. Nutr. Biochem. 2014, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Kim, T.K.; Yong, H.I.; Kim, Y.B.; Kim, H.W.; Choi, Y.S. Edible Insects as a Protein Source: A Review of Public Perception, Processing Technology, and Research Trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [Green Version]
- Bersanetti, P.A.; Park, H.Y.; Bae, K.S.; Son, K.H.; Shin, D.H.; Hirata, I.Y.; Juliano, M.A.; Carmona, A.K.; Juliano, L. Characterization of arazyme, an exocellular metalloprotease isolated from Serratia proteamaculans culture medium. Enz. Microb. Technol. 2005, 37, 574–581. [Google Scholar] [CrossRef]
- Park, J.K.; Jeong, D.H.; Park, H.Y.; Son, K.H.; Shin, D.H.; Do, S.H.; Yang, H.J.; Yuan, D.W.; Hong, I.H.; Goo, M.J.; et al. Hepatoprotective effect of Arazyme on CCl4-induced acute hepatic injury in SMP30 knock-out mice. Toxicology 2008, 246, 132–142. [Google Scholar] [CrossRef]
- Li, H.; Yoo, W.; Park, H.M.; Lim, S.Y.; Shin, D.H.; Kim, S.; Park, H.Y.; Jeong, T.S. Arazyme suppresses hepatic steatosis and steatohepatitis in diet-induced non-alcoholic fatty liver disease-like mouse model. Int. J. Mol. Sci. 2019, 20, 2325. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.S.; Ryu, R.; Seo, Y.R.; Jeong, T.S.; Shin, D.H.; Park, Y.B.; Kim, S.R.; Jung, U.J. The beneficial effect of soybean (Glycine max (L.) Merr.) leaf extracts in adults with prediabetes: A randomized placebo controlled trial. Food Funct. 2014, 5, 1621–1630. [Google Scholar] [CrossRef]
- Li, H.; Ji, H.S.; Kang, J.H.; Shin, D.H.; Park, H.Y.; Choi, M.S.; Lee, C.H.; Lee, I.K.; Yun, B.S.; Jeong, T.S. Soy leaf extract containing kaempferol glycosides and pheophorbides improves glucose homeostasis by enhancing pancreatic beta-cell function and suppressing hepatic lipid accumulation in db/db mice. J. Agric. Food Chem. 2015, 63, 7198–7210. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.J.; Varady, K.A. Are functional foods redefining nutritional requirements? Appl. Physiol. Nutr. Metab. 2008, 33, 118–123. [Google Scholar] [CrossRef] [PubMed]
- Hasler, C.M. Functional foods: Benefits, concerns and challenges—A position paper from the american council on science and health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, F.V.; Ferreira-Guimaraes, C.A.; Paschoalin, T.; Scutti, J.A.; Melo, F.M.; Silva, L.S.; Melo, A.C.; Silva, P.; Tiago, M.; Matsuo, A.L.; et al. A natural bacterial-derived product, the metalloprotease arazyme, inhibits metastatic murine melanoma by inducing MMP-8 cross-reactive antibodies. PLoS ONE 2014, 9, e96141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, F.V.; Melo, A.C.; de Melo, F.M.; Mourao-Sa, D.; Silva, P.; Berzaghi, R.; Herbozo, C.C.; Coelho-Dos-Reis, J.; Scutti, J.A.; Origassa, C.S.; et al. TLR4-mediated immunomodulatory properties of the bacterial metalloprotease arazyme in preclinical tumor models. Oncoimmunology 2016, 5, e1178420. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Yang, E.J.; Shin, D.H.; Son, K.H.; Park, H.Y.; Lee, J.S. Effect of arazyme on the lipopolysaccharide-induced inflammatory response in human endothelial cells. Mol. Med. Rep. 2014, 10, 1025–1029. [Google Scholar] [CrossRef] [Green Version]
- Dewidar, B.; Kahl, S.; Pafili, K.; Roden, M. Metabolic liver disease in diabetes—From mechanisms to clinical trials. Metabolism 2020, 111, 154299. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef]
- Venteclef, N.; Jakobsson, T.; Steffensen, K.R.; Treuter, E. Metabolic nuclear receptor signaling and the inflammatory acute phase response. Trends Endocrinol. Metab. 2011, 22, 333–343. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Kang, H.S.; Park, H.Y.; Moon, Y.A.; Kang, Y.N.; Oh, B.C.; Song, D.K.; Bae, J.H.; Im, S.S. PPARα-dependent Insig2a overexpression inhibits SREBP-1c processing during fasting. Sci. Rep. 2017, 7, 9958. [Google Scholar] [CrossRef] [Green Version]
- Francque, S.; Verrijken, A.; Caron, S.; Prawitt, J.; Paumelle, R.; Derudas, B.; Lefebvre, P.; Taskinen, M.R.; Van Hul, W.; Mertens, I.; et al. PPARα gene expression correlates with severity and histological treatment response in patients with non-alcoholic steatohepatitis. J. Hepatol. 2015, 63, 164–173. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Transcriptional mediators of lipid homeostasis. Cold Spring Harb. Symp. Quant. Biol. 2002, 67, 491–498. [Google Scholar] [CrossRef]
- Musso, G.; Gambino, R.; Cassader, M. Cholesterol metabolism and the pathogenesis of non-alcoholic steatohepatitis. Prog. Lipid. Res. 2013, 52, 175–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodwin, B.; Jones, S.A.; Price, R.R.; Watson, M.A.; McKee, D.D.; Moore, L.B.; Galardi, C.; Wilson, J.G.; Lewis, M.C.; Roth, M.E.; et al. A regulatory cascade of the nuclear receptors FXR, SHP-1, and LRH-1 represses bile acid biosynthesis. Mol. Cell 2000, 6, 517–526. [Google Scholar] [CrossRef]
- Jiang, C.; Xie, C.; Lv, Y.; Li, J.; Krausz, K.W.; Shi, J.; Brocker, C.N.; Desai, D.; Amin, S.G.; Bisson, W.H.; et al. Intestine-selective farnesoid X receptor inhibition improves obesity-related metabolic dysfunction. Nat. Commun. 2015, 6, 10166. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Owsley, E.; Matozel, M.; Hsu, P.; Novak, C.M.; Chiang, J.Y. Transgenic expression of cholesterol 7alpha-hydroxylase in the liver prevents high-fat diet-induced obesity and insulin resistance in mice. Hepatology 2010, 52, 678–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Iqbal, J.; Saha, P.K.; Liu, J.; Chan, L.; Hussain, M.M.; Moore, D.D.; Wang, L. Molecular characterization of the role of orphan receptor small heterodimer partner in development of fatty liver. Hepatology 2007, 46, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Ringseis, R.; Gessner, D.K.; Eder, K. The Gut-Liver Axis in the Control of Energy Metabolism and Food Intake in Animals. Annu. Rev. Anim. Biosci. 2020, 8, 295–319. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Jiang, C.; Krausz, K.W.; Li, Y.; Albert, I.; Hao, H.; Fabre, K.M.; Mitchell, J.B.; Patterson, A.D.; Gonzalez, F.J. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and decreased obesity. Nat. Commun. 2013, 4, 2384. [Google Scholar] [CrossRef]
- Rao, A.; Kosters, A.; Mells, J.E.; Zhang, W.; Setchell, K.D.; Amanso, A.M.; Wynn, G.M.; Xu, T.; Keller, B.T.; Yin, H.; et al. Inhibition of ileal bile acid uptake protects against nonalcoholic fatty liver disease in high-fat diet-fed mice. Sci. Transl. Med. 2016, 8, 357ra122. [Google Scholar] [CrossRef] [Green Version]
| HFD | HFD+Ara | HFD+ESLs | HFD+Ara+ESLs | |
|---|---|---|---|---|
| Body weight | ||||
| Initial (g) | 20.4 ± 0.6 | 20.2 ± 0.8 | 20.9 ± 0.6 | 20.3 ± 0.5 |
| Final (g) | 45.1 ± 1.0 ‡ | 44.1 ± 1.1 ‡ | 45.4 ± 1.5 ‡ | 40.8 ± 1.6 ‡,* |
| Weight gain (g) | 24.3 ± 1.0 | 24.1 ± 0.5 | 25.0 ± 1.5 | 20.9 ± 1.6 * |
| Liver (g) | 1.22 ± 0.08 | 1.17 ± 0.14 | 1.32 ± 0.13 | 1.07 ± 0.08 |
| Food intake (g/day) | 2.4 ± 0.2 | 2.4 ± 0.1 | 2.5 ± 0.2 | 2.4 ± 0.1 |
| WAT | ||||
| Retroperitoneal AT | 1.16 ± 0.07 | 1.17 ± 0.10 | 1.19 ± 0.09 | 1.07 ± 0.11 |
| Gonadal AT | 2.61 ± 0.16 | 2.58 ± 0.21 | 2.58 ± 0.15 | 2.28 ± 0.15 |
| Inguinal AT | 1.95 ± 0.18 | 1.72 ± 0.35 | 2.17 ± 0.23 | 1.45 ± 0.25 |
| Total WAT | 5.59 ± 0.32 | 5.30 ± 0.43 | 5.86 ± 0.37 | 4.99 ± 0.35 |
| HFD | HFD+Ara | HFD+ESLs | HFD+Ara+ESLs | |
|---|---|---|---|---|
| Glucose (mg/dL) | 162.9 ± 11.1 ‡ | 148.0 ± 11.7 ‡ | 131.8 ± 5.1 ‡,* | 116.7 ± 6.2 ‡,** |
| Insulin (ng/mL) | 2.32 ± 0.54 | 1.76 ± 0.37 | 2.05 ± 0.59 | 0.97 ± 0.27 * |
| HOMA-IR | 21.5 ± 6.4 | 15.6 ± 2.2 | 17.0 ± 5.2 | 7.6 ± 2.7 * |
| HbA1c (%) | 6.9 ± 0.5 | 6.5 ± 0.9 | 6.3 ± 0.7 | 5.4 ± 0.1 |
| TG (mg/dL) | 146.4 ± 5.2 | 157.2 ± 11.2 | 144.8 ± 11.2 | 141.3 ± 7.5 |
| TC (mg/dL) | 211.4 ± 9.1 | 205.1 ± 2.1 | 220.4 ± 7.0 | 185.1 ± 9.6 * |
| NEFA (mEq/L) | 2.25 ± 0.08 | 2.16 ± 0.11 | 2.22 ± 0.07 | 2.35 ± 0.10 |
| AST (IU/L) | 67.4 ± 4.4 | 62.4 ± 2.7 | 65.0 ± 5.7 | 54.9 ± 2.5 * |
| ALT (IU/L) | 31.5 ± 3.9 | 24.8 ± 3.5 | 29.0 ± 4.6 | 15.1 ± 0.8 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.; Cho, S.; Kang, A.; Shin, D.-H.; Park, H.-Y.; Jeong, T.-S. Combination Treatment of Arazyme and Soy Leaf Extract Attenuates Hyperglycemia and Hepatic Steatosis in High-Fat Diet-Fed C57BL/6J Mice. Life 2021, 11, 645. https://doi.org/10.3390/life11070645
Lee H, Cho S, Kang A, Shin D-H, Park H-Y, Jeong T-S. Combination Treatment of Arazyme and Soy Leaf Extract Attenuates Hyperglycemia and Hepatic Steatosis in High-Fat Diet-Fed C57BL/6J Mice. Life. 2021; 11(7):645. https://doi.org/10.3390/life11070645
Chicago/Turabian StyleLee, Hwa, Seona Cho, Anna Kang, Dong-Ha Shin, Ho-Yong Park, and Tae-Sook Jeong. 2021. "Combination Treatment of Arazyme and Soy Leaf Extract Attenuates Hyperglycemia and Hepatic Steatosis in High-Fat Diet-Fed C57BL/6J Mice" Life 11, no. 7: 645. https://doi.org/10.3390/life11070645
APA StyleLee, H., Cho, S., Kang, A., Shin, D.-H., Park, H.-Y., & Jeong, T.-S. (2021). Combination Treatment of Arazyme and Soy Leaf Extract Attenuates Hyperglycemia and Hepatic Steatosis in High-Fat Diet-Fed C57BL/6J Mice. Life, 11(7), 645. https://doi.org/10.3390/life11070645






