Oxidized LDL Modifies the Association between Proteinuria and Deterioration of Kidney Function in Proteinuric Diabetic Kidney Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Study Protocol
2.3. Laboratory Analyses
2.4. Follow-up
2.5. Statistics
3. Results
3.1. Baseline Characteristics
3.2. Outcomes
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed]
- Looker, H.C.; Colombo, M.; Hess, S.; Brosnan, M.J.; Farran, B.; Dalton, R.N.; Wong, M.C.; Turner, C.; Palmer, C.N.; Nogoceke, E. Biomarkers of rapid chronic kidney disease progression in type 2 diabetes. Kidney Int. 2015, 88, 888–896. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Eleftheriadis, T.; Mertens, P.R. Oxidative Stress in Patients Undergoing Peritoneal Dialysis: A Current Review of the Literature. Oxid. Med. Cell. Longev. 2017, 2017, 3494867. [Google Scholar] [CrossRef] [PubMed]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Kamiński, T.W.; Pawlak, K.; Karbowska, M.; Myśliwiec, M.; Pawlak, D. Indoxyl sulfate—The uremic toxin linking hemostatic system disturbances with the prevalence of cardiovascular disease in patients with chronic kidney disease. BMC Nephrol. 2017, 18, 1–12. [Google Scholar] [CrossRef]
- Lara-Guzmán, O.J.; Gil-Izquierdo, Á.; Medina, S.; Osorio, E.; Álvarez-Quintero, R.; Zuluaga, N.; Oger, C.; Galano, J.-M.; Durand, T.; Muñoz-Durango, K. Oxidized LDL triggers changes in oxidative stress and inflammatory biomarkers in human macrophages. Redox Biol. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Hulthe, J.; Fagerberg, B. Circulating oxidized LDL is associated with subclinical atherosclerosis development and inflammatory cytokines (AIR Study). Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1162–1167. [Google Scholar] [CrossRef]
- Ujihara, N.; Sakka, Y.; Takeda, M.; Hirayama, M.; Ishii, A.; Tomonaga, O.; Babazono, T.; Takahashi, C.; Yamashita, K.; Iwamoto, Y. Association between plasma oxidized low-density lipoprotein and diabetic nephropathy. Diabetes Res. Clin. Pract. 2002, 58, 109–114. [Google Scholar] [CrossRef]
- Moro, E.; Zambon, C.; Pianetti, S.; Cazzolato, G.; Pais, M.; Bon, G.B. Electronegative low density lipoprotein subform (LDL-) is increased in type 2 (non-insulin-dependent) microalbuminuric diabetic patients and is closely associated with LDL susceptibility to oxidation. Acta Diabetol. 1998, 35, 161–164. [Google Scholar] [CrossRef]
- Lopes-Virella, M.F.; Carter, R.E.; Baker, N.L.; Lachin, J.; Virella, G.; Group, D.E.R. High levels of oxidized LDL in circulating immune complexes are associated with increased odds of developing abnormal albuminuria in Type 1 diabetes. Nephrol. Dial. Transplant. 2012, 27, 1416–1423. [Google Scholar] [CrossRef]
- Theuwissen, E.; Magdeleyns, E.J.; Braam, L.A.; Teunissen, K.J.; Knapen, M.H.; Binnekamp, I.A.; van Summeren, M.J.; Vermeer, C. Vitamin K status in healthy volunteers. Food Funct. 2014, 5, 229–234. [Google Scholar] [CrossRef]
- Tavridou, A.; Georgoulidou, A.; Roumeliotis, A.; Roumeliotis, S.; Giannakopoulou, E.; Papanas, N.; Passadakis, P.; Manolopoulos, V.G.; Vargemezis, V. Association of Plasma Adiponectin and Oxidized Low-Density Lipoprotein with Carotid Intima-Media Thickness in Diabetic Nephropathy. J. Diabetes Res. 2015, 2015, 507265. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Liakopoulos, V.; Roumeliotis, A.; Stamou, A.; Panagoutsos, S.; D’Arrigo, G.; Tripepi, G. Mutual effect modification between adiponectin and HDL as risk factors of cardiovascular events in Type 2 diabetes individuals: A cohort study. Int. Urol. Nephrol. 2021, 1–9. [Google Scholar] [CrossRef]
- De Mutsert, R.; Jager, K.J.; Zoccali, C.; Dekker, F.W. The effect of joint exposures: Examining the presence of interaction. Kidney Int. 2009, 75, 677–681. [Google Scholar] [CrossRef]
- Fine, J.P.; Gray, R.J. A proportional hazards model for the subdistribution of a competing risk. J. Am. Stat. Assoc. 1999, 94, 496–509. [Google Scholar] [CrossRef]
- Rossing, K.; Christensen, P.K.; Hovind, P.; Tarnow, L.; Rossing, P.; Parving, H.-H. Progression of nephropathy in type 2 diabetic patients. Kidney Int. 2004, 66, 1596–1605. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Panagoutsos, S.; Giannakopoulou, E.; Papanas, N.; Manolopoulos, V.G.; Passadakis, P.; Tavridou, A. Matrix Gla protein T-138C polymorphism is associated with carotid intima media thickness and predicts mortality in patients with diabetic nephropathy. J. Diabetes Complicat. 2017, 31, 1527–1532. [Google Scholar] [CrossRef]
- Kastarinen, H.; Vasunta, R.; Ukkola, O.; Kesäniemi, Y. Glomerular filtration rate is related to dipping pattern in ambulatory blood pressure monitoring—A cross-sectional population-based study. J. Hum. Hypertens. 2010, 24, 247–253. [Google Scholar] [CrossRef]
- Bian, S.-Y.; Guo, H.-Y.; Ye, P.; Luo, L.-M.; Wu, H.-M.; Xiao, W.-K.; Qi, L.-P.; Yu, H.-P.; Duan, L.-F. Association of glomerular filtration rate with arterial stiffness in Chinese women with normal to mildly impaired renal function. J. Geriatr. Cardiol. 2012, 9, 158. [Google Scholar]
- Agarwal, R.; Duffin, K.L.; Laska, D.A.; Voelker, J.R.; Breyer, M.D.; Mitchell, P.G. A prospective study of multiple protein biomarkers to predict progression in diabetic chronic kidney disease. Nephrol. Dial. Transplant. 2014, 29, 2293–2302. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Stamou, A.; Leivaditis, K.; Kantartzi, K.; Panagoutsos, S.; Liakopoulos, V. The Association of dp-ucMGP with Cardiovascular Morbidity and Decreased Renal Function in Diabetic Chronic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 6035. [Google Scholar] [CrossRef]
- Koye, D.N.; Magliano, D.J.; Reid, C.M.; Jepson, C.; Feldman, H.I.; Herman, W.H.; Shaw, J.E. Risk of progression of nonalbuminuric CKD to end-stage kidney disease in people with diabetes: The CRIC (Chronic Renal Insufficiency Cohort) Study. Am. J. Kidney Dis. 2018, 72, 653–661. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Liakopoulos, V.; Roumeliotis, A.; Stamou, A.; Panagoutsos, S.; D’Arrigo, G.; Tripepi, G. Prognostic Factors of Fatal and Nonfatal Cardiovascular Events in Patients With Type 2 Diabetes: The Role of Renal Function Biomarkers. Clin. Diabetes 2021, 39, 188–196. [Google Scholar] [CrossRef]
- Vistisen, D.; Andersen, G.S.; Hulman, A.; Persson, F.; Rossing, P.; Jørgensen, M.E. Progressive decline in estimated glomerular filtration rate in patients with diabetes after moderate loss in kidney function—Even without albuminuria. Diabetes Care 2019, 42, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Muskiet, M.H.; Smits, M.M.; Morsink, L.M.; Diamant, M. The gut–renal axis: Do incretin-based agents confer renoprotection in diabetes? Nat. Rev. Nephrol. 2014, 10, 88. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Eleftheriadis, T.; Liakopoulos, V. Is oxidative stress an issue in peritoneal dialysis? Semin. Dial. 2019, 32, 463–466. [Google Scholar] [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mechanisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2019, 32, 58–71. [Google Scholar] [CrossRef]
- Wetzel, M.D.; Gao, T.; Stanley, K.; Cooper, T.K.; Morris, S.M., Jr.; Awad, A.S. Enhancing kidney DDAH-1 expression by adenovirus delivery reduces ADMA and ameliorates diabetic nephropathy. Am. J. Physiol.-Ren. Physiol. 2020, 318, F509–F517. [Google Scholar] [CrossRef]
- Wetzel, M.D.; Stanley, K.; Maity, S.; Madesh, M.; Bopassa, J.C.; Awad, A.S. Homoarginine ameliorates diabetic nephropathy independent of nitric oxide synthase-3. Physiol. Rep. 2021, 9, e14766. [Google Scholar] [CrossRef]
- Yamamoto, N.; Toyoda, M.; Abe, M.; Kobayashi, T.; Kobayashi, K.; Kato, M.; Miyauchi, M.; Kimura, M.; Umezono, T.; Suzuki, D. Lectin-like oxidized LDL receptor-1 (LOX-1) expression in the tubulointerstitial area likely plays an important role in human diabetic nephropathy. Intern. Med. 2009, 48, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Kulah, E.; Tascilar, O.; Acikgoz, S.; Karadeniz, G.; Ozel Tekin, I.; Can, M.; Gun, B.; Barut, F.; Comert, M. Oxidized LDL accumulation in experimental renal ischemia reperfusion injury model. Ren. Fail. 2007, 29, 409–415. [Google Scholar] [CrossRef]
- Gutwein, P.; Abdel-Bakky, M.S.; Doberstein, K.; Schramme, A.; Beckmann, J.; Schaefer, L.; Amann, K.; Doller, A.; Kämpfer-Kolb, N.; Abdel-Aziz, A.A.H. CXCL16 and oxLDL are induced in the onset of diabetic nephropathy. J. Cell. Mol. Med. 2009, 13, 3809–3825. [Google Scholar] [CrossRef] [PubMed]
- Chatauret, N.; Favreau, F.; Giraud, S.; Thierry, A.; Rossard, L.; Le Pape, S.; Lerman, L.O.; Hauet, T. Diet-induced increase in plasma oxidized LDL promotes early fibrosis in a renal porcine auto-transplantation model. J. Transl. Med. 2014, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Bosmans, J.-L.; Holvoet, P.; Dauwe, S.E.; Ysebaert, D.K.; Chapelle, T.; Jürgens, A.; Kovacic, V.; Van Marck, E.A.; De Broe, M.E.; Verpooten, G.A. Oxidative modification of low-density lipoproteins and the outcome of renal allografts at 11/2 years. Kidney Int. 2001, 59, 2346–2356. [Google Scholar] [CrossRef]
- Menne, J.; Park, J.-K.; Boehne, M.; Elger, M.; Lindschau, C.; Kirsch, T.; Meier, M.; Gueler, F.; Fiebeler, A.; Bahlmann, F.H. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-α—Deficient diabetic mice. Diabetes 2004, 53, 2101–2109. [Google Scholar] [CrossRef]
- Spoto, B.; Mattace-Raso, F.; Sijbrands, E.; Pizzini, P.; Cutrupi, S.; D’Arrigo, G.; Tripepi, G.; Zoccali, C.; Mallamaci, F. Resistin and all-cause and cardiovascular mortality: Effect modification by adiponectin in end-stage kidney disease patients. Nephrol. Dial. Transplant. 2013, 28, iv181–iv187. [Google Scholar] [CrossRef]
- Spoto, B.; D’Arrigo, G.; Tripepi, G.; Bolignano, D.; Zoccali, C. Serum gamma-glutamyltransferase, oxidized LDL and mortality in the elderly. Aging Clin. Exp. Res. 2019, 1–5. [Google Scholar] [CrossRef]

| Estimated Glomerular Filtration Rate (mL/min/1.73 m2) | |||||
|---|---|---|---|---|---|
| All Subjects (n = 91) | Tertile 1 (n = 30) | Tertile 2 (n = 31) | Tertile 3 (n = 30) | p | |
| eGFR (mL/min/1.73 m2) | 59.6 (18–89.6) | 33.1 (18–41.3) | 59.6 (42–72) | 83.3 (73.3–89.6) | <0.0001 |
| Age (years) | 67 (47–84) | 71 (47–81) | 70 (50–84) | 63 (50–78) | 0.026 |
| Gender, Male (%) | 47 | 63 | 42 | 36 | 0.09 |
| History of CV events (yes, %) | 70.3 | 83.3 | 71 | 56.7 | 0.08 |
| Duration of T2DM (years) | 13 (7–35) | 16 (7–35) | 13 (7–28) | 10.5 (7–26) | 0.05 |
| Duration of hypertension (years) | 13.0 (2–42) | 16.5 (3–34) | 17 (3–42) | 12 (2–25) | 0.09 |
| Waist circumference (cm) | 106.4 (12.3) | 106.1 (11.6) | 108.8 (13.4) | 104.3 (11.9) | 0.36 |
| SBP (mm Hg) | 140 (100–180) | 145 (100–180) | 140 (120–180) | 130 (120–165) | 0.024 |
| DBP (mm Hg) | 80 (50–95) | 80 (50–95) | 80 (60–95) | 75 (60–90) | 0.12 |
| Hemoglobin (g/dL) | 12.6 (1.7) | 11.8 (1.8) | 13.0 (1.2) | 12.9 (1.6) | 0.011 |
| Fasting Glucose (mg/dL) | 157.8 (50.9) | 165.4 (68.2) | 150.5 (36.9) | 157.7 (43.2) | 0.86 |
| HbA1c (%) | 7.2 (5.0–11.6) | 7.6 (5.7–11.6) | 7.1 (5.0–10.8) | 7.2 (6.3–10.7) | 0.69 |
| Albumin (g/dL) | 4.3 (3.2–5.0) | 3.9 (3.2–5.0) | 4.4 (3.8–4.8) | 4.3 (4.0–4.9) | 0.009 |
| Total cholesterol (mg/dL) | 176 (103–345) | 171 (112–240) | 177 (128–300) | 174 (103–345) | 0.62 |
| LDL cholesterol (mg/dL) | 94.5 (41–245) | 96.5 (52–157) | 95.5 (64–206) | 92 (41–245) | 0.81 |
| HDL cholesterol (mg/dL) | 47 (27–105) | 42 (29–59) | 48 (27–84) | 49 (31–105) | 0.04 |
| Triglycerides (mg/dL) | 140 (52–450) | 180.5 (59–450) | 164 (66–292) | 100 (52–320) | <0.0001 |
| Oxidized LDL (U/L) | 66.2 (22.9–123.4) | 72.2 (33.2–96.7) | 70.1 (45.4–105.3) | 53.8 (22.9–123.4) | 0.022 |
| UPCR (g/g) | 0.15 (0.007–6) | 0.5 (0.007–6) | 0.14 (0.02–1.6) | 0.1 (0.01–0.98) | <0.001 |
| CRP (mg/dL) | 0.2 (0–11) | 0.31 (0–2.6) | 0.2 (0–11) | 0.1 (0–4) | 0.004 |
| eGFR Decline over 30% from Baseline or Progression to ESKD | ||
|---|---|---|
| Crude model | Adjusted model | |
| Variables (units of measurement) | SHR (95% CI), p | SHR (95% CI), p |
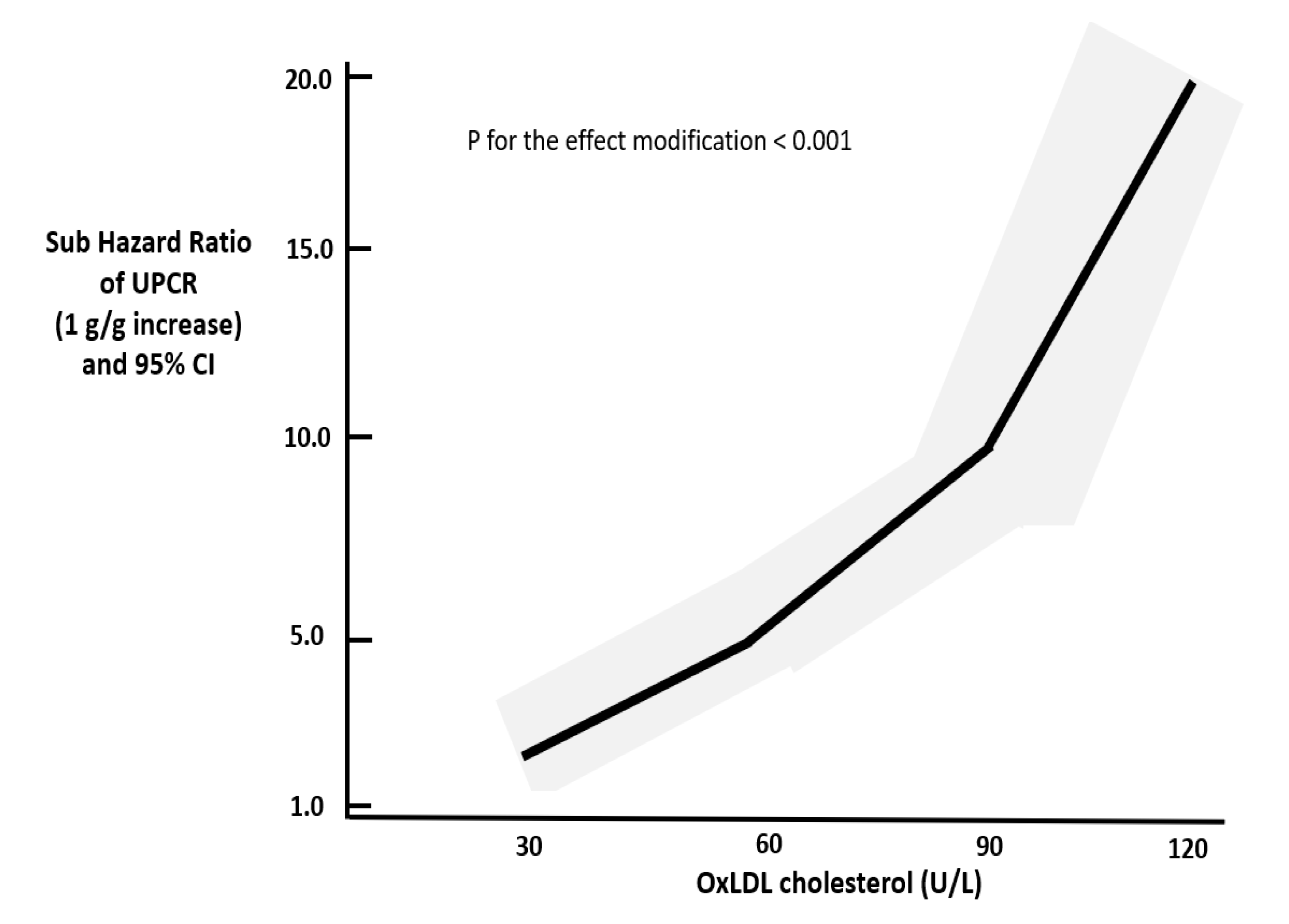
| Ox-LDL × UPCR interaction(U.L/g.g) Adjusted for the main effect of UPCR and Ox-LDL | 1.01 (1.00–1.01), p < 0.001 (see Figure 1) | 1.01 (0.98–1.04), p = 0.4 |
| Ox-LDL (U/L) | 1.05 (1.02–1.08) p < 0.0001 | 1.07 (1.03–1.12) p = 0.002 |
| UPCR (g/g) | 1.53 (1.15–2.03) p = 0.003 | 0.58 (0.06–5.9) p = 0.65 |
| eGFR(mL/min/1.73 m2) | 0.97 (0.95–0.99) p = 0.03 | 0.99 (0.96–1.04) p = 0.96 |
| SBP (mm Hg) | 0.96 (0.93–0.99) p = 0.01 | 0.97 (0.94–0.99) p = 0.05 |
| Duration of T2DM (years) | 1.05 (1.00–1.11) p = 0.03 | 1.07 (0.99–1.17) p = 0.09 |
| Serum albumin (g/dL) | 0.25 (0.11–0.60) p = 0.002 | 0.99 (0.95–1.05) p = 0.97 |
| β | Standard Error | p | |
|---|---|---|---|
| Model 1 | |||
| Ox-LDL × UPCR interaction | −0.001 | 0.003 | <0.0001 (see Figure 2) |
| Model 2 | |||
| Ox-LDL × UPCR interaction | −0.05 | 0.002 | 0.04 (see Figure 2) |
| Ox-LDL | −0.003 | 0.001 | 0.08 |
| UPCR | 0.30 | 0.17 | 0.08 |
| Duration of T2DM | −0.004 | 0.003 | 0.24 |
| Serum albumin | 0.02 | 0.07 | 0.81 |
| History of CV disease | 0.004 | 0.06 | 0.94 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roumeliotis, S.; Georgianos, P.I.; Roumeliotis, A.; Eleftheriadis, T.; Stamou, A.; Manolopoulos, V.G.; Panagoutsos, S.; Liakopoulos, V. Oxidized LDL Modifies the Association between Proteinuria and Deterioration of Kidney Function in Proteinuric Diabetic Kidney Disease. Life 2021, 11, 504. https://doi.org/10.3390/life11060504
Roumeliotis S, Georgianos PI, Roumeliotis A, Eleftheriadis T, Stamou A, Manolopoulos VG, Panagoutsos S, Liakopoulos V. Oxidized LDL Modifies the Association between Proteinuria and Deterioration of Kidney Function in Proteinuric Diabetic Kidney Disease. Life. 2021; 11(6):504. https://doi.org/10.3390/life11060504
Chicago/Turabian StyleRoumeliotis, Stefanos, Panagiotis I. Georgianos, Athanasios Roumeliotis, Theodoros Eleftheriadis, Aikaterini Stamou, Vangelis G. Manolopoulos, Stylianos Panagoutsos, and Vassilios Liakopoulos. 2021. "Oxidized LDL Modifies the Association between Proteinuria and Deterioration of Kidney Function in Proteinuric Diabetic Kidney Disease" Life 11, no. 6: 504. https://doi.org/10.3390/life11060504
APA StyleRoumeliotis, S., Georgianos, P. I., Roumeliotis, A., Eleftheriadis, T., Stamou, A., Manolopoulos, V. G., Panagoutsos, S., & Liakopoulos, V. (2021). Oxidized LDL Modifies the Association between Proteinuria and Deterioration of Kidney Function in Proteinuric Diabetic Kidney Disease. Life, 11(6), 504. https://doi.org/10.3390/life11060504











