Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor
Abstract
:1. Introduction
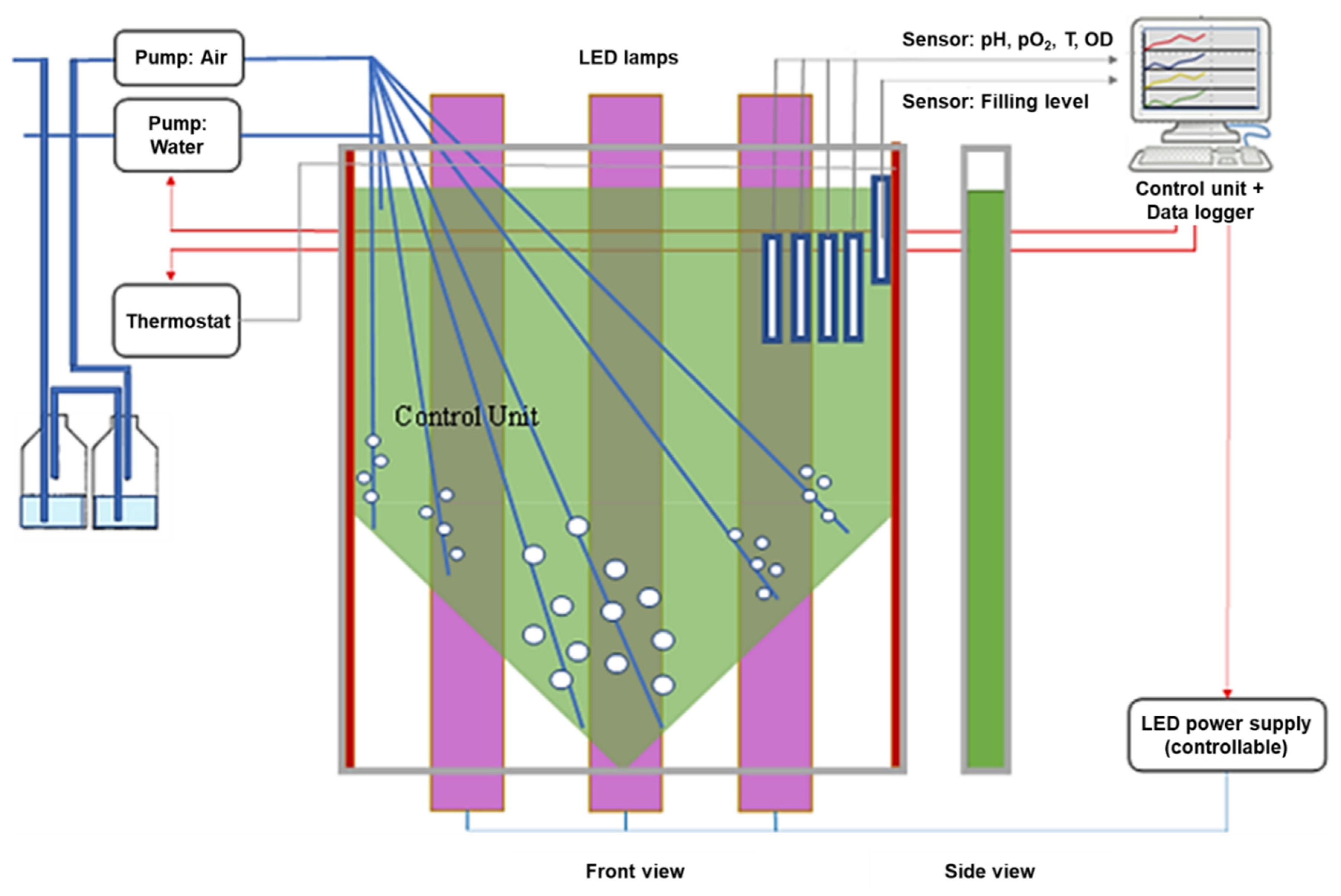
2. Materials and Methods
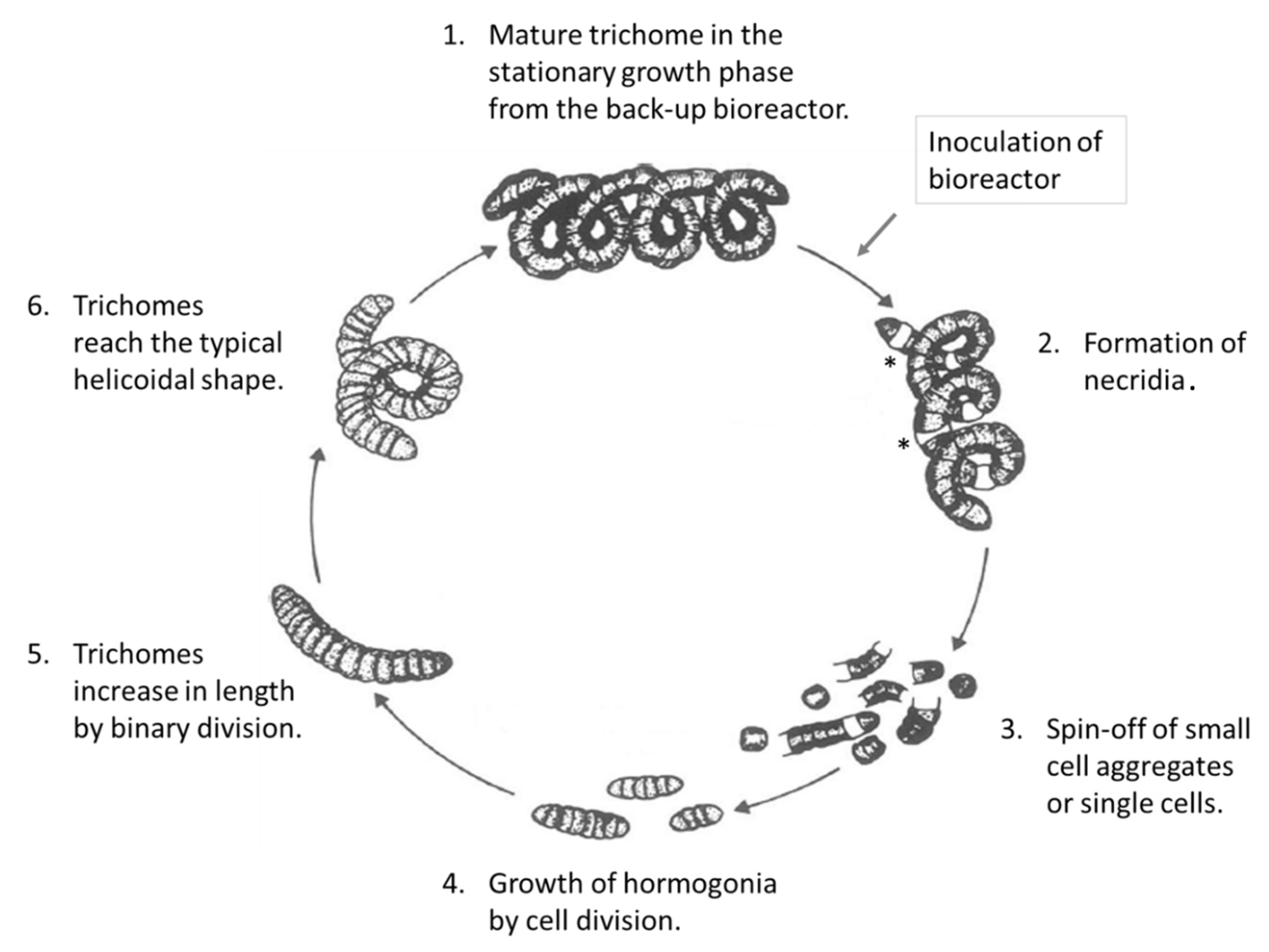
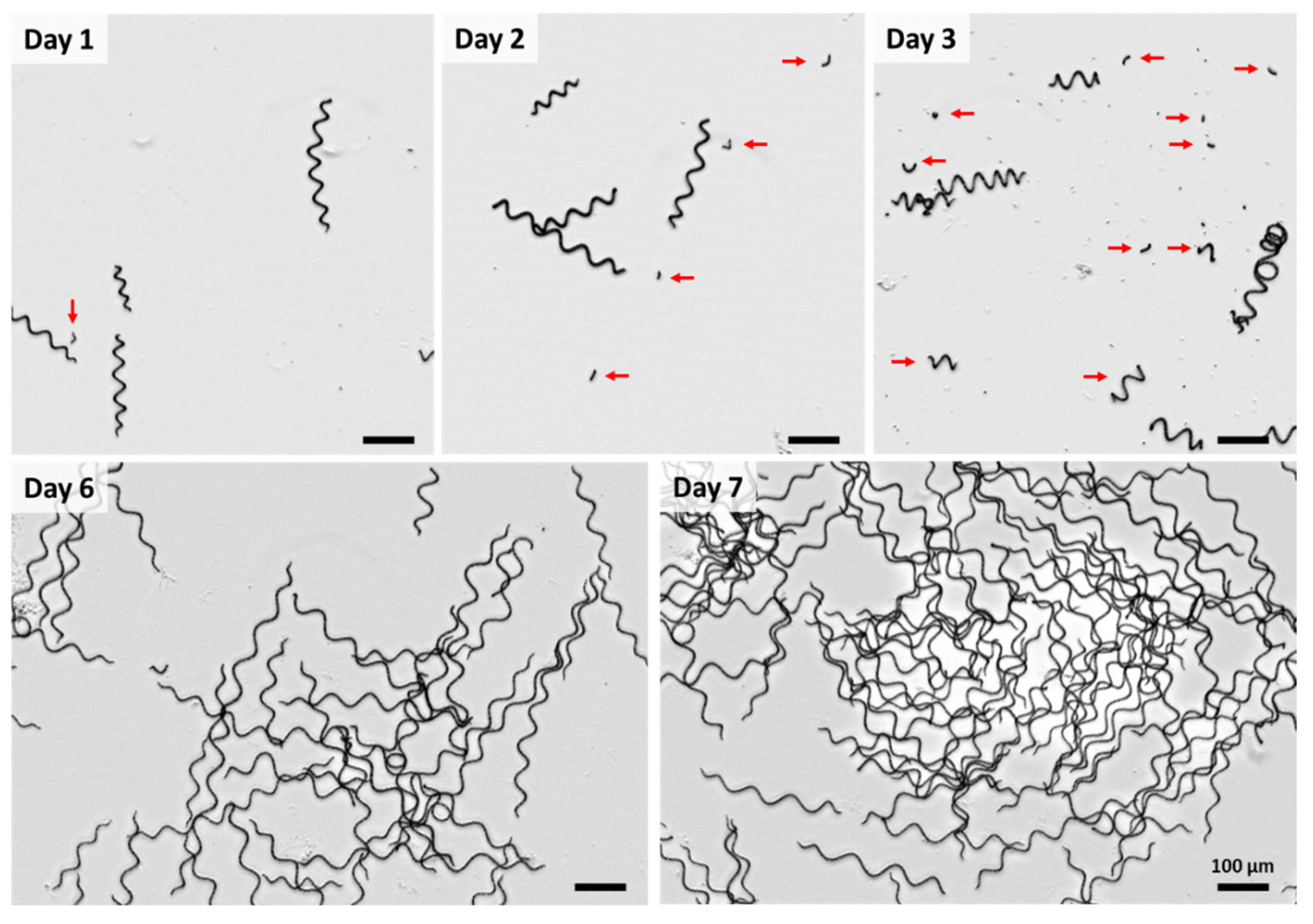
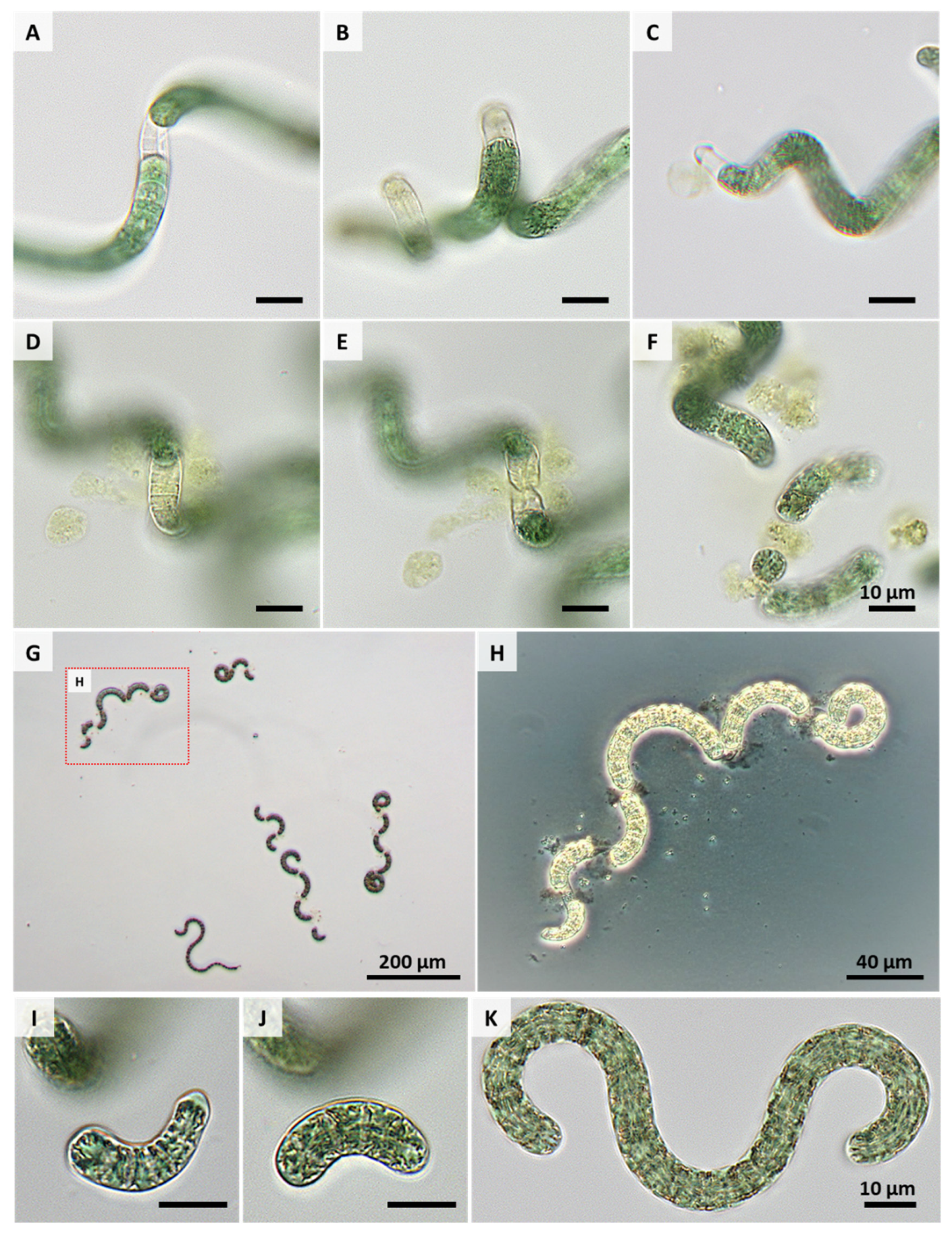
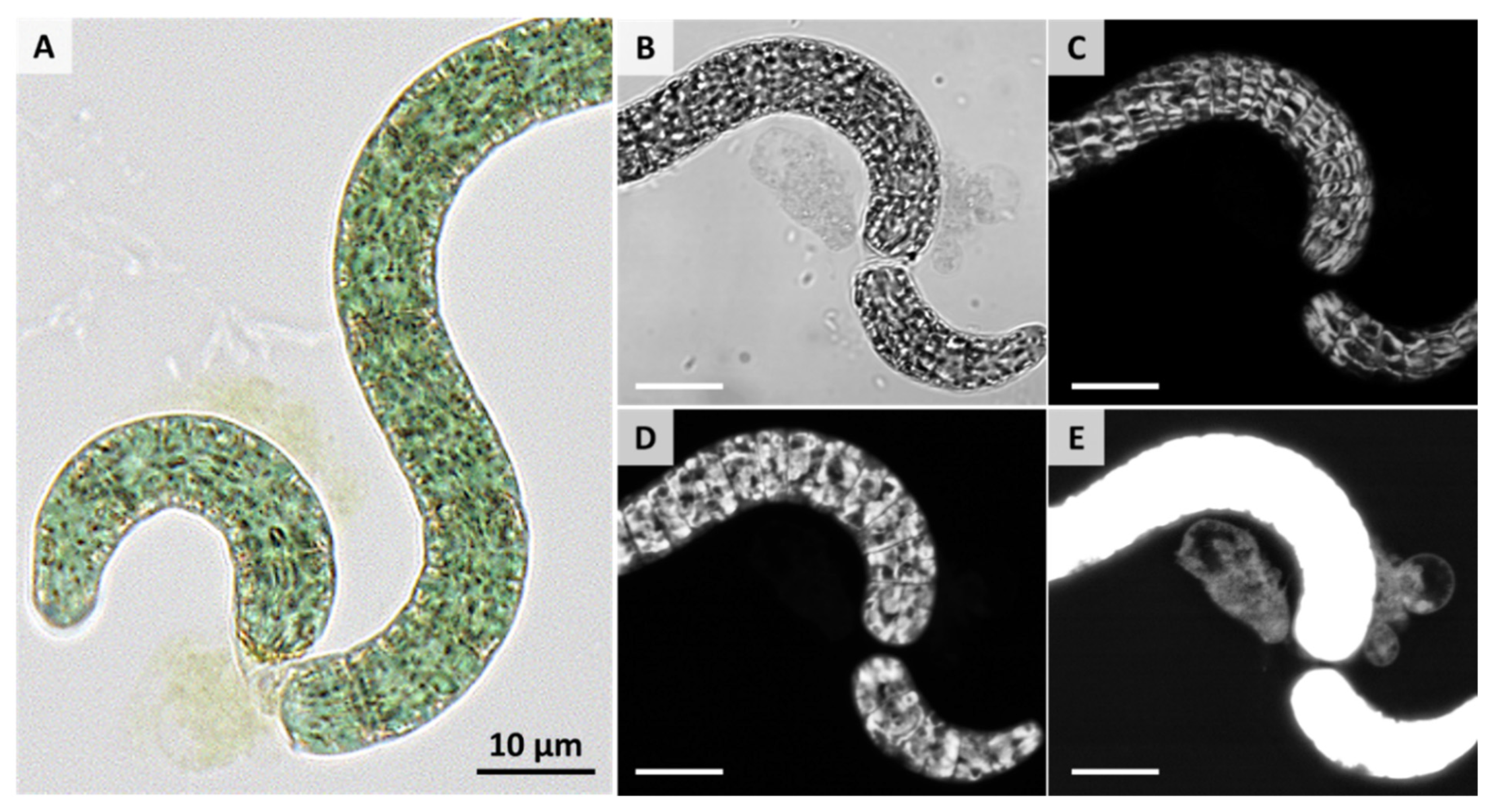
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Veaudor, T.; Blanc-Garin, V.; Chenebault, C.; Diaz-Santos, E.; Sassi, J.-F.; Cassier-Chauvat, C.; Chauvat, F. Recent Advances in the Photoautotrophic Metabolism of Cyanobacteria: Biotechnological Implications. Life 2020, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Habib, M.A.B.; Parvin, M.; Huntington, T.C.; Hasan, M.R. A Review on Culture, Production and Use of Spirulina as Food for Humans and Feed for Domestic Animals and Fish; FAO Fisheries and Aquaculture Circular No. 1034; Food and Agriculture Organization of the United Nations: Rome, Italy, 2008. [Google Scholar]
- Braune, S.; Krüger-Genge, A.; Kammerer, S.; Jung, F.; Küpper, J.-H. Phycocyanin from Arthrospira platensis as Potential Anti-Cancer Drug: Review of In Vitro and In Vivo Studies. Life 2021, 11, 91. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Steinbrecht, S.; Jung, C.G.H.; Westphal, S.; Klöpzig, S.; Waldeck, P.; Küpper, J.-H.; Storsberg, J.; Jung, F. Arthrospira platensis Accelerates the Formation of an Endothelial Cell Monolayer and Protects against Endothelial Cell Detachment after Bacterial Contamination. Clin. Hemorheol. Microcirc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.-H. Spirulina platensis, a Super Food? JCB 2019, 5, 43–54. [Google Scholar] [CrossRef]
- Qiang, H.; Zarmi, Y.; Richmond, A. Combined Effects of Light Intensity, Light-Path and Culture Density on Output Rate of Spirulina platensis (Cyanobacteria). Eur. J. Phycol. 1998, 33, 165–171. [Google Scholar] [CrossRef]
- Zeng, X.; Danquah, M.K.; Zhang, S.; Zhang, X.; Wu, M.; Chen, X.D.; Ng, I.-S.; Jing, K.; Lu, Y. Autotrophic Cultivation of Spirulina platensis for CO2 Fixation and Phycocyanin Production. Chem. Eng. J. 2012, 183, 192–197. [Google Scholar] [CrossRef]
- Rachedi, R.; Foglino, M.; Latifi, A. Stress Signaling in Cyanobacteria: A Mechanistic Overview. Life 2020, 10, 312. [Google Scholar] [CrossRef] [PubMed]
- Ciferri, O. Spirulina, the Edible Microorganism. Microbiol. Rev. 1983, 47, 551–578. [Google Scholar] [CrossRef] [PubMed]
- Zarrouk, C. Contribution a l’etude d’une Cianophycee: Influence de Divers Facteurs Physiques et Chimiques Sur La Croissance et La Photosynthese de Spirulina Maxima (Setch. et Garndner) Geitler. Ph.D. Thesis, Faculte des Sciences, Universite de Paris, Paris, France, 1966. [Google Scholar]
- Jung, C.G.H.; Waldeck, P.; Petrick, I.; Braune, S.; Küpper, J.-H.; Jung, F. Bioreactor for the Cultivation of Arthrospira platensis under Controlled Conditions. JCB 2021, 1–6. [Google Scholar] [CrossRef]
- Collins, T.J. ImageJ for Microscopy. BioTechniques 2007, 43, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Tomaselli, L.; Giovannetti, L.; Margheri, M.C. On the Mechanism of Trichome Breakage in Spirulina platensis and S. maxima. Ann. Microbiol. 1981, 31, 27–33. [Google Scholar]
- Springstein, B.L.; Nürnberg, D.J.; Weiss, G.L.; Pilhofer, M.; Stucken, K. Structural Determinants and Their Role in Cyanobacterial Morphogenesis. Life 2020, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Jung, C.G.H.; Krüger-Genge, A.; Waldeck, P.; Küpper, J.-H. Factors Influencing the Growth of Spirulina platensis in Closed Photobioreactors under CO2–O2 Conversion. JCB 2019, 5, 125–134. [Google Scholar] [CrossRef]
- Tomaselli, L. Morphology, Ultrastructure and Taxonomy of Arthrospira (Spirulina) maxima and Arthrospira (Spirulina) platensis. In Spirulina Platensis (Arthrospira): Physiology, Cell-Biology and Biotechnology; Taylor and Francis: London, UK, 2002. [Google Scholar]
- Ma, Z.; Gao, K. Spiral Breakage and Photoinhibition of Arthrospira platensis (Cyanophyta) Caused by Accumulation of Reactive Oxygen Species under Solar Radiation. Environ. Exp. Bot. 2010, 68, 208–213. [Google Scholar] [CrossRef]
- Kebede, E. Response of Spirulina platensis (= Arthrospira fusiformis) from Lake Chitu, Ethiopia, to Salinity Stress from Sodium Salts. Hydrobiologia 1997, 9, 551–558. [Google Scholar] [CrossRef]
- Wu, L.; Ho, J.A.; Shieh, M.-C.; Lu, I.-W. Antioxidant and Antiproliferative Activities of Spirulina and Chlorella Water Extracts. J. Agric. Food Chem. 2005, 53, 4207–4212. [Google Scholar] [CrossRef]
- Jeeji Bai, N.; Seshadri, C.V. On Coiling and Uncoiling of Trichomes in the Genus Spirulina. Algol. Stud. Arch. Für Hydrobiol. Suppl. Vol. 1980, 26, 32–47. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, C.H.G.; Braune, S.; Waldeck, P.; Küpper, J.-H.; Petrick, I.; Jung, F. Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor. Life 2021, 11, 536. https://doi.org/10.3390/life11060536
Jung CHG, Braune S, Waldeck P, Küpper J-H, Petrick I, Jung F. Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor. Life. 2021; 11(6):536. https://doi.org/10.3390/life11060536
Chicago/Turabian StyleJung, Conrad H. G., Steffen Braune, Peter Waldeck, Jan-Heiner Küpper, Ingolf Petrick, and Friedrich Jung. 2021. "Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor" Life 11, no. 6: 536. https://doi.org/10.3390/life11060536
APA StyleJung, C. H. G., Braune, S., Waldeck, P., Küpper, J.-H., Petrick, I., & Jung, F. (2021). Morphology and Growth of Arthrospira platensis during Cultivation in a Flat-Type Bioreactor. Life, 11(6), 536. https://doi.org/10.3390/life11060536






