Diabetic Retinopathy in Children with Type 1 Diabetes—Occurrence and Screening Using Optical Coherence Tomography
Abstract
1. Introduction
1.1. Incidence of Diabetic Retinopathy in Pediatric Population
1.2. Risk Factors for Diabetic Retinopathy
1.3. Classification
- Recurrent vitreous hemorrhages from neovascular vessels;
- Retinal detachment complicating proliferative vitreo-retinopathy;
- Severe glaucoma.
1.4. Screening Recommendations
- Screening for DR should start from age 11 with 2 to 5 years of diabetes duration; screening should be performed by an ophthalmologist, optometrist, or a trained experienced observer through dilatated pupils via bio-microscopy examination or fundus photography;
- In the case of children with diabetes duration <10 years, mild non-proliferative retinopathy (only microaneurysms) and a good glycemic control, it is sufficient to have a medical check-up every two years (biomicroscopy, or a color fundus photography);
- Due to the risk of developing retinopathy in children who undergo rapid improvement of treatment after prolonged poor glycemic control, an ophthalmological examination is recommended prior to such treatment and then every three months for one year;
- In the case of retinopathy stages carrying the risk of blindness (severe non-proliferative retinopathy or worse and/or diabetic macular edema), laser photocoagulation and intravitreal injections of anty—vascular endothelial growth factor (anty–VEGF) are recommended, which statistically reduce the risk of vision loss;
- Intensive education and treatment should be implemented to prevent or delay the beginning of vessel problems.
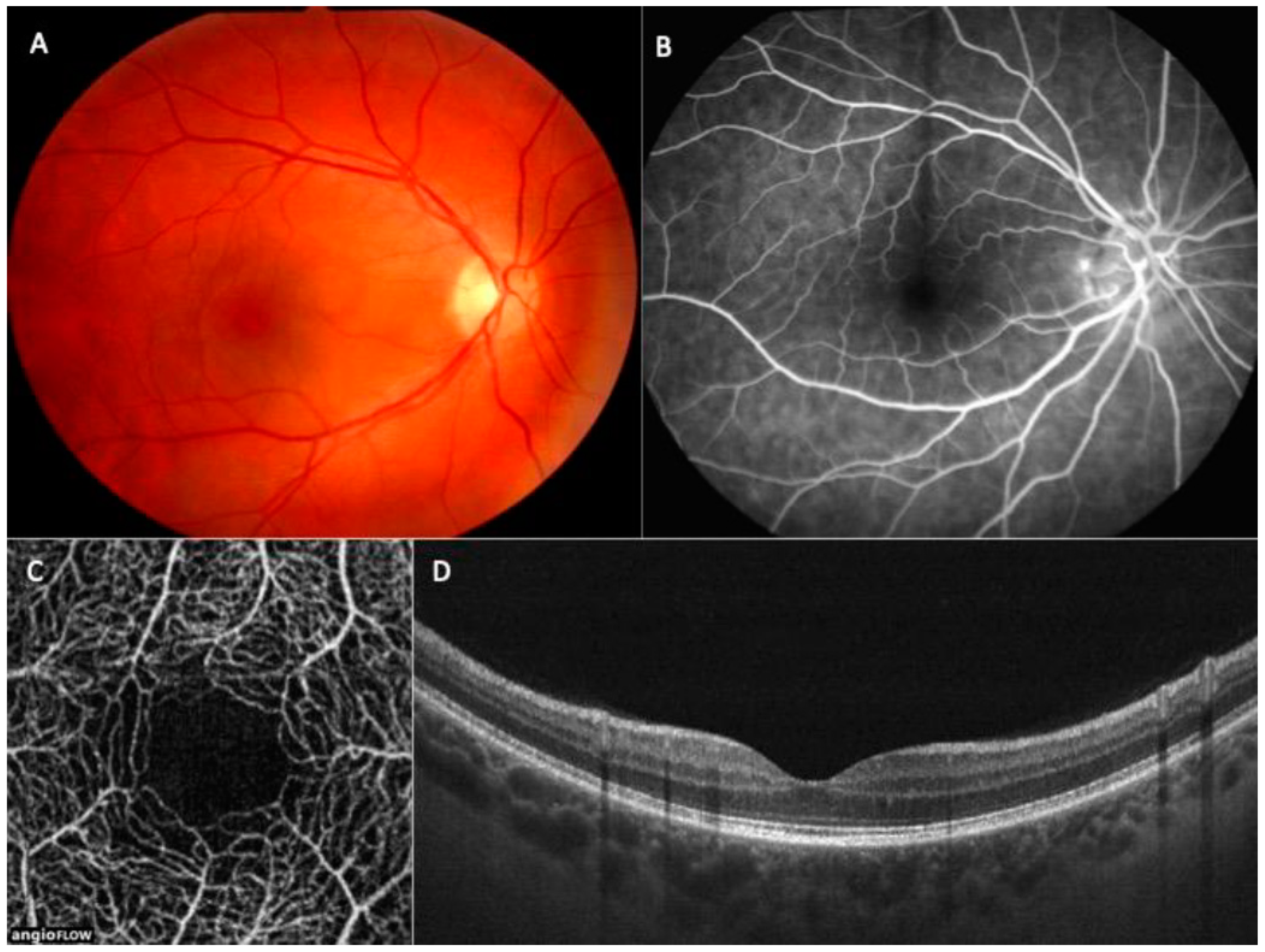
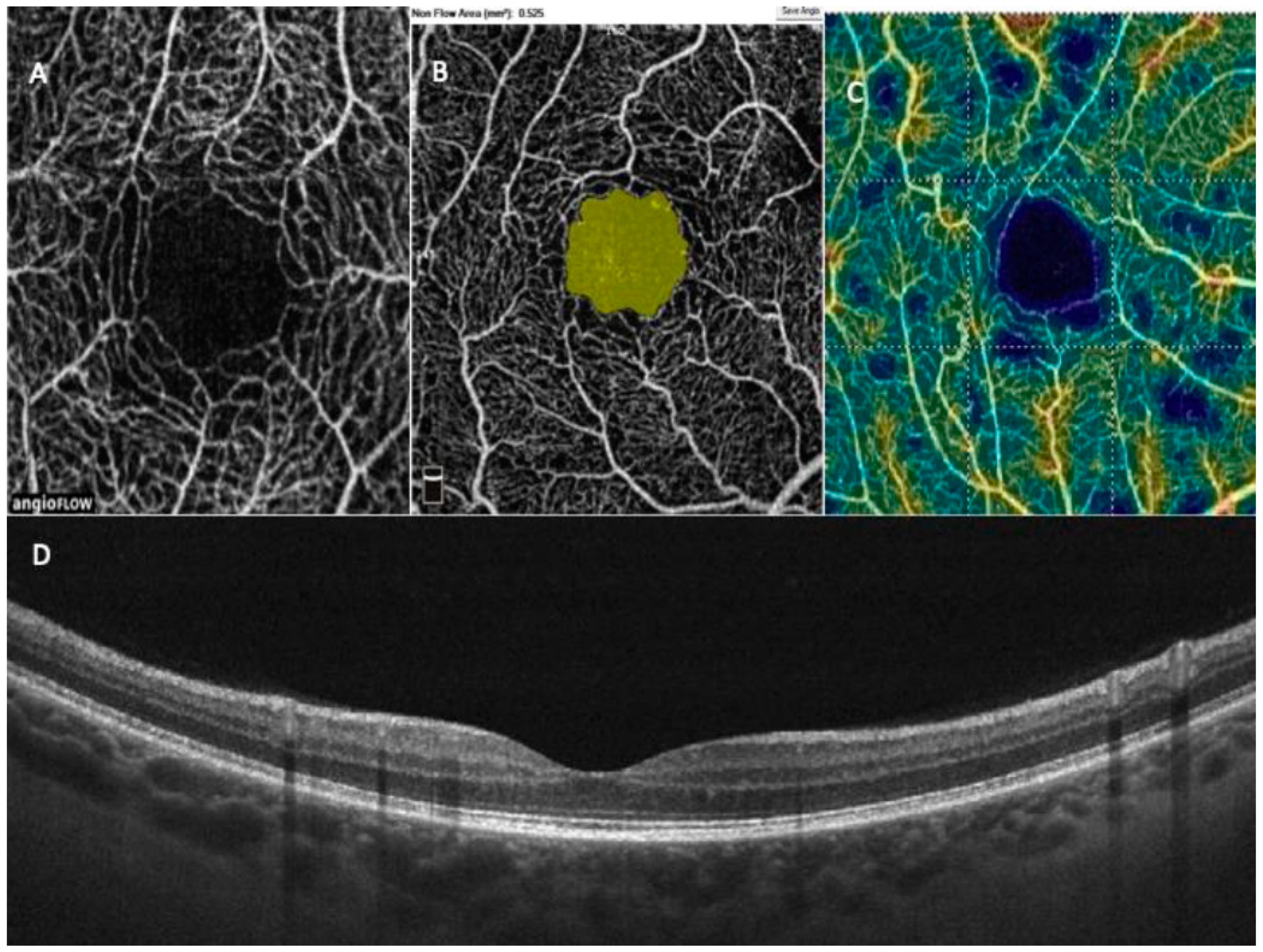
1.5. OCT in Paediatric Population
2. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N. Engl. J. Med. 1993, 329, 977–986. [Google Scholar] [CrossRef] [PubMed]
- Writing Team for the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications Research Group. Effect of intensive therapy on the microvascular complications of type 1 diabetes mellitus. JAMA 2002, 287, 2563–2569. [Google Scholar] [CrossRef] [PubMed]
- Karvonen, M.; Viik-Kajander, M.; Moltchanova, E.; Libman, I.; Laporte, R.; Tuomilehto, J. Incidence of childhood type 1 diabetes worldwide. Diabetes Mondiale (DiaMond) Project Group. Diabetes Care 2000, 23, 1516–1526. [Google Scholar] [CrossRef]
- Sultan, M.B.; Starita, C.; Huang, K. Epidemiology, risk factors and management of paediatric diabetic retinopathy: Table 1. Br. J. Ophthalmol. 2012, 96, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, G.P.; Steward, M.W. Diabetic retinopathy in children. Ped. Endocrinol. Rev. 2013, 10, 217–227. [Google Scholar]
- Geloneck, M.M.; Forbes, B.J.; Shaffer, J.; Ying, G.-S.; Binenbaum, G. Ocular Complications in Children with Diabetes Mellitus. Opthalmology 2015, 122, 2457–2464. [Google Scholar] [CrossRef]
- Thomas, R.L.; Ng, S.M. Risks and Prevalence of Diabetic Retinopathy in Children and Young People with Type 1 Diabetes Mellitus. J. Diabetes Clin. Res. 2020, 2, 68–74. [Google Scholar] [CrossRef]
- Royal College of Pediatrics and Child Health: National Paediatric Diabetes Audit Report 2018–2019. Available online: https://www.rcpchacuk/sites/default/files/2020-03/final_npda_core_report_2018-2019pdf2019 (accessed on 15 March 2020).
- Beauchamp, G.; Boyle, C.T.; Tamborlane, W.V.; Miller, K.M.; Libman, I.M.; Haller, M.J.; Beck, R.W. Treatable Diabetic Retinopathy Is Extremely Rare Among Pediatric T1D Exchange Clinic Registry Participants. Diabetes Care 2016, 39, e218–e219. [Google Scholar] [CrossRef] [PubMed]
- Huo, B.; Steffen, A.T.; Swan, K.; Sikes, K.; Weinzimer, S.A.; Tamborlane, W.V.; Pnp, M. Clinical Outcomes and Cost-Effectiveness of Retinopathy Screening in Youth with Type 1 Diabetes. Diabetes Care 2007, 30, 362–363. [Google Scholar] [CrossRef]
- Hamid, A.; Wharton, H.M.; Mills, A.; Gibson, J.M.; Clarke, M.; Dodson, P.M. Diagnosis of retinopathy in children younger than 12 years of age: Implications for the diabetic eye screening guidelines in the UK. Eye 2016, 30, 949–951. [Google Scholar] [CrossRef][Green Version]
- Downie, E.; Craig, M.; Hing, S.; Cusumano, J.; Chan, A.K.F.; Donaghue, K.C. Continued reduction in the prevalence of retinopathy in adolescents with type 1 diabetes: Role of insulin therapy and glycemic control. Diabetes Care 2011, 34, 2368–2373. [Google Scholar] [CrossRef] [PubMed]
- Lecaire, T.; Palta, M.; Zhang, H.; Allen, C.; Klein, R.; D’Alessio, D. Lower-than-expected prevalence and severity of retinopathy in an incident cohort followed during the first 4–14 years of type 1 diabetes: The Wisconsin Diabetes Registry Study. Am. J. Epidem. 2006, 164, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Scanlon, P.H.; Stratton, I.M.; Bachmann, M.O.; Jones, C.; Leese, G.P. The Four Nations Diabetic Retinopathy Screening Study Group Risk of diabetic retinopathy at first screen in children at 12 and 13 years of age. Diabet. Med. 2016, 33, 1655–1658. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gołębiewska, J.; Olechowski, A.; Wysocka-Mincewicz, M.; Odrobina, D.; Baszyńska-Wilk, M.; Groszek, A.; Szalecki, M.; Hautz, W. Optical coherence tomography angiography vessel density in children with type 1 diabetes. PLoS ONE 2017, 12, e0186479. [Google Scholar] [CrossRef]
- Raczyńska, D.; Zorena, K.; Urban, B.; Zalewski, D.; Skorek, A.; Malukiewicz, G.; Sikorski, B.L. Current Trends in the Monitoring and Treatment of Diabetic Retinopathy in Young Adults. Mediat. Inflamm. 2014, 2014, 492926. [Google Scholar] [CrossRef]
- Holl, R.; Lang, G.; Grabert, M.; Heinze, E.; Lang, G.; Debatin, K. Diabetic retinopathy in pediatric patients with type-1 diabetes: Effect of diabetes duration, prepubertal and pubertal onset of diabetes, and metabolic control. J. Pediatr. 1998, 132, 790–794. [Google Scholar] [CrossRef]
- Donaghue, K.C.; Fung, A.T.; Hing, S.; Fairchild, J.; King, J.; Chan, A.; Howard, N.J.; Silink, M. The Effect of Prepubertal Diabetes Duration on Diabetes: Microvascular Complications in Early and Late Adolescence. Diabetes Care 1997, 20, 77–80. [Google Scholar] [CrossRef]
- Cho, Y.H.; Craig, M.E.; Donaghue, K.C. Puberty as an accelerator for diabetes complications. Pediatr. Diabetes 2014, 15, 18–26. [Google Scholar] [CrossRef]
- Ng, S.; Ayoola, O.; McGuigan, M.; Chandrasekaran, S.A. Multicentre study evaluating the risk and prevalence of diabetic reti-nopathy in children and young people with type 1 diabetes mellitus. Diabetes Metab. Syndr. Clin. Res. Rev. 2019, 13, 744–774. [Google Scholar] [CrossRef]
- Hainsworth, D.P.; Bebu, I.; Aiello, L.P.; Sivitz, W.; Gubitosi-Klug, R.; Malone, J.; White, N.H.; Danis, R.; Wallia, A.; Gao, X.; et al. Risk Factors for Retinopathy in Type 1 Diabetes: The DCCT/EDIC Study. Diabetes Care 2019, 42, 875–882. [Google Scholar] [CrossRef]
- Yau, J.; Rogers, S.; Kawasaki, R.; Lamoureux, E.; Kowalski, J.; Bek, T. Global Prevalence and Major Risk Factors of Diabetic Retinopathy. Diabetes Care 2012, 35, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.Y.; Andrews, C.; Herman, W.H.; Gardner, T.W.; Stein, J.D. Incidence and Risk Factors for Developing Diabetic Retinopathy among Youths with Type 1 or Type 2 Diabetes throughout the United States. Opthalmology 2017, 124, 424–430. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Grading diabetic retinopathy from stereoscopic color fundus photographs—An extension of the modified Airlie House classification. ETDRS report number 10. Ophthalmology 1991, 98, 786–806. [Google Scholar] [CrossRef]
- Wilkinson, C.P.; Ferris, F.L., III; Klein, R.E.; Lee, P.P.; Agardh, C.D.; Davis, M.; Dills, D.; Kampik, A.; Pararajasegaram, R.; Verdaguer, J.T.; et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology 2003, 110, 1679. [Google Scholar] [CrossRef]
- American Diabetes Association 13. Children and Adolescents: Standards of Medical Care in Diabetes—2021. Diabetes Care 2021, 44, S180–S199. [Google Scholar] [CrossRef]
- NICE Guideline 18. Methods, Evidence, and Recommendations. Diabetes (Type 1 and Type 2) in Children and Young People: Diagnosis and Management August 2015. p. 236. Available online: https://www.nice.org.uk/guidance/ng18/evidence/full-guideline-pdf-435396352 (accessed on 21 June 2021).
- Donaghue, K.C.; Marcovecchio, M.L.; Wadwa, R.P. ISPAD Clinical Practice Consensus Guidelines 2018: Microvascular and macrovascular complications in children and adolescens. Pediatr. Diabetes 2018, 19 (Suppl. 27), 262–274. [Google Scholar] [CrossRef] [PubMed]
- Early Treatment Diabetic Retinopathy Study Research Group. Fluorescein angiographic risk factors for progression. Ophthalmology 1991, 98 (Suppl. 5), 834–840. [Google Scholar] [CrossRef]
- Early Treatment Diabetic Retinopathy Study Research Group. Classification of diabetic retinopathy from fluorescein angiograms: ETDRS report number 11. Ophthalmology 1991, 98, 807–822. [Google Scholar] [CrossRef]
- Wiley, H.E.; Ferris, F.L. Nonproliferative Diabetic Retinopathy and Diabetic Macular Edema; Elsevier BV: Amsterdam, The Netherlands, 2013; pp. 940–968. [Google Scholar]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44. [Google Scholar] [CrossRef]
- Hwang, T.S.; Jia, Y.; Gao, S.; Bailey, S.T.; Lauer, A.K.; Flaxel, C.J.; Wilson, D.J.; Huang, D. Optical coherence tomography angiography features of diabetic retinopathy. Retina 2015, 35, 2371–2376. [Google Scholar] [CrossRef]
- Matsunaga, D.R.; Yi, J.J.; De Koo, L.O.; Ameri, H.; Puliafito, C.A.; Kashani, A.H. Optical Coherence Tomography Angiography of Diabetic Retinopathy in Human Subjects. Ophthalmic Surg. Lasers Imaging Retin. 2015, 46, 796–805. [Google Scholar] [CrossRef]
- Johannesen, S.K.; Viken, J.N.; Vergmann, A.S.; Grauslund, J. Optical coherence tomography angiography and microvascular changes in diabetic retinopathy: A systematic review. Acta Ophthalmol. 2019, 97, 7–14. [Google Scholar] [CrossRef]
- Gołębiewska, J.; Olechowski, A.; Wysocka-Mincewicz, M.; Baszyńska-Wilk, M.; Groszek, A.; Czeszyk, A.; Szalecki, M.; Hautz, W. Choroidal Thickness and Ganglion Cell Complex in Pubescent Children with Type 1 Diabetes without Diabetic Retinopathy Analyzed by Spectral Domain Optical Coherence Tomography. J. Diabetes Res. 2018, 2018, 1–8. [Google Scholar] [CrossRef]
- Niestrata-Ortiz, M.; Fichna, P.; Stankiewicz, W.; Stopa, M. Determining the effect of diabetes duration on retinal and choroidal thicknesses in children with type 1 diabetes mellitus. Retina 2020, 40, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Mameli, C.; Invernizzi, A.; Bolchini, A.; Bedogni, G.; Giani, E.; MacEdoni, M.; Zuccotti, G.; Preziosa, C.; Pellegrini, M. Analysis of Retinal Perfusion in Children, Adolescents, and Young Adults with Type 1 Diabetes Using Optical Coherence Tomography Angiography. J. Diabetes Res. 2019, 2019, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wysocka-Mincewicz, M.; Baszyńska-Wilk, M.; Gołębiewska, J.; Olechowski, A.; Byczyńska, A.; Hautz, W.; Szalecki, M. Influence of Metabolic Parameters and Treatment Method on OCT Angiography Results in Children with Type 1 Diabetes. J. Diabetes Res. 2020, 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Kara, O.; Can, M.E. Evaluation of microvascular changes in retinal zones and optic disc in pediatric with type 1 diabetes mellitus. Graefes. Arch. Clin. Exp. Ophthalmol. 2021, 259, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Veiby, N.C.B.B.; Simeunovic, A.; Heier, M.; Brunborg, C.; Saddique, N.; Moe, M.C.; Dahl-Jørgensen, K.; Margeirsdottir, H.D.; Petrovski, G. Associations between Macular OCT Angiography and Nonproliferative Diabetic Retinopathy in Young Patients with Type 1 Diabetes Mellitus. J. Diabetes Res. 2020, 2020, 8849116. [Google Scholar] [CrossRef]
- Demir, S.T.; Ucar, A.; Elitok, G.K.; Karatas, M.E.; Karapapak, M.; Kutucu, O.K.; Uzun, S.U.; Guven, D. Evaluation of retinal neurovascular structures by optical coherence tomography and optical coherence tomography angiography in children and adolescents with type 1 diabetes mellitus without clinical sign of diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020, 258, 2363–2372. [Google Scholar] [CrossRef] [PubMed]
- Onoe, H.; Kitagawa, Y.; Shimada, H.; Shinojima, A.; Aoki, M.; Urakami, T. Foveal avascular zone area analysis in juvenile-onset type 1 diabetes using optical coherence tomography angiography. Jpn. J. Ophthalmol. 2020, 64, 271–277. [Google Scholar] [CrossRef]
- Niestrata-Ortiz, M.; Fichna, P.; Stankiewicz, W.; Stopa, M. Enlargement of the foveal avascular zone detected by optical coherence tomography angiography in diabetic children without diabetic retinopathy. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 689–697. [Google Scholar] [CrossRef] [PubMed]
- Wysocka-Mincewicz, M.; Gołębiewska, J.; Baszyńska-Wilk, M.; Olechowski, A.; Byczyńska, A.; Szalecki, M. Influence of puberty on retinal microcirculation in children with type 1 diabetes without retinopathy using optical coherence tomography angiography. Diabetes Vasc. Dis. Res. 2021, 18. [Google Scholar] [CrossRef] [PubMed]
- Wysocka-Mincewicz, M.; Baszyńska-Wilk, M.; Gołębiewska, J.; Olechowski, A.; Byczyńska, A.; Hautz, W.; Szalecki, M. The effect of coexisting autoimmune thyroiditis in children with Type 1 diabetes on OCT results. Pediatr. Diabetes 2020, 22, 329–334. [Google Scholar] [CrossRef] [PubMed]
| Non-Proliferative Diabetic Retinopathy (NPDR) | |
|---|---|
| Mild diabetic retinopathy (simple) | microaneurysms only |
| Moderate non-proliferative retinopathy | more than just microaneurysms, but less than severe non-proliferative retinopathy |
| Severe non-proliferative diabetic retinopathy | any of the following (governed by the 4/2/1 rule), and no signs of proliferative DR —>20 intra-retinal hemorrhages in each 4 quadrants of retinal circumference, —definite venous beading in at least 2 retinal quadrants, prominent intra-retinal microvascular abnormalities (IRMA) in at least 1 quadrant, —no signs of proliferative retinopathy. |
| Proliferative diabetic retinopathy (PDR)—one or more of the following: neovascularization and/or vitreous or preretinal hemorrhages) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wysocka-Mincewicz, M.; Gołębiewska, J.; Olechowski, A.; Szalecki, M. Diabetic Retinopathy in Children with Type 1 Diabetes—Occurrence and Screening Using Optical Coherence Tomography. Life 2021, 11, 590. https://doi.org/10.3390/life11060590
Wysocka-Mincewicz M, Gołębiewska J, Olechowski A, Szalecki M. Diabetic Retinopathy in Children with Type 1 Diabetes—Occurrence and Screening Using Optical Coherence Tomography. Life. 2021; 11(6):590. https://doi.org/10.3390/life11060590
Chicago/Turabian StyleWysocka-Mincewicz, Marta, Joanna Gołębiewska, Andrzej Olechowski, and Mieczysław Szalecki. 2021. "Diabetic Retinopathy in Children with Type 1 Diabetes—Occurrence and Screening Using Optical Coherence Tomography" Life 11, no. 6: 590. https://doi.org/10.3390/life11060590
APA StyleWysocka-Mincewicz, M., Gołębiewska, J., Olechowski, A., & Szalecki, M. (2021). Diabetic Retinopathy in Children with Type 1 Diabetes—Occurrence and Screening Using Optical Coherence Tomography. Life, 11(6), 590. https://doi.org/10.3390/life11060590






