Effect of Increased Flexor Hallucis Longus Muscle Activity on Ground Reaction Force during Landing
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects
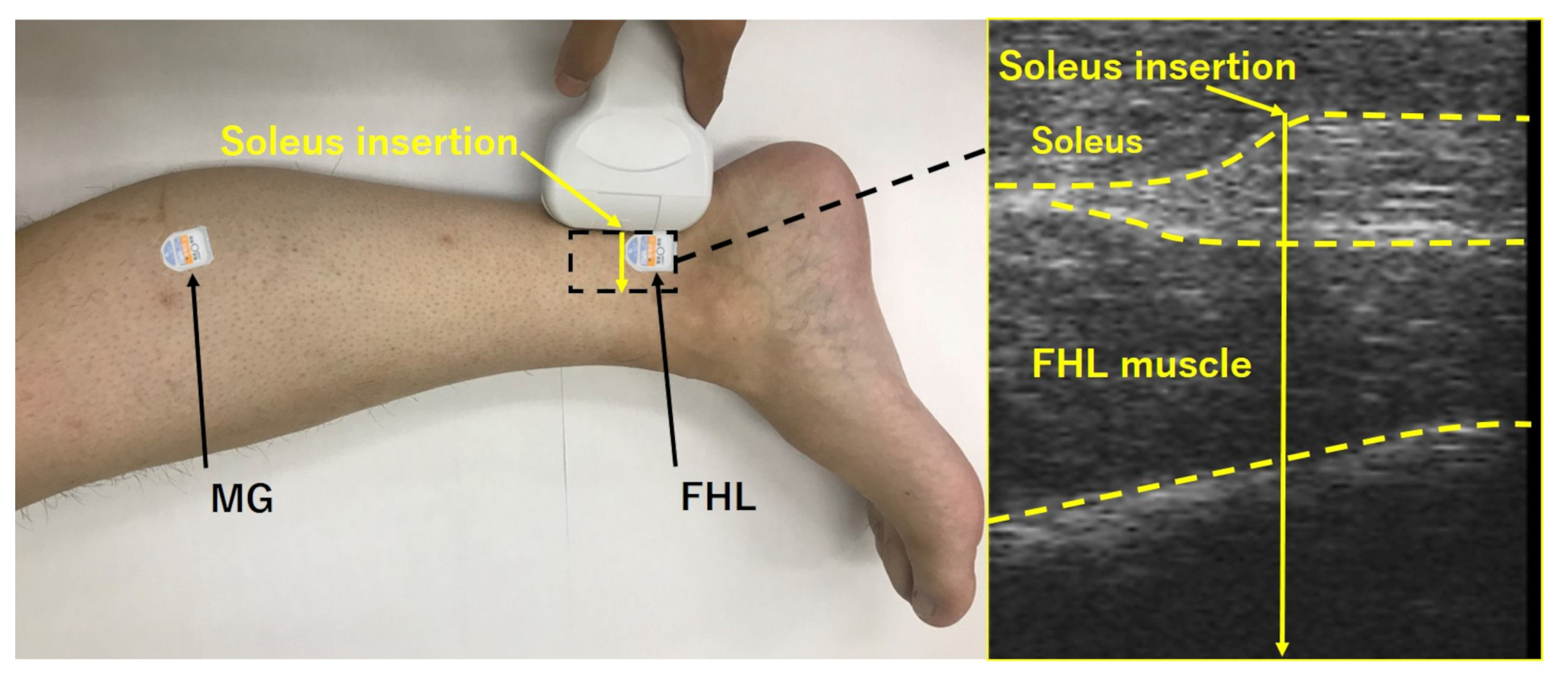
2.2. Instrumentation
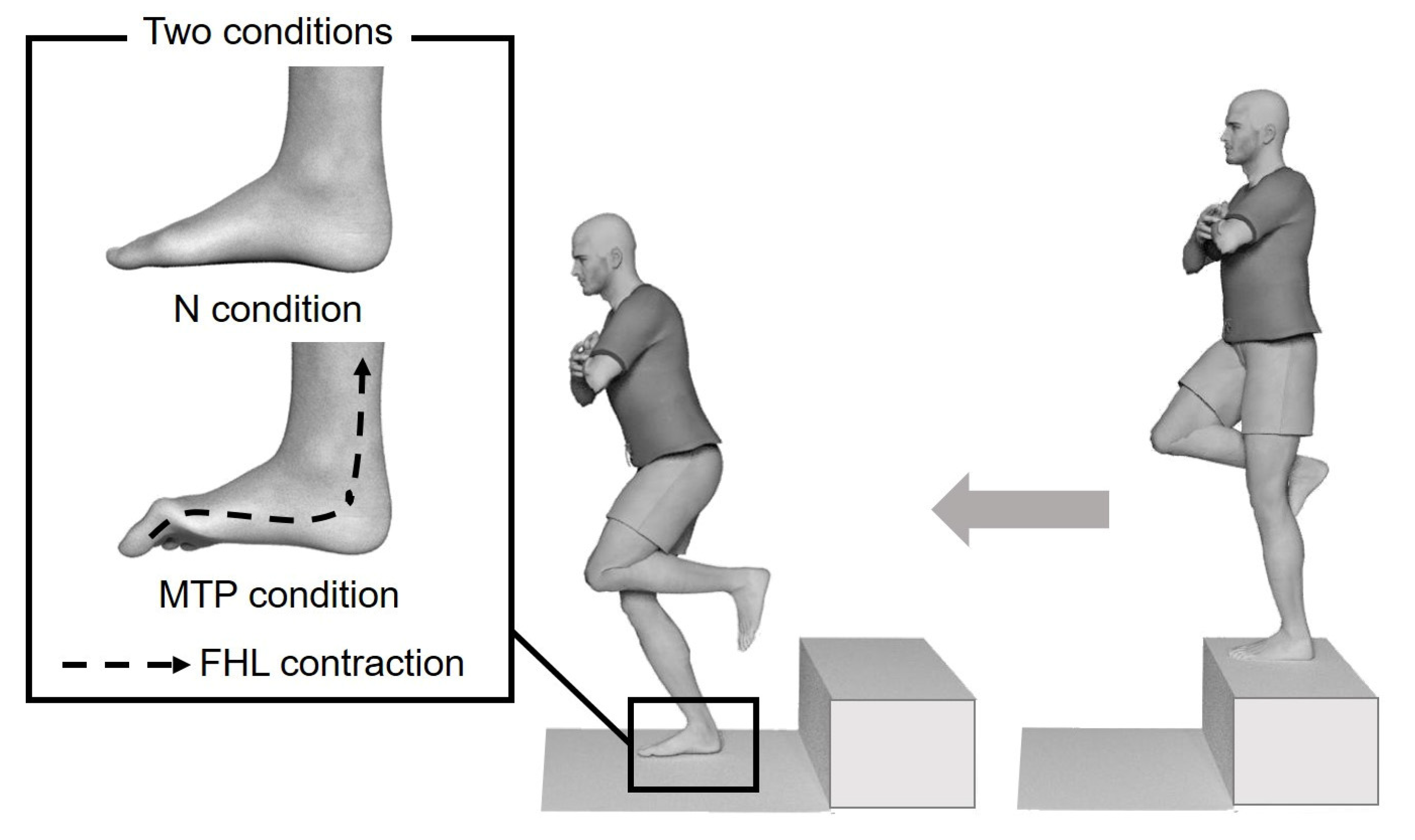
2.3. Landing Protocol
2.4. Electromyography and Ground Reaction Force Signal Processing
2.5. Sample Size
2.6. Statistical Analyses
3. Results
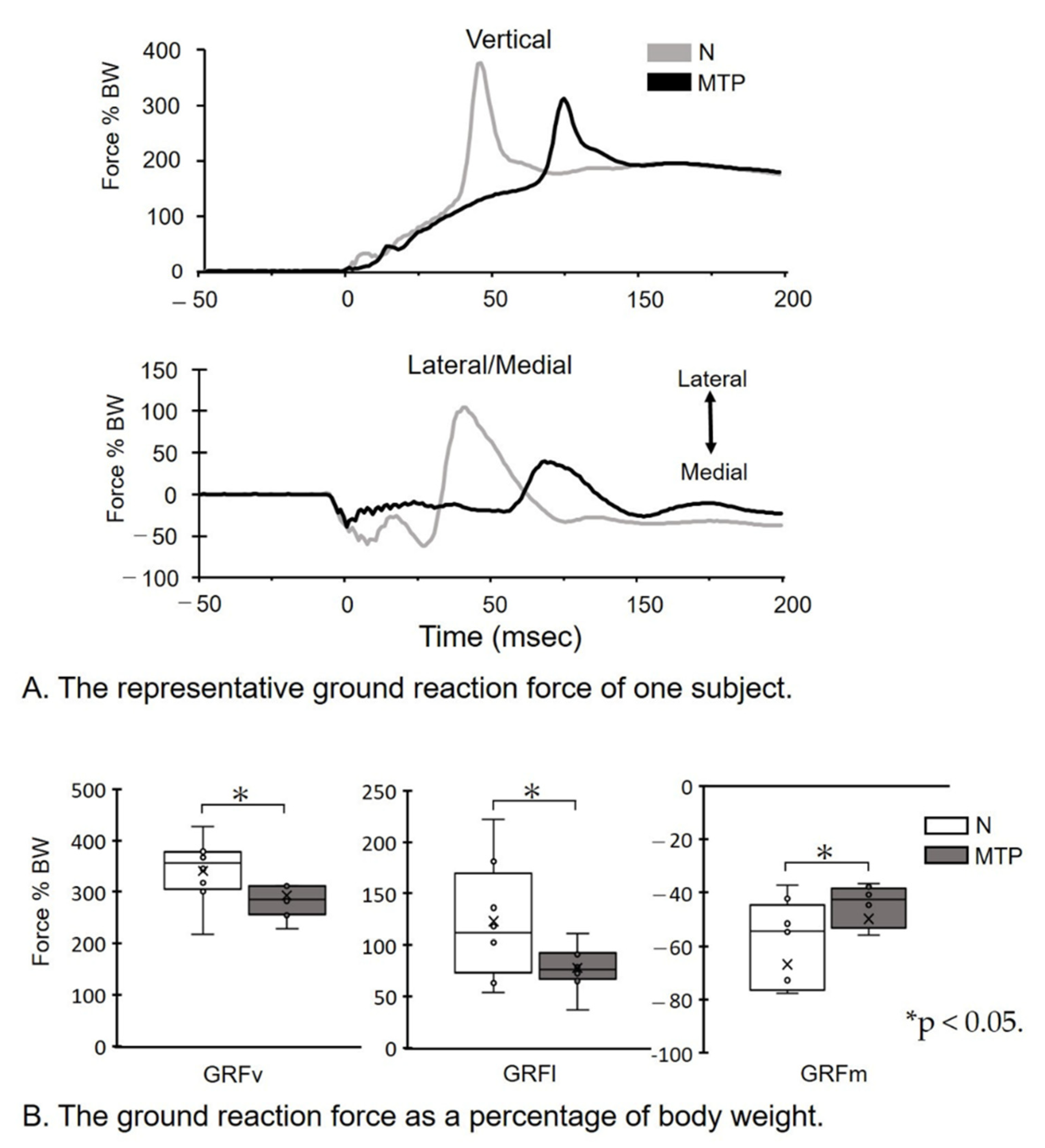
- (A)
- The representative GRF of one subject. GRF data during the 50 ms before and 100 ms following the initial ground contact at 0 ms are shown. Note that the GRF amplitude in the MTP condition is decreased, compared to the N condition.
- (B)
- The GRF as a percentage of body weight (BW). Magnitudes of peak GRFv, GRFl, and GRFm forces as a percentage of BW were identified for each jump, and individual and group means were subsequently calculated. * p < 0.05. Lines represent the range of the minimum and maximum. Boxes represent the lower, median, and upper quartiles. The GRF as a percentage of BW in the MTP condition was decreased compared to the N condition.
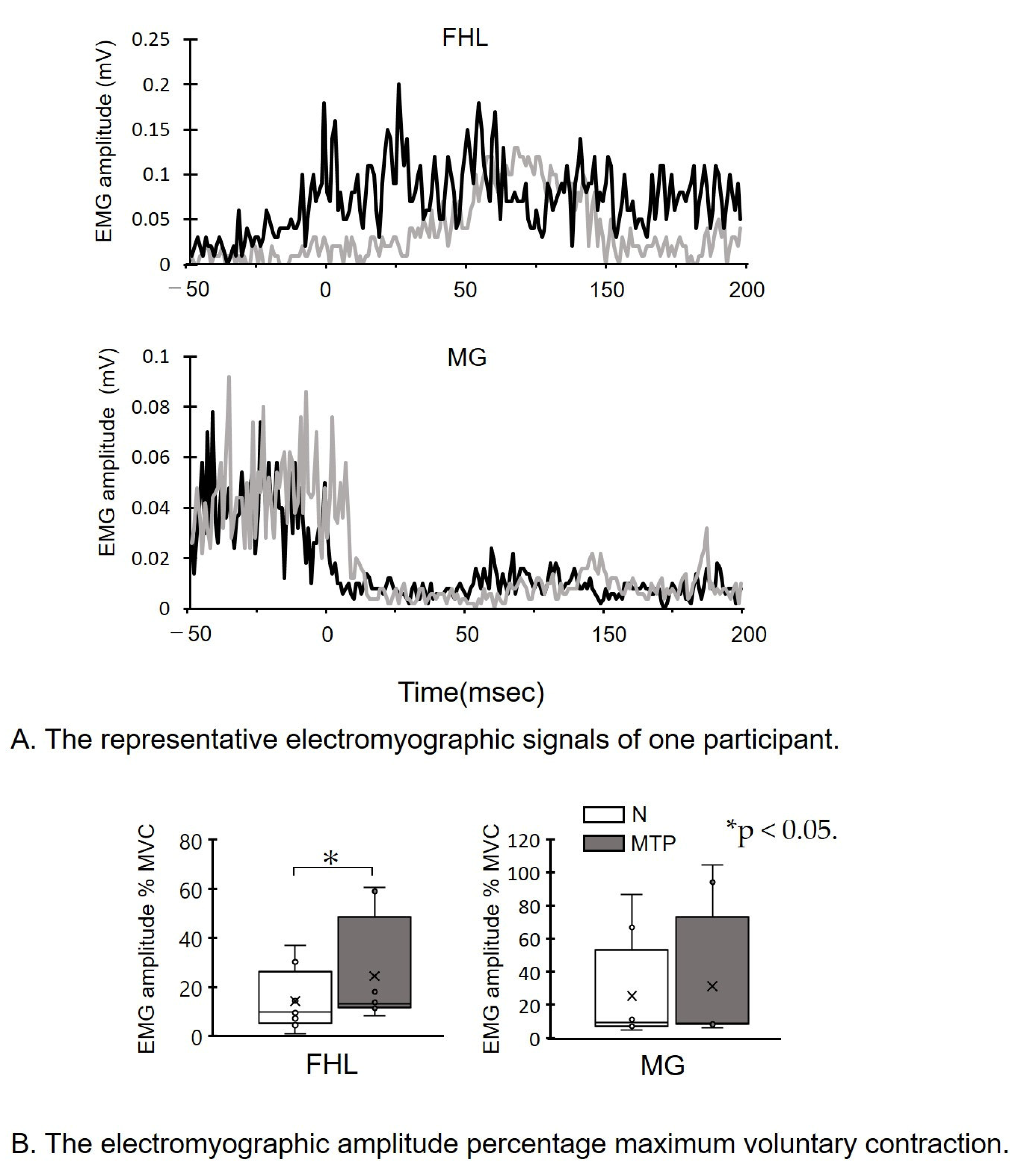
- (A)
- The representative electromyographic signals of one participant. sEMG data during the 50 ms before and 100 ms following initial ground contact at 0 msec are shown. Note that the FHL amplitude in the MTP condition is increased compared to the N condition.
- (B)
- The electromyographic amplitude percentage maximum voluntary contraction (MVC). sEMG amplitude of FHL and MG percentage MVC. * p < 0.05. Lines represent the range of the minimum and maximum. Boxes represent the lower, median, and upper quartiles. The sEMG of FHL in the MTP condition was increased compared to the N condition.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lafortune, M.A.; Hennig, E.M.; Lake, M.J. Dominant role of interface over knee angle for cushioning impact loading and regulating initial leg stiffness. J. Biomech. 1996, 29, 1523–1529. [Google Scholar] [CrossRef]
- Yeow, C.; Lee, P.V.; Goh, J.C. Regression relationships of landing height with ground reaction forces, knee flexion angles, angular velocities and joint powers during double-leg landing. Knee 2009, 16, 381–386. [Google Scholar] [CrossRef]
- Mei, Q.; Fernandez, J.; Fu, W.; Feng, N.; Gu, Y. A comparative biomechanical analysis of habitually unshod and shod runners based on a foot morphological difference. Hum. Mov. Sci. 2015, 42, 38–53. [Google Scholar] [CrossRef]
- Backes, A.; Skejø, S.D.; Gette, P.; Nielsen, R.Ø.; Sørensen, H.; Morio, C.; Malisoux, L. Predicting cumulative load during running using field-based measures. Scandinavian J. Med. Sci. Sports 2020, 30, 2399–2407. [Google Scholar] [CrossRef] [PubMed]
- Sasimontonkul, S.; Bay, B.K.; Pavol, M.J. Bone contact forces on the distal tibia during the stance phase of running. J. Biomech. 2007, 40, 3503–3509. [Google Scholar] [CrossRef]
- van der Worp, H.; Vrielink, J.W.; Bredeweg, S.W. Do runners who suffer injuries have higher vertical ground reaction forces than those who remain injury-free? A systematic review and meta-analysis. Br. J. Sports Med. 2016, 50, 450–457. [Google Scholar] [CrossRef]
- Zadpoor, A.A.; Nikooyan, A.A. The relationship between lower-extremity stress fractures and the ground reaction force: A systematic review. Clin. Biomech. 2011, 26, 23–28. [Google Scholar] [CrossRef]
- Nigg, B.M. Biomechanics of Sport Shoes; University of Calgary: Calgary, AB, Canada, 2010. [Google Scholar]
- Nigg, B.; Mohr, M.; Nigg, S.R. Muscle tuning and preferred movement path-a paradigm shift. Curr. Issues Sport Sci. (CISS) 2017, 2, 1–12. [Google Scholar] [CrossRef]
- Lieberman, D.E.; Venkadesan, M.; Werbel, W.A.; Daoud, A.I.; D’andrea, S.; Davis, I.S.; Mang’Eni, R.O.; Pitsiladis, Y. Foot strike patterns and collision forces in habitually barefoot versus shod runners. Nature 2010, 463, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Breine, B.; Malcolm, P.; Galle, S.; Fiers, P.; Frederick, E.C.; De Clercq, D. Running speed-induced changes in foot contact pattern influence impact loading rate. Eur. J. Sport Sci. 2019, 19, 774–783. [Google Scholar] [CrossRef]
- Devita, P.; Skelly, W.A. Effect of landing stiffness on joint kinetics and energetics in the lower extremity. Med. Sci. Sports Exerc. 1992, 24, 108–115. [Google Scholar] [CrossRef]
- Williams, D.S., III; McClay, I.S.; Hamill, J. Arch structure and injury patterns in runners. Clin. Biomech. 2001, 16, 341–347. [Google Scholar] [CrossRef]
- Angin, S.; Mickle, K.J.; Nester, C.J. Contributions of foot muscles and plantar fascia morphology to foot posture. Gait Posture 2018, 61, 238–242. [Google Scholar] [CrossRef]
- McKeon, P.O.; Hertel, J.; Bramble, D.; Davis, I. The foot core system: A new paradigm for understanding intrinsic foot muscle function. Br. J. Sports Med. 2015, 49, 290. [Google Scholar] [CrossRef]
- Péter, A.; Hegyi, A.; Stenroth, L.; Finni, T.; Cronin, N.J. EMG and force production of the flexor hallucis longus muscle in isometric plantarflexion and the push-off phase of walking. J. Biomech. 2015, 48, 3413–3419. [Google Scholar] [CrossRef] [PubMed]
- Chapman, D. Planning for conjunctive goals. Artif. Intell. 1987, 32, 333–377. [Google Scholar] [CrossRef]
- Zelik, K.E.; La Scaleia, V.; Ivanenko, Y.P.; Lacquaniti, F. Coordination of intrinsic and extrinsic foot muscles during walking. Eur. J. Appl. Physiol. 2015, 115, 691–701. [Google Scholar] [CrossRef]
- Fukunaga, T.; Roy, R.; Shellock, F.; Hodgson, J.; Day, M.; Lee, P.; Kwong-Fu, H.; Edgerton, V. Physiological cross-sectional area of human leg muscles based on magnetic resonance imaging. J. Orthop. Res. 1992, 10, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Péter, A.; Hegyi, A.; Finni, T.; Cronin, N.J. In vivo fascicle behavior of the flexor hallucis longus muscle at different walking speeds. Scand. J. Med. Sci. Sports 2017, 27, 1716–1723. [Google Scholar] [CrossRef]
- Caulfield, B.; Garrett, M. Changes in ground reaction force during jump landing in participants with functional instability of the ankle joint. Clin. Biomech. 2004, 19, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Potvin, J.; Brown, S. Less is more: High pass filtering, to remove up to 99% of the surface EMG signal power, improves EMG-based biceps brachii muscle force estimates. J. Electromyogr. Kinesiol. 2004, 14, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Nanbancha, A.; Tretriluxana, J.; Limroongreungrat, W.; Sinsurin, K. Decreased supraspinal control and neuromuscular function controlling the ankle joint in athletes with chronic ankle instability. Eur. J. Appl. Physiol. 2019, 119, 2041–2052. [Google Scholar] [CrossRef] [PubMed]
- Tretriluxana, J.; Nanbancha, M.A.; Sinsurin, K.; Limroongreungrat, W.; Wang, H.-K. Neuromuscular control of the ankle during pre-landing in athletes with chronic ankle instability: Insights from statistical parametric mapping and muscle co-contraction analysis. Phys. Ther. Sport 2021, 47, 46–52. [Google Scholar] [CrossRef]
- Kwon, O.-Y.; Minor, S.D.; Maluf, K.S.; Mueller, M.J. Comparison of muscle activity during walking in participants with and without diabetic neuropathy. Gait Posture 2003, 18, 105–113. [Google Scholar] [CrossRef]
- Faul, F.; Erdfelder, E.; Lang, A.-G.; Buchner, A. G* Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef]
- Cohen, J. A power primer. Psychol. Bull. 1992, 112, 155. [Google Scholar] [CrossRef] [PubMed]
- Friederich, J.A.; Brand, R.A. Muscle fiber architecture in the human lower limb. J. Biomech. 1990, 23, 91–95. [Google Scholar] [CrossRef]
- Kura, H.; Luo, Z.P.; Kitaoka, H.B.; An, K.N. Quantitative analysis of the intrinsic muscles of the foot. Anat. Rec. 1997, 249, 143–151. [Google Scholar] [CrossRef]
- Fiolkowski, P.; Brunt, D.; Bishop, M.; Woo, R.; Horodyski, M. Intrinsic pedal musculature support of the medial longitudinal arch: An electromyography study. J. Foot Ankle Surg. 2003, 42, 327–333. [Google Scholar] [CrossRef]
- Franco, A.H. Pes cavus and pes planus: Analyses and treatment. Phys. Ther. 1987, 67, 688–694. [Google Scholar] [CrossRef]
- Jam, B. Evaluation and retraining of the intrinsic foot muscles for pain syndromes related to abnormal control of pronation. Adv. Phys. Ther. Educ. Inst. 2006, 21, 1–8. [Google Scholar]
- Bates, K.T.; Collins, D.; Savage, R.; McClymont, J.; Webster, E.; Pataky, T.C.; D’Aout, K.; Sellers, W.I.; Bennett, M.R.; Crompton, R.H. The evolution of compliance in the human lateral mid-foot. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131818. [Google Scholar] [CrossRef]
- Pataky, T.C.; Caravaggi, P.; Savage, R.; Parker, D.; Goulermas, J.Y.; Sellers, W.I.; Crompton, R.H. New insights into the plantar pressure correlates of walking speed using pedobarographic statistical parametric mapping (pSPM). J. Biomech. 2008, 41, 1987–1994. [Google Scholar] [CrossRef] [PubMed]
- Barrios, J.A.; Heitkamp, C.A.; Smith, B.P.; Sturgeon, M.M.; Suckow, D.W.; Sutton, C.R. Three-dimensional hip and knee kinematics during walking, running, and single-limb drop landing in females with and without genu valgum. Clin. Biomech. 2016, 31, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Dufek, J.S.; Bates, B.T. Biomechanical factors associated with injury during landing in jump sports. Sports Med. 1991, 12, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Bennell, K.; Crossley, K.; Jayarajan, J.; Walton, E.; Warden, S.; Kiss, Z.S.; Wrigley, T. Ground reaction forces and bone parameters in females with tibial stress fracture. Med. Sci. Sports Exerc. 2004, 36, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Powers, C.M. The influence of abnormal hip mechanics on knee injury: A biomechanical perspective. J. Orthop. Sports Phys. Ther. 2010, 40, 42–51. [Google Scholar] [CrossRef]
- Castro, A.; Goethel, M.F.; Gáspari, A.F.; Crozara, L.F.; Gonçalves, M. Ankle brace attenuates the medial-lateral ground reaction force during basketball rebound jump. Rev. Bras. Med. Esporte 2017, 23, 232–236. [Google Scholar] [CrossRef]
- Sacco, I.d.C.; Takahasi, H.Y.; Vasconcellos, Â.A.; Suda, E.Y.; Bacarin, T.d.A.; Pereira, C.S.; Battistella, L.R.; Kavamoto, C.; Lopes, J.A.F.; Vasconcelos, J.C.P.d. Influence of ankle devices in the jump and landing biomechanical responses in basketball. Rev. Bras. Med. Esporte 2004, 10, 447–452. [Google Scholar] [CrossRef][Green Version]
- Hertel, J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J. Athl. Train. 2002, 37, 364. [Google Scholar]
- Matijevich, E.S.; Branscombe, L.M.; Scott, L.R.; Zelik, K.E. Ground reaction force metrics are not strongly correlated with tibial bone load when running across speeds and slopes: Implications for science, sport and wearable tech. PLoS ONE 2019, 14, e0210000. [Google Scholar] [CrossRef] [PubMed]
- Kelly, L.A.; Kuitunen, S.; Racinais, S.; Cresswell, A.G. Recruitment of the plantar intrinsic foot muscles with increasing postural demand. Clin. Biomech. 2012, 27, 46–51. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oku, K.; Kimura, D.; Ito, T.; Matsugi, A.; Sugioka, T.; Kobayashi, Y.; Satake, H.; Kumai, T. Effect of Increased Flexor Hallucis Longus Muscle Activity on Ground Reaction Force during Landing. Life 2021, 11, 630. https://doi.org/10.3390/life11070630
Oku K, Kimura D, Ito T, Matsugi A, Sugioka T, Kobayashi Y, Satake H, Kumai T. Effect of Increased Flexor Hallucis Longus Muscle Activity on Ground Reaction Force during Landing. Life. 2021; 11(7):630. https://doi.org/10.3390/life11070630
Chicago/Turabian StyleOku, Kosuke, Daisuke Kimura, Tomotaka Ito, Akiyoshi Matsugi, Tatsuya Sugioka, Yusuke Kobayashi, Hayato Satake, and Tsukasa Kumai. 2021. "Effect of Increased Flexor Hallucis Longus Muscle Activity on Ground Reaction Force during Landing" Life 11, no. 7: 630. https://doi.org/10.3390/life11070630
APA StyleOku, K., Kimura, D., Ito, T., Matsugi, A., Sugioka, T., Kobayashi, Y., Satake, H., & Kumai, T. (2021). Effect of Increased Flexor Hallucis Longus Muscle Activity on Ground Reaction Force during Landing. Life, 11(7), 630. https://doi.org/10.3390/life11070630






