Injectable Hydrogel for Cu2+ Controlled Release and Potent Tumor Therapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Isolation of Hierarchical Microstructures
2.3. Synthesis of Different Hydrogel
2.4. Structural Characterizations of the Hydrogel
2.5. Rheological Test
2.6. Photothermal Conversion Efficiency
2.7. In Vitro Phototoxicity of CRC
2.8. In Vivo Toxicity
3. Results
3.1. Characterization of CRC NPs
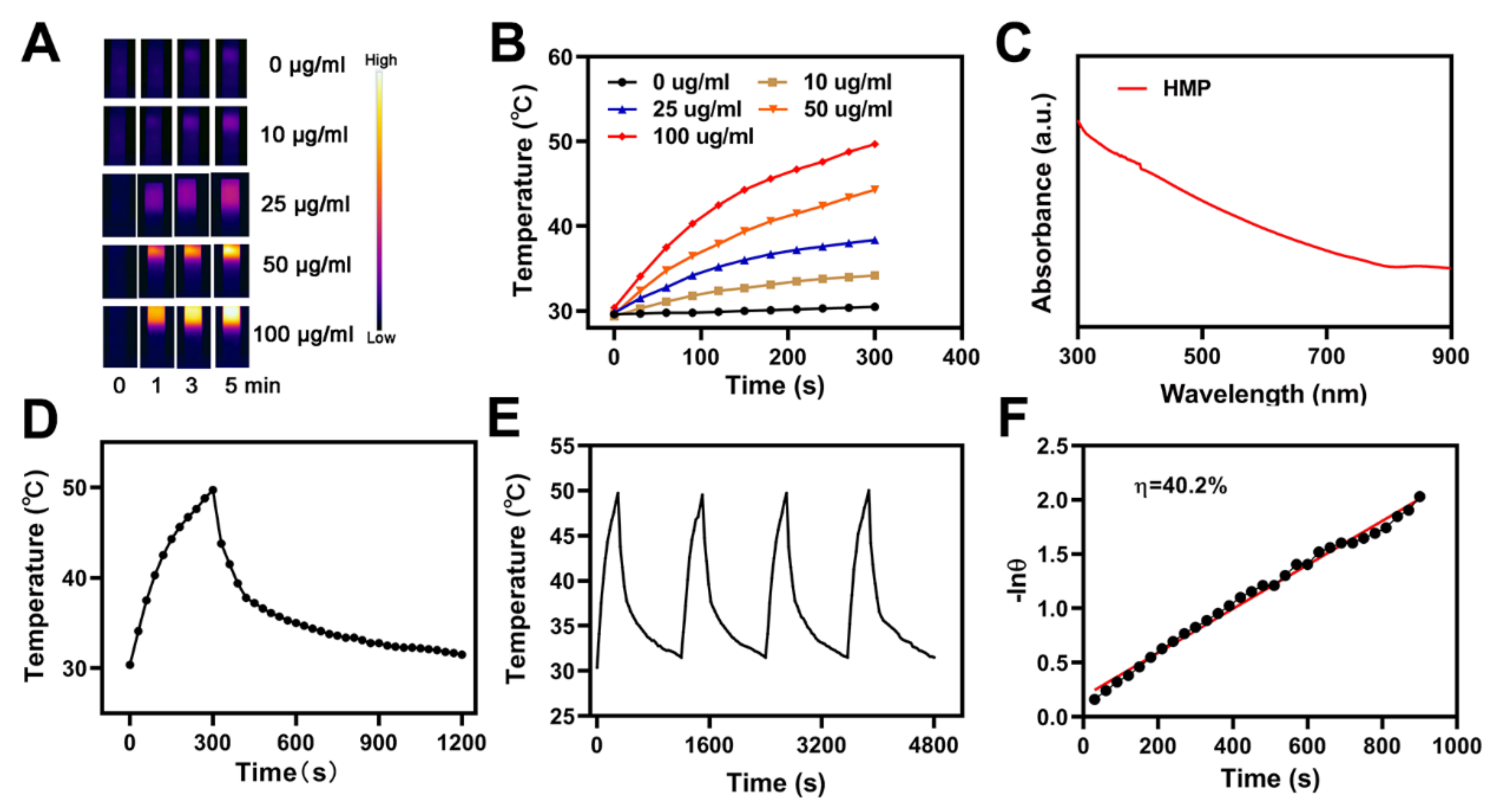
3.2. Photo-Thermal of the CRC for PTT
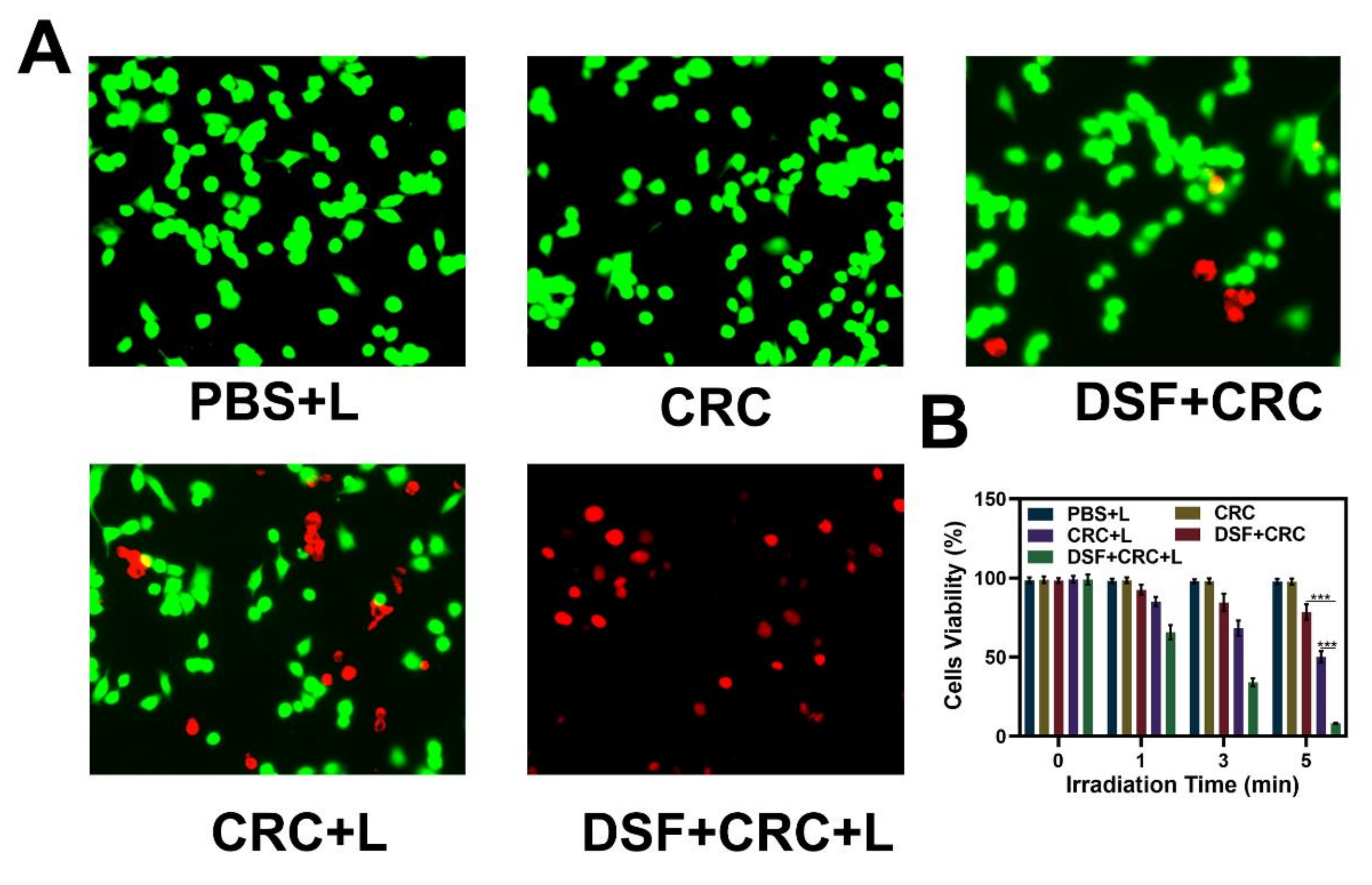
3.3. In vitro Combination Therapy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Wang, D.; Lee, M.M.S.; Shan, G.; Kwok, R.T.K.; Lam, J.W.Y.; Su, H.; Cai, Y.; Tang, B.Z. Highly efficient photosensitizers with far-red/near-infrared aggregation-induced emission for in vitro and in vivo cancer theranostics. Adv. Mater. 2018, 30, e1802105. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.; Liu, H.; Zhai, S.; Zhang, H.; Shan, G.; Kwok, R.T.K.; Ma, C.; Sung, H.H.Y.; Williams, I.D.; Lam, J.W.Y.; et al. Highly efficient singlet oxygen generation, two-photon photodynamic therapy and melanoma ablation by rationally designed mitochondria-specific near-infrared aiegens. Chem. Sci. 2020, 11, 2494–2503. [Google Scholar] [CrossRef]
- Jiang, W.; Zhang, H.; Wu, J.; Zhai, G.; Li, Z.; Luan, Y.; Garg, S. Cus@mof-based well-designed quercetin delivery system for chemo-photothermal therapy. ACS Appl. Mater. Interfaces 2018, 10, 34513–34523. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tan, L.; Meng, X. Nanoscale metal-organic frameworks: Synthesis, biocompatibility, imaging applications, and thermal and dynamic therapy of tumors. Adv. Funct. Mater. 2020, 30, 1908924. [Google Scholar] [CrossRef]
- Zhang, Z.; Sang, W.; Xie, L.; Dai, Y. Metal-organic frameworks for multimodal bioimaging and synergistic cancer chemotherapy. Coord. Chem. Rev. 2019, 399, 213022. [Google Scholar] [CrossRef]
- Wang, S.B.; Liu, X.H.; Li, B.; Fan, J.X.; Ye, J.J.; Cheng, H.; Zhang, X.Z. Bacteria-assisted selective photothermal therapy for precise tumor inhibition. Adv. Funct. Mater. 2019, 29, 1904093. [Google Scholar] [CrossRef]
- Kang, M.; Zhou, C.; Wu, S.; Yu, B.; Zhang, Z.; Song, N.; Lee, M.M.S.; Xu, W.; Xu, F.J.; Wang, D.; et al. Evaluation of structure-function relationships of aggregation-induced emission luminogens for simultaneous dual applications of specific discrimination and efficient photodynamic killing of gram-positive bacteria. J. Am. Chem. Soc. 2019, 141, 16781–16789. [Google Scholar] [CrossRef]
- Akin, D.; Sturgis, J.; Ragheb, K.; Sherman, D.; Burkholder, K.; Robinson, J.P.; Bhunia, A.K.; Mohammed, S.; Bashir, R. Bacteria-mediated delivery of nanoparticles and cargo into cells. Nat. Nanotechnol. 2007, 2, 441–449. [Google Scholar] [CrossRef]
- Lyu, M.; Zhu, D.; Li, Y.; Quan, H. Bimetallic nanodots for tri-modal ct/mri/pa imaging and hypoxia-resistant thermoradiotherapy in the nir-ii biological windows. Biomaterials 2019, 233, 119656. [Google Scholar] [CrossRef]
- Zhu, D.; Lyu, M.; Jiang, W.; Suo, M.; Huang, Q.; Li, K. A biomimetic nanozyme/camptothecin hybrid system for synergistically enhanced radiotherapy. J. Mater. Chem. B 2020, 8, 5312–5319. [Google Scholar] [CrossRef]
- Zhu, D.; Duo, Y.; Suo, M.; Zhao, Y.; Xia, L.; Zheng, Z.; Li, Y.; Tang, B.Z. Tumor-exocytosed exosome/aggregation-induced emission luminogen hybrid nanovesicles facilitate efficient tumor penetration and photodynamic therapy. Angew. Chem. 2020, 132, 13940–13947. [Google Scholar] [CrossRef]
- Zhu, D.; Lyu, M.; Huang, Q.; Suo, M.; Liu, Y.; Jiang, W.; Duo, Y.; Fan, K. Stellate plasmonic exosomes for penetrative targeting tumor nir-ii thermo-radiotherapy. ACS Appl. Mater. Interfaces 2020, 12, 36928–36937. [Google Scholar] [CrossRef]
- Dong, Z.; Feng, L.; Hao, Y.; Li, Q.; Chen, M.; Yang, Z.; Zhao, H.; Liu, Z. Synthesis of caco3-based nanomedicine for enhanced sonodynamic therapy via amplification of tumor oxidative stress. Chem 2020, 6, 1391–1407. [Google Scholar] [CrossRef]
- Han, W.; Zhang, S.; Deng, R.; Du, Y.; Qian, J.; Zheng, X.; Xu, B.; Xie, Z.; Yan, F.; Tian, W. Self-assembled nanostructured photosensitizer with aggregation-induced emission for enhanced photodynamic anticancer therapy. Sci. China Mater. 2019, 63, 136–146. [Google Scholar] [CrossRef]
- Wan, S.S.; Zhang, L.; Zhang, X.Z. An atp-regulated ion transport nanosystem for homeostatic perturbation therapy and sensitizing photodynamic therapy by autophagy inhibition of tumors. ACS Cent. Sci. 2019, 5, 327–340. [Google Scholar] [CrossRef]
- Chen, S.Y.; Xing, C.Y.; Huang, D.Z.; Zhou, C.H.; Ding, B.; Guo, Z.H.; Peng, Z.C.; Wang, D.; Zhu, X.; Liu, S.Z.; et al. Eradication of tumor growth by delivering novel photothermal selenium-coated tellurium nanoheterojunctions. Sci. Adv. 2020, 6, eaay6825. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Qiu, M.; Liang, X.; Liu, Q.; Zou, Q.; Li, Z.; Xie, Z.; Wang, D.; Dong, B.; et al. Conceptually novel black phosphorus/cellulose hydrogels as promising photothermal agents for effective cancer therapy. Adv. Healthc. Mater. 2018, 7, e1701510. [Google Scholar] [CrossRef]
- Tao, W.; Ji, X.; Zhu, X.; Li, L.; Wang, J.; Zhang, Y.; Saw, P.E.; Li, W.; Kong, N.; Islam, M.A.; et al. Two-dimensional antimonene-based photonic nanomedicine for cancer theranostics. Adv. Mater. 2018, 30, e1802061. [Google Scholar] [CrossRef]
- Xing, C.; Chen, S.; Liang, X.; Liu, Q.; Qu, M.; Zou, Q.; Li, J.; Tan, H.; Liu, L.; Fan, D.; et al. Two-dimensional mxene (ti3c2)-integrated cellulose hydrogels: Toward smart three-dimensional network nanoplatforms exhibiting light-induced swelling and bimodal photothermal/chemotherapy anticancer activity. ACS Appl. Mater. Interfaces 2018, 10, 27631–27643. [Google Scholar] [CrossRef] [PubMed]
- Morales, D.R.; Morris, A.D. Metformin in cancer treatment and prevention. Annu. Rev. Med. 2015, 66, 17–29. [Google Scholar] [CrossRef]
- Pernicova, I.; Korbonits, M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat. Rev. Endocrinol. 2014, 10, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Liang, S.; Cai, X.; Huang, S.; Cheng, Z.; Shi, Y.; Pang, M.; Ma, P.; Lin, J. Yolk-shell structured au nanostar@metal-organic framework for synergistic chemo-photothermal therapy in the second near-infrared window. Nano Lett. 2019, 19, 6772–6780. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yu, L.; Jiang, Q.; Huo, M.; Lin, H.; Wang, L.; Chen, Y.; Shi, J. Enhanced tumor-specific disulfiram chemotherapy by in situ cu(2+) chelation-initiated nontoxicity-to-toxicity transition. J. Am. Chem. Soc. 2019, 141, 11531–11539. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Cui, Q.C.; Yang, H.; Dou, Q.P. Disulfiram, a clinically used anti-alcoholism drug and copper-binding agent, induces apoptotic cell death in breast cancer cultures and xenografts via inhibition of the proteasome activity. Cancer Res. 2006, 66, 10425–10433. [Google Scholar] [CrossRef]
- Hao, Y.; Dong, Z.; Chen, M.; Chao, Y.; Liu, Z.; Feng, L.; Hao, Y.; Dong, Z.L.; Chen, M.C.; Chao, Y.; et al. Near-infrared light and glucose dual-responsive cascading hydroxyl radical generation for in situ gelation and effective breast cancer treatment. Biomaterials 2020, 228, 119568. [Google Scholar] [CrossRef]
- Chen, Z.; Meng, H.; Xing, G.; Chen, C.; Zhao, Y.; Jia, G.; Wang, T.; Yuan, H.; Ye, C.; Zhao, F.; et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol. Lett. 2006, 163, 109–120. [Google Scholar] [CrossRef]
- Ma, B.; Wang, S.; Liu, F.; Zhang, S.; Duan, J.; Li, Z.; Kong, Y.; Sang, Y.; Liu, H.; Bu, W.; et al. Self-assembled copper-amino acid nanoparticles for in situ glutathione “and” h2o2 sequentially triggered chemodynamic therapy. J. Am. Chem. Soc. 2019, 141, 849–857. [Google Scholar] [CrossRef]
- Wei, Y.; Zhu, N.; Lavoie, M.; Wang, J.; Qian, H.; Fu, Z. Copper toxicity to phaeodactylum tricornutum: A survey of the sensitivity of various toxicity endpoints at the physiological, biochemical, molecular and structural levels. BioMetals 2014, 27, 527–537. [Google Scholar] [CrossRef]
- Kennedy, D.C.; McKay, C.S.; Legault, M.C.B.; Danielson, D.C.; Blake, J.A.; Pegoraro, A.F.; Stolow, A.; Mester, Z.; Pezacki, J.P. Cellular consequences of copper complexes used to catalyze bioorthogonal click reactions. J. Am. Chem. Soc. 2011, 133, 17993–18001. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, S.; Yi, J.; Zhang, H.F.; Ameer, G.A. A cooperative copper metal-organic framework-hydrogel system improves wound healing in diabetes. Adv. Funct. Mater. 2017, 27, 1604872. [Google Scholar] [CrossRef]
- Gopal, A.; Kant, V.; Gopalakrishnan, A.; Tandan, S.K.; Kumar, D. Chitosan-based copper nanocomposite accelerates healing in excision wound model in rats. Eur. J. Pharmacol. 2014, 731, 8–19. [Google Scholar] [CrossRef]
- Qiu, M.; Wang, D.; Liang, W.; Liu, L.; Zhang, Y.; Chen, X.; Sang, D.K.; Xing, C.; Li, Z.; Dong, B.; et al. Novel concept of the smart nir-light-controlled drug release of black phosphorus nanostructure for cancer therapy. Proc. Natl. Acad. Sci. USA 2018, 115, 501–506. [Google Scholar] [CrossRef]
- Meng, Z.; Zhou, X.; Xu, J.; Han, X.; Dong, Z.; Wang, H.; Zhang, Y.; She, J.; Xu, L.; Wang, C.; et al. Light-triggered in situ gelation to enable robust photodynamic-immunotherapy by repeated stimulations. Adv. Mater. 2019, 31, e1900927. [Google Scholar] [CrossRef]
- Qiu, M.; Singh, A.; Wang, D.; Qu, J.; Swihart, M.; Zhang, H.; Prasad, P.N. Biocompatible and biodegradable inorganic nanostructures for nanomedicine: Silicon and black phosphorus. Nano Today 2019, 25, 135–155. [Google Scholar] [CrossRef]
- Luo, M.; Fan, T.; Zhou, Y.; Zhang, H.; Mei, L. 2d black phosphorus–based biomedical applications. Adv. Funct. Mater. 2019, 29, 1808306. [Google Scholar] [CrossRef]
- Hong, S.; Zhang, Q.L.; Zheng, D.W.; Zhang, C.; Zhang, Y.; Ye, J.J.; Cheng, H.; Zhang, X.Z. Enzyme mimicking based on the natural melanin particles from human hair. iScience 2020, 23, 100778. [Google Scholar] [CrossRef]
- Zheng, D.W.; Hong, S.; Xu, L.; Li, C.X.; Li, K.; Cheng, S.X.; Zhang, X.Z. Hierarchical micro-/nanostructures from human hair for biomedical applications. Adv. Mater. 2018, 30, e1800836. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.; Chen, B.; Chen, M.; Jiang, W.; Liu, W. Injectable Hydrogel for Cu2+ Controlled Release and Potent Tumor Therapy. Life 2021, 11, 391. https://doi.org/10.3390/life11050391
Huang C, Chen B, Chen M, Jiang W, Liu W. Injectable Hydrogel for Cu2+ Controlled Release and Potent Tumor Therapy. Life. 2021; 11(5):391. https://doi.org/10.3390/life11050391
Chicago/Turabian StyleHuang, Chunyu, Bei Chen, Mingzhu Chen, Wei Jiang, and Wei Liu. 2021. "Injectable Hydrogel for Cu2+ Controlled Release and Potent Tumor Therapy" Life 11, no. 5: 391. https://doi.org/10.3390/life11050391
APA StyleHuang, C., Chen, B., Chen, M., Jiang, W., & Liu, W. (2021). Injectable Hydrogel for Cu2+ Controlled Release and Potent Tumor Therapy. Life, 11(5), 391. https://doi.org/10.3390/life11050391





