Lipopolysaccharide-Induced Nitric Oxide and Prostaglandin E2 Production Is Inhibited by Tellimagrandin II in Mouse and Human Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Antibodies
2.2. Cell Culture
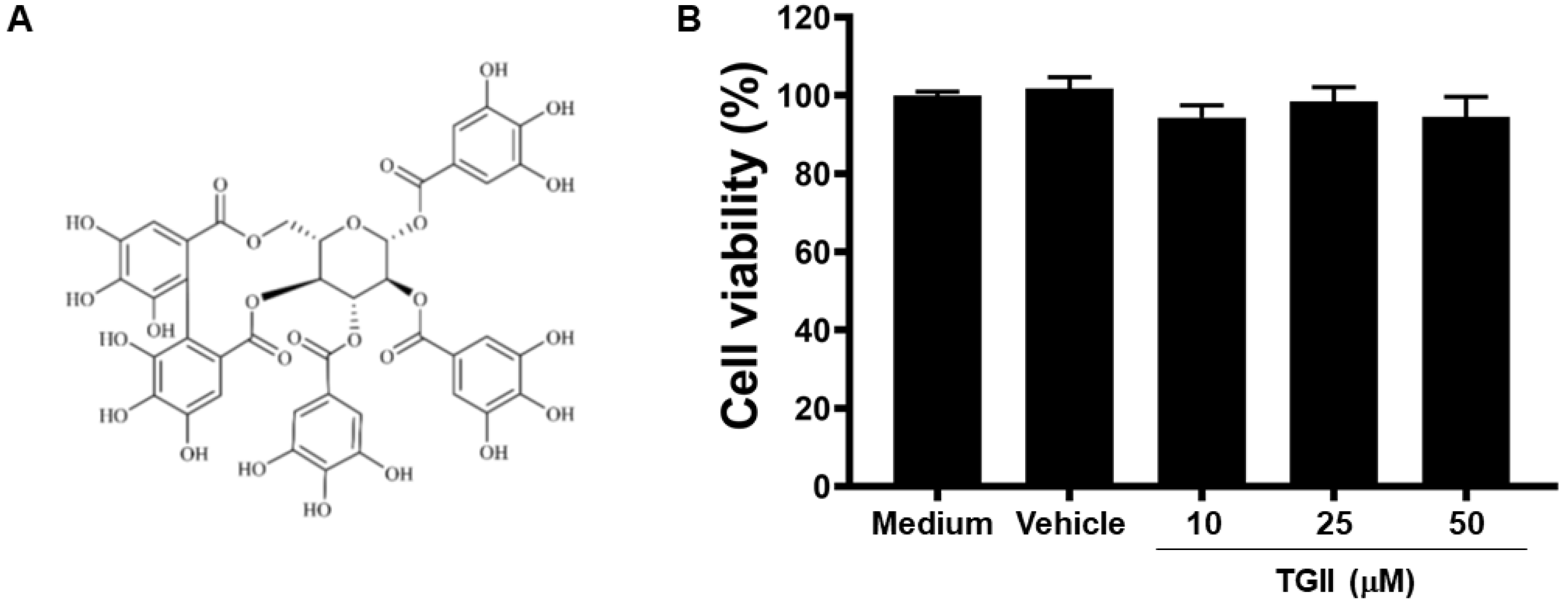
2.3. Cell Viability
2.4. Measurement of NO Production
2.5. Preparation of Nuclear and Cytosolic Fractions
2.6. RT-PCR and Western Blot Analysis
2.7. Enzyme-Linked Immunosorbent Assay (ELISA)
2.8. Isolation of Human CD14+ Monocytes and Stimulation into Macrophages
2.9. Statistical Analysis
3. Results
3.1. TGII Does Not Induce Macrophage Cytotoxicity
3.2. TGII Reduces LPS-Stimulated NO Production in Macrophages by Regulating NOS2 Transcription
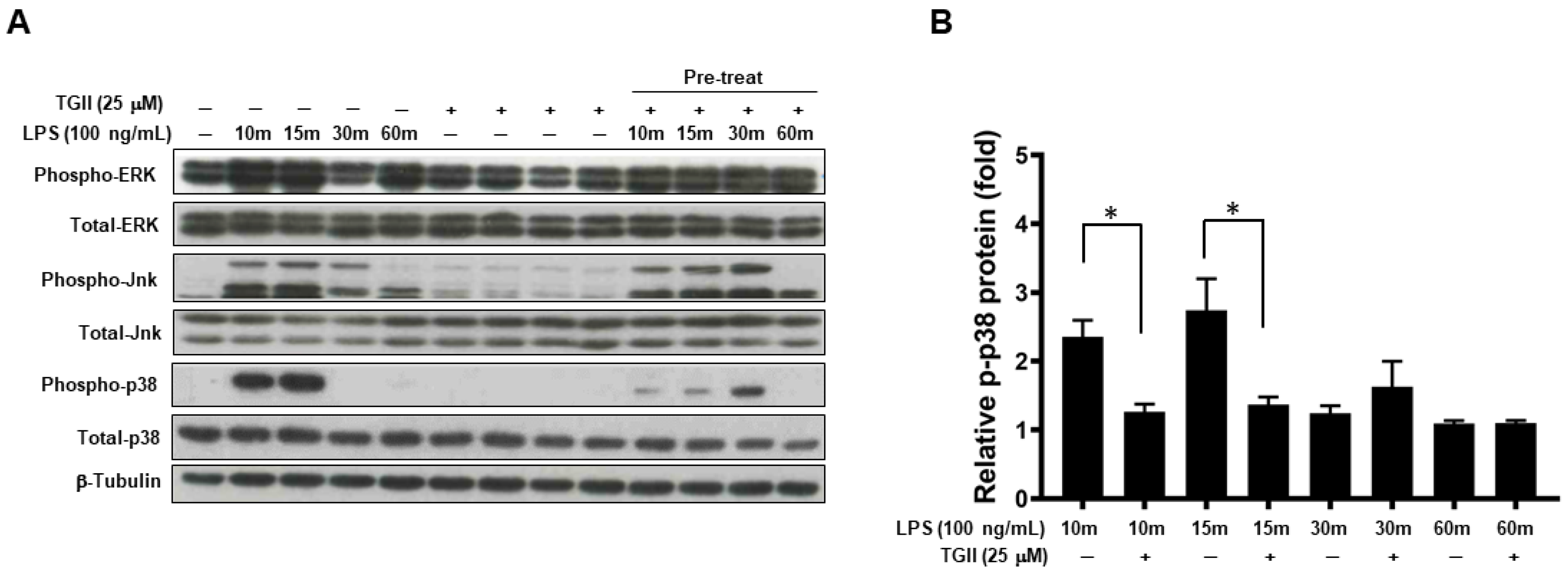
3.3. TGII Suppressed LPS-Stimulated Macrophage Activation Is Associated with Increased Phosphorylated-p-38 Expression
3.4. TGII Suppressed LPS-Stimulated Macrophage Activation Is Associated with the Increase in p-38 Downstream
3.5. TGII Inhibits COX-2 Signaling in LPS-Activated Macrophages
3.6. Effects of TGII on PGE2 Production in CD14+ Monocyte-Derived Macrophages after LPS Stimulation
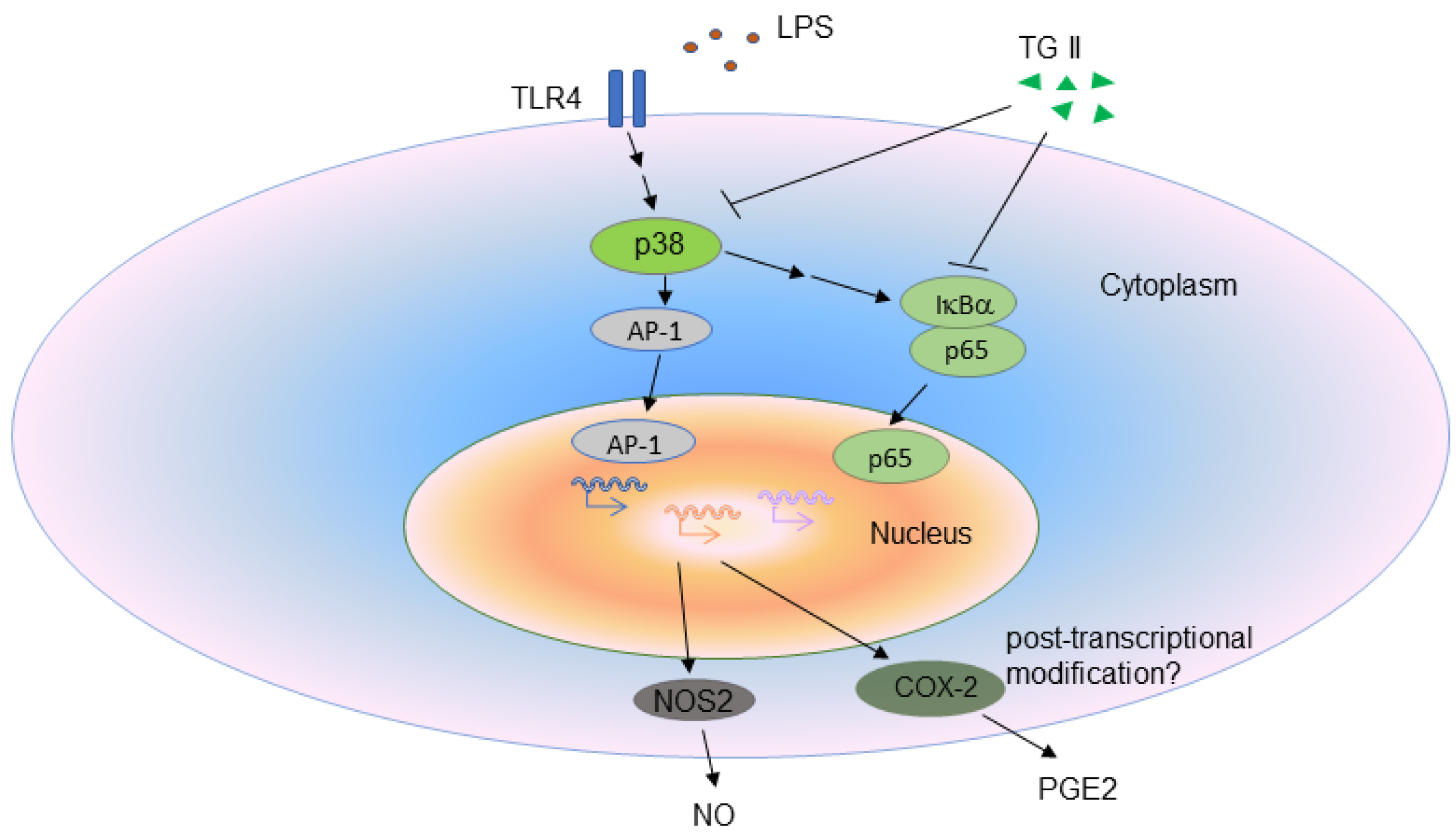
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grande, E.; Grippo, F.; Frova, L.; Pantosti, A.; Pezzotti, P.; Fedeli, U. The increase of sepsis-related mortality in Italy: A nationwide study, 2003–2015. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 1701–1708. [Google Scholar] [CrossRef] [PubMed]
- Rannikko, J.; Syrjänen, J.; Seiskari, T.; Aittoniemi, J.; Huttunen, R. Sepsis-related mortality in 497 cases with blood culture-positive sepsis in an emergency department. Int. J. Infect. Dis. 2017, 58, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Kakihana, Y.; Ito, T.; Nakahara, M.; Yamaguchi, K.; Yasuda, T. Sepsis-induced myocardial dysfunction: Pathophysiology and management. J. Intensiv. Care 2016, 4, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Shenoy, A.; Dous, G.; Kamran, H.; El-Sherif, N. Sepsis-Induced Takotsubo Cardiomyopathy Leading to Torsades de Pointes. Case Rep. Cardiol. 2016, 2016, 2384752. [Google Scholar] [CrossRef] [PubMed]
- Cavaillon, J.-M. Exotoxins and endotoxins: Inducers of inflammatory cytokines. Toxicon 2018, 149, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Kerfoot, S.M.; Kubes, P. Local coordination verses systemic disregulation: Complexities in leukocyte recruitment revealed by local and systemic activation of TLR4 in vivo. J. Leukoc. Biol. 2005, 77, 862–867. [Google Scholar] [CrossRef]
- Rosadini, C.V.; Kagan, J.C. Early innate immune responses to bacterial LPS. Curr. Opin. Immunol. 2017, 44, 14–19. [Google Scholar] [CrossRef]
- Chaudhry, H.; Zhou, J.; Zhong, Y.; Ali, M.M.; McGuire, F.; Nagarkatti, P.S.; Nagarkatti, M. Role of cytokines as a double-edged sword in sepsis. In Vivo 2013, 27, 669–684. [Google Scholar]
- Russell, J.A. Management of Sepsis. N. Engl. J. Med. 2006, 355, 1699–1713. [Google Scholar] [CrossRef]
- Zhong, Y.; Chiou, Y.-S.; Pan, M.-H.; Shahidi, F. Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem. 2012, 134, 742–748. [Google Scholar] [CrossRef]
- Murakami, A.; Ohigashi, H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. Int. J. Cancer 2007, 121, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Bambouskova, M.; Gorvel, L.; Lampropoulou, V.; Sergushichev, A.; Loginicheva, E.; Johnson, K.; Korenfeld, D.; Mathyer, M.E.; Kim, H.; Huang, L.; et al. Electrophilic properties of itaconate and derivatives regulate the IkappaBzeta-ATF3 inflammatory axis. Nature 2018, 556, 501–504. [Google Scholar] [CrossRef]
- Costa, A.S.H.; Higgins, M.; Hams, E.; Szpyt, J.; Runtsch, M.C.; King, M.S.; McGouran, J.F.; Fischer, R.; Kessler, B.M.; McGettrick, A.F.; et al. Itaconate is an anti-inflammatory metabolite that activates Nrf2 via alkylation of KEAP1. Nature 2018, 556, 113–117. [Google Scholar]
- Adkar, P.; Dongare, A.; Ambavade, S.; Bhaskar, V.H. Trapa bispinosa Roxb.: A Review on Nutritional and Pharmacological Aspects. Adv. Pharmacol. Sci. 2014, 2014, 959830. [Google Scholar] [CrossRef] [PubMed]
- Nitta, Y.; Kikuzaki, H.; Azuma, T.; Ye, Y.; Sakaue, M.; Higuchi, Y.; Komori, H.; Ueno, H. Inhibitory activity of Filipendula ulmaria constituents on recombinant human histidine decarboxylase. Food Chem. 2013, 138, 1551–1556. [Google Scholar] [CrossRef] [PubMed]
- Beckman, C.H. Phenolic-storing cells: Keys to programmed cell death and periderm formation in wilt disease resistance and in general defence responses in plants? Plant Pathol. 2000, 57, 101–110. [Google Scholar] [CrossRef]
- Focaccetti, C.; Izzi, V.; Benvenuto, M.; Fazi, S.; Ciuffa, S.; Giganti, M.G.; Potenza, V.; Manzari, V.; Modesti, A.; Bei, R. Polyphenols as Immunomodulatory Compounds in the Tumor Microenvironment: Friends or Foes? Int. J. Mol. Sci. 2019, 20, 1714. [Google Scholar] [CrossRef] [PubMed]
- Gaudry, A.; Bos, S.; Viranaicken, W.; Roche, M.; Krejbich-Trotot, P.; Gadea, G.; Desprès, P.; El-Kalamouni, C. The Flavonoid Isoquercitrin Precludes Initiation of Zika Virus Infection in Human Cells. Int. J. Mol. Sci. 2018, 19, 1093. [Google Scholar] [CrossRef]
- Matsumoto, T.; Kaneko, A.; Koseki, J.; Matsubara, Y.; Aiba, S.; Yamasaki, K. Pharmacokinetic Study of Bioactive Flavonoids in the Traditional Japanese Medicine Keigairengyoto Exerting Antibacterial Effects against Staphylococcus aureus. Int. J. Mol. Sci. 2018, 19, 328. [Google Scholar] [CrossRef]
- Pacheco-Ordaz, R.; Antunes-Ricardo, M.; Gutiérrez-Uribe, J.A.; González-Aguilar, G.A. Intestinal Permeability and Cellular Antioxidant Activity of Phenolic Compounds from Mango (Mangifera indica cv. Ataulfo) Peels. Int. J. Mol. Sci. 2018, 19, 514. [Google Scholar] [CrossRef]
- Bachmeier, B.E.; Melchart, D. Therapeutic Effects of Curcumin-From Traditional Past to Present and Future Clinical Applications. Int. J. Mol. Sci. 2019, 20, 3757. [Google Scholar] [CrossRef]
- Daglia, M. Polyphenols as antimicrobial agents. Curr. Opin. Biotechnol. 2012, 23, 174–181. [Google Scholar] [CrossRef]
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552. [Google Scholar] [CrossRef] [PubMed]
- Toro, M.D.; Nowomiejska, K.; Avitabile, T.; Rejdak, R.; Tripodi, S.; Porta, A.; Reibaldi, M.; Figus, M.; Posarelli, C.; Fiedorowicz, M. Effect of Resveratrol on In Vitro and In Vivo Models of Diabetic Retinophathy: A Systematic Review. Int. J. Mol. Sci. 2019, 20, 3503. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Huang, W.-C.; Lin, C.-Y.; Wang, W.-H.; Hung, L.-C.; Chen, Y.-H. Tellimagrandin II, A Type of Plant Polyphenol Extracted from Trapa bispinosa Inhibits Antibiotic Resistance of Drug-Resistant Staphylococcus aureus. Int. J. Mol. Sci. 2019, 20, 5790. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef]
- Huang, S.-H.; Lee, C.-H.; Wang, H.-M.; Chang, Y.-W.; Lin, C.-Y.; Chen, C.-Y.; Chen, Y.-H. 6-Dehydrogingerdione Restrains Lipopolysaccharide-Induced Inflammatory Responses in RAW 264.7 Macrophages. J. Agric. Food Chem. 2014, 62, 9171–9179. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Lee, C.-H.; Chang, Y.-W.; Wang, H.-M.; Chen, C.-Y.; Chen, Y.-H. Pheophytin a Inhibits Inflammation via Suppression of LPS-Induced Nitric Oxide Synthase-2, Prostaglandin E2, and Interleukin-1β of Macrophages. Int. J. Mol. Sci. 2014, 15, 22819–22834. [Google Scholar] [CrossRef]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell. Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef]
- van Grevenynghe, J.; Rion, S.; Le Ferrec, E.; le Vee, M.; Amiot, L.; Fauchet, R.; Fardel, O. Polycyclic aromatic hydrocarbons inhibit differentiation of human monocytes into macrophages. J. Immunol. 2003, 170, 2374–2381. [Google Scholar] [CrossRef]
- Shin, J.-S.; Park, Y.M.; Choi, J.-H.; Park, H.-J.; Shin, M.C.; Lee, Y.S.; Lee, K.-T. Sulfuretin isolated from heartwood of Rhus verniciflua inhibits LPS-induced inducible nitric oxide synthase, cyclooxygenase-2, and pro-inflammatory cytokines expression via the down-regulation of NF-κB in RAW 264.7 murine macrophage cells. Int. Immunopharmacol. 2010, 10, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Lee, M.S.; Shin, T.S.; Hua, H.; Jang, B.; Choi, J.; Byun, D.; Utsuki, T.; Ingram, D.; Kim, H. Phlorofucofuroeckol A inhibits the LPS-stimulated iNOS and COX-2 expressions in macrophages via inhibition of NF-kappaB, Akt, and p38 MAPK. Toxicol. In Vitro 2011, 25, 1789–1795. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1beta, IL-6 and TNF-alpha production by AP-1 and NF-kappaB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Ling-Chien, H.; Wang, W.-H.; Chen, S.-H.; Chang, Y.-W.; Hung, L.-C.; Chen, C.-Y.; Chen, Y.-H. Lipopolysaccharide-Induced Nitric Oxide, Prostaglandin E2, and Cytokine Production of Mouse and Human Macrophages Are Suppressed by Pheophytin-b. Int. J. Mol. Sci. 2017, 18, 2637. [Google Scholar] [CrossRef]
- Weinberg, J.B.; Misukonis, M.A.; Shami, P.J.; Mason, S.N.; Sauls, D.L.; Dittman, W.A.; Wood, E.R.; Smith, G.K.; McDonald, B.; Bachus, K.E.; et al. Human mononuclear phagocyte inducible nitric oxide synthase (iNOS): Analysis of iNOS mRNA, iNOS protein, biopterin, and nitric oxide production by blood monocytes and peritoneal macrophages. Blood 1995, 86, 1184–1195. [Google Scholar] [CrossRef] [PubMed]
- Fink, M.P.; Warren, H.S. Strategies to improve drug development for sepsis. Nat. Rev. Drug Discov. 2014, 13, 741–758. [Google Scholar] [CrossRef] [PubMed]
- Rice, T.W.; Wheeler, A.P.; Bernard, G.R.; Vincent, J.-L.; Angus, D.C.; Aikawa, N.; Demeyer, I.; Sainati, S.; Amlot, N.; Cao, C.; et al. A randomized, double-blind, placebo-controlled trial of TAK-242 for the treatment of severe sepsis. Crit. Care Med. 2010, 38, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Bernard, G.R.; Vincent, J.-L.; Laterre, P.-F.; LaRosa, S.P.; Dhainaut, J.-F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef]
- Nacira, S.; Meziani, F.; Dessebe, O.; Cattan, V.; Collin, S.; Montemont, C.; Gibot, S.; Asfar, P.; Ramaroson, A.; Regnault, V.; et al. Activated protein C improves lipopolysaccharide-induced cardiovascular dysfunction by decreasing tissular inflammation and oxidative stress. Crit. Care Med. 2009, 37, 246–255. [Google Scholar] [CrossRef]
- Lupp, C.; Baasner, S.; Ince, C.; Nocken, F.; Stover, J.F.; Westphal, M. Differentiated control of deranged nitric oxide metabolism: A therapeutic option in sepsis? Crit. Care 2013, 17, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Ejima, K.; Layne, M.D.; Carvajal, I.M.; Kritek, P.A.; Baron, R.M.; Chen, Y.-H.; Saal, J.V.; Levy, B.D.; Yet, S.-F.; Perrella, M.A. Cyclooxygenase-2 deficient mice are resistant to endotoxin-induced inflammation and death. FASEB J. 2003, 17, 1325–1327. [Google Scholar] [CrossRef] [PubMed]
- Reddy, R.C.; Chen, G.H.; Tateda, K.; Tsai, W.C.; Phare, S.M.; Mancuso, P.; Peters-Golden, M.; Standiford, T.J. Selective inhibition of COX-2 improves early survival in murine endotoxemia but not in bacterial peritonitis. Am. J. Physiol. Cell. Mol. Physiol. 2001, 281, L537–L543. [Google Scholar] [CrossRef]
- Tunçtan, B.; Altug, S.; Uludag, O.; Demirkay, B.; Abacioǧlu, N. Effects of cyclooxygenase inhibitors on nitric oxide production and survival in a mice model of sepsis. Pharmacol. Res. 2003, 48, 37–48. [Google Scholar] [CrossRef]
- Aronoff, D.M.; Canetti, C.; Peters-Golden, M. Prostaglandin E2Inhibits Alveolar Macrophage Phagocytosis through an E-Prostanoid 2 Receptor-Mediated Increase in Intracellular Cyclic AMP. J. Immunol. 2004, 173, 559–565. [Google Scholar] [CrossRef]
- Goldmann, O.; Hertzen, E.; Hecht, A.; Schmidt, H.; Lehne, S.; Norrby-Teglund, A.; Medina, E. Inducible cyclooxygenase released prostaglandin E2 modulates the severity of infection caused by Streptococcus pyogenes. J. Immunol. 2010, 185, 2372–2381. [Google Scholar] [CrossRef]
- Aronoff, D.M. Cyclooxygenase Inhibition in Sepsis: Is There Life after Death? Mediat. Inflamm. 2012, 2012, 696897. [Google Scholar] [CrossRef]
- Bone, R.C.; Jacobs, E.R.; Wilson, F.J. Increased hemodynamic and survival with endotoxin and septic shock with ibuprofen treatment. Prog. Clin. Biol. Res. 1987, 236, 327–332. [Google Scholar]
- Short, B.L.; Miller, M.K.; Pan, J. Group B streptococcal (GBSS) newborn septic shock model: The role of prostaglandins. Prog. Clin. Biol. Res. 1988, 264, 333–336. [Google Scholar]
- Baghaki, S.; Yalcin, C.E.; Baghaki, H.S.; Aydin, S.Y.; Daghan, B.; Yavuz, E. COX2 inhibition in the treatment of COVID-19: Review of literature to propose repositioning of celecoxib for randomized controlled studies. Int. J. Infect. Dis. 2020, 101, 29–32. [Google Scholar] [CrossRef]
- Kim, A.R.; Shin, T.S.; Lee, M.S.; Park, J.; Park, K.; Yoon, N.; Kim, J.; Choi, J.; Jang, B.; Byun, D.; et al. Isolation and identification of phlorotannins from Ecklonia stolonifera with antioxidant and anti-inflammatory properties. J. Agric. Food Chem. 2009, 57, 3483–3489. [Google Scholar] [CrossRef] [PubMed]
- Hiroi, M.; Sakaeda, Y.; Yamaguchi, H.; Ohmori, Y. Anti-Inflammatory Cytokine Interleukin-4 Inhibits Inducible Nitric Oxide Synthase Gene Expression in the Mouse Macrophage Cell Line RAW264.7 through the Repression of Octamer-Dependent Transcription. Mediat. Inflamm. 2013, 2013, 369693. [Google Scholar] [CrossRef] [PubMed]
- Lowenstein, C.J. iNOS (NOS2) at a glance. J. Cell Sci. 2004, 117, 2865–2867. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, C.-Y.; Kao, S.-H.; Hung, L.-C.; Chien, H.-J.; Wang, W.-H.; Chang, Y.-W.; Chen, Y.-H. Lipopolysaccharide-Induced Nitric Oxide and Prostaglandin E2 Production Is Inhibited by Tellimagrandin II in Mouse and Human Macrophages. Life 2021, 11, 411. https://doi.org/10.3390/life11050411
Lin C-Y, Kao S-H, Hung L-C, Chien H-J, Wang W-H, Chang Y-W, Chen Y-H. Lipopolysaccharide-Induced Nitric Oxide and Prostaglandin E2 Production Is Inhibited by Tellimagrandin II in Mouse and Human Macrophages. Life. 2021; 11(5):411. https://doi.org/10.3390/life11050411
Chicago/Turabian StyleLin, Chun-Yu, Shih-Han Kao, Ling-Chien Hung, Hsin-Ju Chien, Wen-Hung Wang, Yu-Wei Chang, and Yen-Hsu Chen. 2021. "Lipopolysaccharide-Induced Nitric Oxide and Prostaglandin E2 Production Is Inhibited by Tellimagrandin II in Mouse and Human Macrophages" Life 11, no. 5: 411. https://doi.org/10.3390/life11050411
APA StyleLin, C.-Y., Kao, S.-H., Hung, L.-C., Chien, H.-J., Wang, W.-H., Chang, Y.-W., & Chen, Y.-H. (2021). Lipopolysaccharide-Induced Nitric Oxide and Prostaglandin E2 Production Is Inhibited by Tellimagrandin II in Mouse and Human Macrophages. Life, 11(5), 411. https://doi.org/10.3390/life11050411






