Gender Difference in Architectural and Mechanical Properties of Medial Gastrocnemius–Achilles Tendon Unit In Vivo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurement Procedure
2.2.1. Architectural Properties of the gMTU
2.2.2. Mechanical Properties of the gMTU
2.3. Data Processing
2.4. Statistics
3. Results
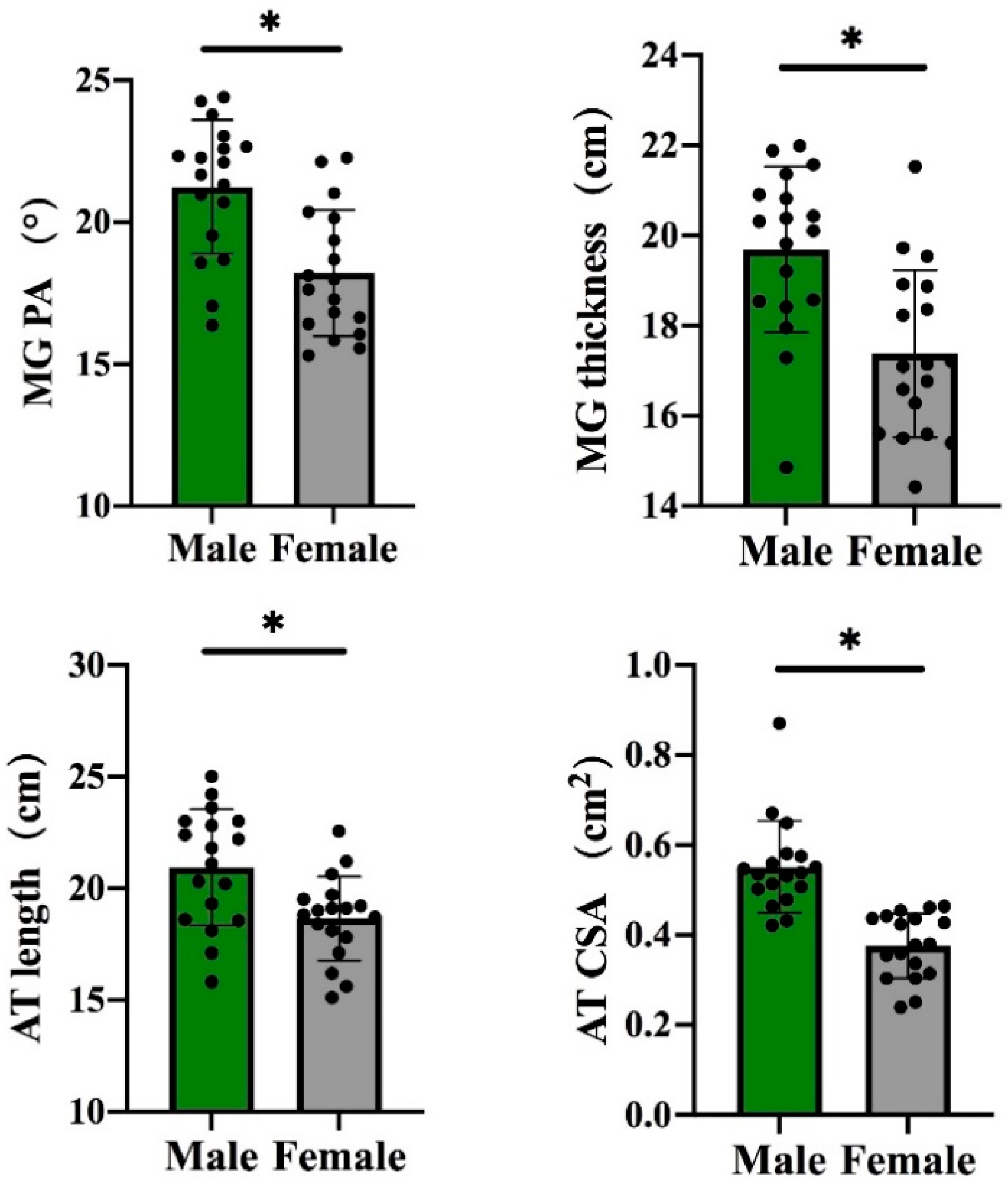
3.1. Gender Differences in the Architectural Properties of the gMTU
3.2. Gender Differences in the Mechanical Properties of the gMTU
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chang, T.T.; Li, Z.; Wang, X.Q.; Zhang, Z.J. Stiffness of the Gastrocnemius-Achilles Tendon Complex Between Amateur Basketball Players and the Non-athletic General Population. Front. Physiol. 2020, 11, 606706. [Google Scholar] [CrossRef]
- Kakouris, N.; Yener, N.; Fong, D.T.P. A systematic review of running-related musculoskeletal injuries in runners. J. Sport Health Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Hamner, S.R.; Seth, A.; Delp, S.L. Muscle contributions to propulsion and support during running. J. Biomech. 2010, 43, 2709–2716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longo, U.G.; Ronga, M.; Maffulli, N. Achilles Tendinopathy. Sports Med. Arthrosc. Rev. 2018, 26, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Kraeutler, M.J.; Purcell, J.M.; Hunt, K.J. Chronic Achilles Tendon Ruptures. Foot Ankle Int. 2017, 38, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Gebert, A.; Gerber, M.; Pühse, U.; Gassmann, P.; Stamm, H.; Lamprecht, M. Costs resulting from nonprofessional soccer injuries in Switzerland: A detailed analysis. J. Sport Health Sci. 2020, 9, 240–247. [Google Scholar] [CrossRef]
- Timmins, R.G.; Shield, A.J.; Williams, M.D.; Lorenzen, C.; Opar, D.A. Architectural adaptations of muscle to training and injury: A narrative review outlining the contributions by fascicle length, pennation angle and muscle thickness. Br. J. Sports Med. 2016, 50, 1467–1472. [Google Scholar] [CrossRef] [Green Version]
- Onambele, G.L.; Narici, M.V.; Maganaris, C.N. Calf muscle-tendon properties and postural balance in old age. J. Appl. Physiol. 2006, 100, 2048–2056. [Google Scholar] [CrossRef] [PubMed]
- Epro, G.; McCrum, C.; Mierau, A.; Leyendecker, M.; Brüggemann, G.P.; Karamanidis, K. Effects of triceps surae muscle strength and tendon stiffness on the reactive dynamic stability and adaptability of older female adults during perturbed walking. J. Appl. Physiol. 2018, 124, 1541–1549. [Google Scholar] [CrossRef] [Green Version]
- Arampatzis, A.; De Monte, G.; Karamanidis, K.; Morey-Klapsing, G.; Stafilidis, S.; Brüggemann, G.P. Influence of the muscle-tendon unit’s mechanical and morphological properties on running economy. J. Exp. Biol. 2006, 209, 3345–3357. [Google Scholar] [CrossRef] [Green Version]
- Kumagai, K.; Abe, T.; Brechue, W.F.; Ryushi, T.; Takano, S.; Mizuno, M. Sprint performance is related to muscle fascicle length in male 100-m sprinters. J. Appl. Physiol. 2000, 88, 811–816. [Google Scholar] [CrossRef]
- Timmins, R.G.; Bourne, M.N.; Shield, A.J.; Williams, M.D.; Lorenzen, C.; Opar, D.A. Short biceps femoris fascicles and eccentric knee flexor weakness increase the risk of hamstring injury in elite football (soccer): A prospective cohort study. Br. J. Sports Med. 2016, 50, 1524–1535. [Google Scholar] [CrossRef]
- Štefan, L.; Paradžik, P.; Sporiš, G. Sex and age correlations of reported and estimated physical fitness in adolescents. PLoS ONE 2019, 14, e0219217. [Google Scholar] [CrossRef] [Green Version]
- Chow, R.S.; Medri, M.K.; Martin, D.C.; Leekam, R.N.; Agur, A.M.; McKee, N.H. Sonographic studies of human soleus and gastrocnemius muscle architecture: Gender variability. Eur. J. Appl. Physiol. 2000, 82, 236–244. [Google Scholar] [CrossRef]
- Daniels, J.; Daniels, N. Running economy of elite male and elite female runners. Med. Sci. Sports Exerc. 1992, 24, 483–489. [Google Scholar] [CrossRef]
- Lepers, R. Sex Difference in Triathlon Performance. Front. Physiol. 2019, 10, 973. [Google Scholar] [CrossRef]
- Marques, A.; Henriques-Neto, D.; Peralta, M.; Martins, J.; Gomes, F.; Popovic, S.; Masanovic, B.; Demetriou, Y.; Schlund, A.; Ihle, A. Field-Based Health-Related Physical Fitness Tests in Children and Adolescents: A Systematic Review. Front. Pediatrics 2021, 9, 640028. [Google Scholar] [CrossRef]
- Fields, K.B.; Rigby, M.D. Muscular Calf Injuries in Runners. Curr. Sports Med. Rep. 2016, 15, 320–324. [Google Scholar] [CrossRef] [PubMed]
- Kvist, M. Achilles tendon injuries in athletes. Sports Med. 1994, 18, 173–201. [Google Scholar] [CrossRef] [PubMed]
- Nolan, L.; Patritti, B.L.; Stana, L.; Tweedy, S.M. Is increased residual shank length a competitive advantage for elite transtibial amputee long jumpers? Adapt Phys. Activ. Q. 2011, 28, 267–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geremia, J.M.; Baroni, B.M.; Bini, R.R.; Lanferdini, F.J.; de Lima, A.R.; Herzog, W.; Vaz, M.A. Triceps Surae Muscle Architecture Adaptations to Eccentric Training. Front. Physiol. 2019, 10, 1456. [Google Scholar] [CrossRef] [Green Version]
- Wren, T.A.; Cheatwood, A.P.; Rethlefsen, S.A.; Hara, R.; Perez, F.J.; Kay, R.M. Achilles tendon length and medial gastrocnemius architecture in children with cerebral palsy and equinus gait. J. Pediatr. Orthop. 2010, 30, 479–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, K.A.; Johnson, A.W.; Hunter, I.; Myrer, J.W. Does achilles tendon cross sectional area differ after downhill, level and uphill running in trained runners? J. Sports Sci. Med. 2014, 13, 823–828. [Google Scholar]
- Huang, J.; Qin, K.; Tang, C.; Zhu, Y.; Klein, C.S.; Zhang, Z.; Liu, C. Assessment of Passive Stiffness of Medial and Lateral Heads of Gastrocnemius Muscle, Achilles Tendon, and Plantar Fascia at Different Ankle and Knee Positions Using the MyotonPRO. Med. Sci. Monit. 2018, 24, 7570–7576. [Google Scholar] [CrossRef]
- Feng, Y.N.; Li, Y.P.; Liu, C.L.; Zhang, Z.J. Assessing the elastic properties of skeletal muscle and tendon using shearwave ultrasound elastography and MyotonPRO. Sci. Rep. 2018, 8, 17064. [Google Scholar] [CrossRef] [PubMed]
- Schneebeli, A.; Falla, D.; Clijsen, R.; Barbero, M. Myotonometry for the evaluation of Achilles tendon mechanical properties: A reliability and construct validity study. BMJ Open Sport Exerc. Med. 2020, 6, e000726. [Google Scholar] [CrossRef] [Green Version]
- Aggeloussis, N.; Giannakou, E.; Albracht, K.; Arampatzis, A. Reproducibility of fascicle length and pennation angle of gastrocnemius medialis in human gait in vivo. Gait Posture 2010, 31, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Shavandi, N.; Talebian, S.; Aslanpoor, S.; Sheikhhoseini, R.; Zeinali, S. The effect of strength training and combination technique on preserving the strength of plantar flexor muscles after a period of detraining. J. Sports Med. Phys. Fit. 2016, 56, 990–996. [Google Scholar]
- Abe, T.; Brechue, W.F.; Fujita, S.; Brown, J.B. Gender differences in FFM accumulation and architectural characteristics of muscle. Med. Sci. Sports Exerc. 1998, 30, 1066–1070. [Google Scholar] [CrossRef] [PubMed]
- Stenroth, L.; Peltonen, J.; Cronin, N.J.; Sipilä, S.; Finni, T. Age-related differences in Achilles tendon properties and triceps surae muscle architecture in vivo. J. Appl. Physiol. 2012, 113, 1537–1544. [Google Scholar] [CrossRef] [Green Version]
- Pearson, M.B.; Bassey, E.J.; Bendall, M.J. Muscle strength and anthropometric indices in elderly men and women. Age Ageing 1985, 14, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Alegre, L.M.; Lara, A.J.; Elvira, J.L.; Aguado, X. Muscle morphology and jump performance: Gender and intermuscular variability. J. Sports Med. Phys. Fit. 2009, 49, 320–326. [Google Scholar]
- Mangine, G.T.; Fukuda, D.H.; LaMonica, M.B.; Gonzalez, A.M.; Wells, A.J.; Townsend, J.R.; Jajtner, A.R.; Fragala, M.S.; Stout, J.R.; Hoffman, J.R. Influence of gender and muscle architecture asymmetry on jump and sprint performance. J. Sports Sci. Med. 2014, 13, 904–911. [Google Scholar] [PubMed]
- Fujiwara, K.; Asai, H.; Toyama, H.; Kunita, K.; Yaguchi, C.; Kiyota, N.; Tomita, H.; Jacobs, J.V. Changes in muscle thickness of gastrocnemius and soleus associated with age and sex. Aging Clin. Exp. Res. 2010, 22, 24–30. [Google Scholar] [CrossRef] [PubMed]
- Coratella, G.; Longo, S.; Rampichini, S.; Limonta, E.; Shokohyar, S.; Bisconti, A.V.; Cè, E.; Esposito, F. Quadriceps and Gastrocnemii Anatomical Cross-Sectional Area and Vastus Lateralis Fascicle Length Predict Peak-Power and Time-To-Peak-Power. Res. Q. Exerc. Sport 2020, 91, 158–165. [Google Scholar] [CrossRef]
- Storey, A.; Smith, H.K. Unique aspects of competitive weightlifting: Performance, training and physiology. Sports Med. 2012, 42, 769–790. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, K.W.; Lee, Y.W.; Kim, H.J. Correlation between Cycling Power and Muscle Thickness in Cyclists. Clin. Anat. 2018, 31, 899–906. [Google Scholar] [CrossRef]
- Ueno, H.; Suga, T.; Takao, K.; Tanaka, T.; Misaki, J.; Miyake, Y.; Nagano, A.; Isaka, T. Relationship between Achilles tendon length and running performance in well-trained male endurance runners. Scand. J. Med. Sci. Sports 2018, 28, 446–451. [Google Scholar] [CrossRef]
- Morgan, G.; Martin, R.; Welch, H.; Williams, L.; Morris, K. Objective assessment of stiffness in the gastrocnemius muscle in patients with symptomatic Achilles tendons. BMJ Open Sport Exerc. Med. 2019, 5, e000622. [Google Scholar] [CrossRef] [Green Version]
- Blackburn, J.T.; Padua, D.A.; Weinhold, P.S.; Guskiewicz, K.M. Comparison of triceps surae structural stiffness and material modulus across sex. Clin. Biomech. 2006, 21, 159–167. [Google Scholar] [CrossRef]
- Bell, D.R.; Blackburn, J.T.; Norcross, M.F.; Ondrak, K.S.; Hudson, J.D.; Hackney, A.C.; Padua, D.A. Estrogen and muscle stiffness have a negative relationship in females. Knee Surg. Sports Traumatol. Arthrosc. 2012, 20, 361–367. [Google Scholar] [CrossRef]
- Waugh, C.M.; Korff, T.; Fath, F.; Blazevich, A.J. Rapid force production in children and adults: Mechanical and neural contributions. Med. Sci. Sports Exerc. 2013, 45, 762–771. [Google Scholar] [CrossRef]
- Kocur, P.; Wilski, M.; Goliwąs, M.; Lewandowski, J.; Łochyński, D. Influence of Forward Head Posture on Myotonometric Measurements of Superficial Neck Muscle Tone, Elasticity, and Stiffness in Asymptomatic Individuals with Sedentary Jobs. J. Manip. Physiol. Ther. 2019, 42, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liu, C.; Zhang, Z. Non-uniform Stiffness within Gastrocnemius-Achilles tendon Complex Observed after Static Stretching. J. Sports Sci. Med. 2019, 18, 454–461. [Google Scholar] [PubMed]
- Yu, B.; Liu, H.; Garrett, W.E. Mechanism of hamstring muscle strain injury in sprinting. J. Sport Health Sci. 2017, 6, 130–132. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, F.M.; Gleason, C.N.; Reiter, D.A.; Dunham, J.; Sayyid, S.K.; Labib, S.A. In vivo Sonographic Characterization of The Achilles Tendons in Healthy Young Collegiate Athletes as a Function of Ankle Position. J. Foot Ankle Surg. 2020, 59, 898–902. [Google Scholar] [CrossRef]
- Deng, L.; Zhang, X.; Xiao, S.; Yang, Y.; Li, L.; Fu, W. Changes in the Plantar Flexion Torque of the Ankle and in the Morphological Characteristics and Mechanical Properties of the Achilles Tendon after 12-Week Gait Retraining. Life 2020, 10, 159. [Google Scholar] [CrossRef] [PubMed]
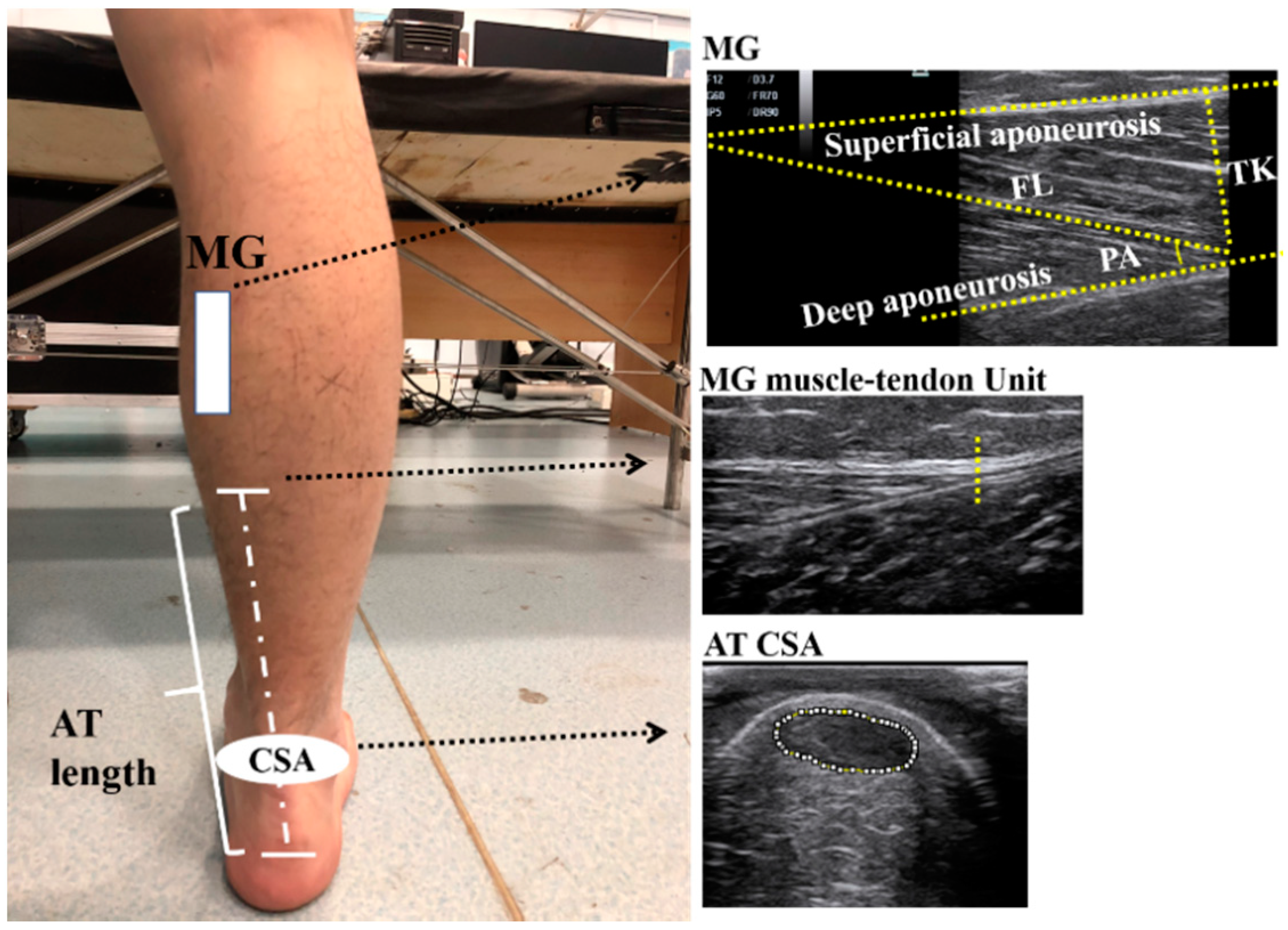
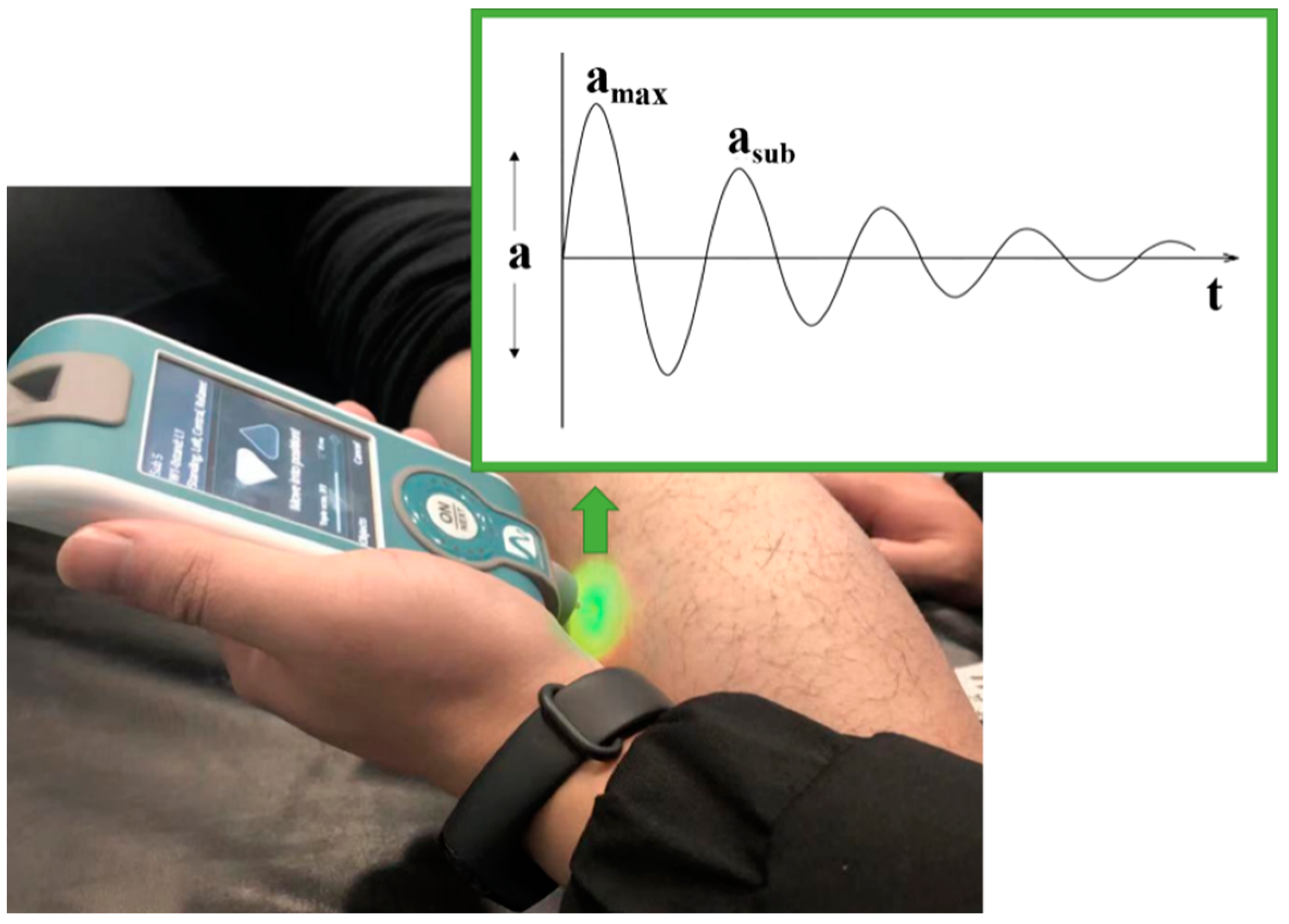

| Group | n | Age (years) | Height (cm) | Weight (kg) |
|---|---|---|---|---|
| Male | 18 | 24.4 ± 2.1 | 175 ± 3.9 | 69.7 ± 7.3 |
| Female | 18 | 25.1 ± 1.6 | 161.6 ± 4.3 | 52.8 ± 5.4 |
| p-Value | 0.261 | <0.001 | <0.001 | |
| Shank Length (cm) | FL (cm) | PA (°) | TK (cm) | Vertical Stiffness MG (N/m) | DMG | Length AT (cm) | CSA (cm2) | Vertical Stiffness AT (N/m) | DAT | |
|---|---|---|---|---|---|---|---|---|---|---|
| Female | 32.1 ± 1.7 | 5.7 ± 0.6 | 18.2 ± 2.2 | 1.7 ± 0.2 | 337.4 ± 89.8 | 1.0 ± 0.2 | 18.7 ± 1.9 | 0.4 ± 0.1 | 1205.4 ± 187.3 | 0.6 ± 0.3 |
| Male | 34.4 ± 1.9 | 5.9 ± 0.4 | 21.2 ± 2.4 | 2.0 ± 0.2 | 438.6 ± 86.4 | 0.9 ± 0.1 | 20.9 ± 2.6 | 0.6 ± 0.1 | 1228.6 ± 162.8 | 0.8 ± 0.3 |
| p | 0.001 | 0.226 | <0.001 | 0.001 | 0.002 | 0.001 | 0.005 | 0.001 | 0.695 | 0.164 |
| Cohen’s d | 1.28 | 0.39 | 1.30 | 1.06 | 0.78 | 1.25 | 1.13 | 2.00 | 0.13 | 0.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, L.; Zhang, X.; Xiao, S.; Wang, B.; Fu, W. Gender Difference in Architectural and Mechanical Properties of Medial Gastrocnemius–Achilles Tendon Unit In Vivo. Life 2021, 11, 569. https://doi.org/10.3390/life11060569
Deng L, Zhang X, Xiao S, Wang B, Fu W. Gender Difference in Architectural and Mechanical Properties of Medial Gastrocnemius–Achilles Tendon Unit In Vivo. Life. 2021; 11(6):569. https://doi.org/10.3390/life11060569
Chicago/Turabian StyleDeng, Liqin, Xini Zhang, Songlin Xiao, Baofeng Wang, and Weijie Fu. 2021. "Gender Difference in Architectural and Mechanical Properties of Medial Gastrocnemius–Achilles Tendon Unit In Vivo" Life 11, no. 6: 569. https://doi.org/10.3390/life11060569
APA StyleDeng, L., Zhang, X., Xiao, S., Wang, B., & Fu, W. (2021). Gender Difference in Architectural and Mechanical Properties of Medial Gastrocnemius–Achilles Tendon Unit In Vivo. Life, 11(6), 569. https://doi.org/10.3390/life11060569






