Transcranial Magnetic Resonance-Guided Focused Ultrasound in X-Linked Dystonia-Parkinsonism
Abstract
1. Introduction
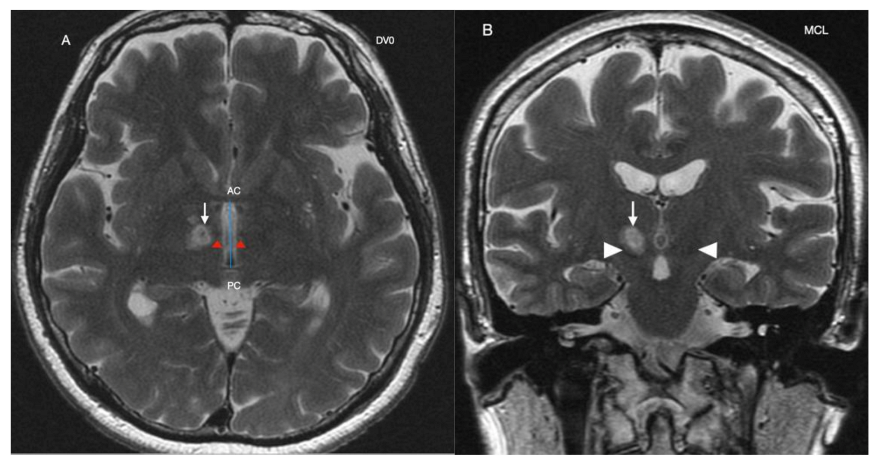
2. Case Reports
Procedure
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, L.V.; Rivera, C.; Teleg, R.A.; Dantes, M.B.; Pasco, P.M.; Jamora, R.D.; Arancillo, J.; Villareal-Jordan, R.F.; Rosales, R.L.; Demaisip, C.; et al. The unique phenomenology of sex-linked dystonia parkinsonism (XDP, DYT3, “Lubag”). Int. J. Neurosci. 2011, 121, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Jamora, R.D.; Ledesma, L.K.; Domingo, A.R.; Cenina, A.R.; Lee, L.V. Nonmotor features in sex-linked dystonia-parkinsonism (XDP, DYT3). Neurodegener. Dis. Manag. 2014, 4, 283–289. [Google Scholar] [CrossRef] [PubMed]
- Jamora, R.D.; Diesta, C.C.; Pasco, P.M.; Lee, L.V. Oral pharmacological treatment of X-linked dystonia parkinsonism: Successes and failures. Int. J. Neurosci. 2011, 121, 18–21. [Google Scholar] [CrossRef] [PubMed]
- Jamora, R.D.; Teleg, R.A.; Cordero, C.P.; Villareal-Jordan, R.F.; Lee, L.V.; Pasco, P.M. Levodopa+carbidopa in X-linked dystonia parkinsonism (XDP/DYT3/Lubag): A randomized, double-blind, placebo-controlled trial. Acta Med. Philipp. 2018, 52, 510–514. [Google Scholar] [CrossRef]
- Rosales, R.L.; Ng, A.R.; Santos, M.M.; Fernandez, H.H. The broadening application of chemodenervation in X-linked dystonia-parkinsonism (part II): An open-label experience with botulinum toxin-A (Dysport®) injections for oromandibular, lingual, and truncal-axial dystonias. Int. J. Neurosci. 2011, 121, 44–56. [Google Scholar] [CrossRef]
- De Roxas, R.C.; Jamora, R.D. Cost-analysis of the different treatment modalities in X-linked dystonia–parkinsonism. Front. Neurol. 2019, 10, 500. [Google Scholar] [CrossRef]
- Abejero, J.E.; Jamora, R.D.; Vesagas, T.S.; Teleg, R.A.; Rosales, R.L.; Anlacan, J.P.; Velasquez, M.S.; Aguilar, J.A. Long term outcomes of pallidal deep brain stimulation in X-linked dystonia parkinsonism (XDP): Up to 84 months follow up and review of literature. Parkinsonism Relat. Disord. 2019, 60, 81–86. [Google Scholar] [CrossRef]
- Kilbane, C.; Witt, J.; Galifianakis, N.B.; Glass, G.A.; Volz, M.; Heath, S.; Starr, P.A.; Ostrem, J.L. Long-term outcomes of bilateral pallidal deep brain stimulation for X-linked dystonia and parkinsonism. Stereotact. Funct. Neurosurg. 2018, 96, 320–326. [Google Scholar] [CrossRef]
- Brüggemann, N.; Domingo, A.; Rasche, D.; Moll, C.K.; Rosales, R.L.; Jamora, R.D.; Hanssen, H.; Münchau, A.; Graf, J.; Weissbach, A.; et al. Association of pallidal neurostimulation and outcome predictors with X-linked dystonia-parkinsonism. JAMA Neurol. 2019, 76, 211–216. [Google Scholar] [CrossRef]
- Chang, W.; Taira, T.; Jamora, R.D.; Chiu, P.; Lin, W. First experience with MR-guided focused ultrasound in the treatment of X-linked dystonia-parkinsonism (XDP). Mov. Disord. 2019, 34. Available online: https://www.mdsabstracts.org/abstract/first-experience-with-mr-guided-focused-ultrasound-in-the-treatment-of-x-linked-dystonia-parkinsonism-xdp/ (accessed on 1 April 2021).
- Horisawa, S.; Fukui, A.; Tanaka, Y.; Wendong, L.; Yamahata, H.; Kawamata, T.; Taira, T. Pallidothalamic tractotomy (Forel’s Field H1-tomy) for dystonia: Preliminary results. World Neurosurg. 2019, 129, e851–e856. [Google Scholar] [CrossRef]
- Gallay, M.N.; Moser, D.; Federau, C.; Jeanmonod, D. Anatomical and technical reappraisal of the pallidothalamic tractotomy with the incisionless transcranial MR-guided focused ultrasound. A technical note. Front. Surg. 2019, 6, 2. [Google Scholar] [CrossRef]
- Meng, Y.; Huang, Y.; Solomon, B.; Hynynen, K.; Scantlebury, N.; Schwartz, M.L.; Lipsman, N. MRI-guided focused ultrasound thalamotomy for patients with. medically-refractory essential tremor. J. Vis. Exp. 2017, 130, 56365. [Google Scholar] [CrossRef]
- Ghanouni, P.; Pauly, K.B.; Elias, W.J.; Henderson, J.; Sheehan, J.; Monteith, S.; Wintermark, M. Transcranial MRI-guided focused ultrasound: A review of the technologic and neurologic applications. AJR Am. J. Roentgenol. 2015, 205, 150–159. [Google Scholar] [CrossRef]
- Magara, A.; Bühler, R.; Moser, D.; Kowalski, M.; Pourtehrani, P.; Jeanmonod, D. First experience with MR-guided focused ultrasound in the treatment of Parkinson’s disease. J. Ther. Ultrasound. 2014, 2, 11. [Google Scholar] [CrossRef]
- Bond, A.E.; Shah, B.B.; Huss, D.S.; Dallapiazza, R.F.; Warren, A.; Harrison, M.B.; Sperling, S.A.; Wang, X.Q.; Gwinn, R.; Witt, J.; et al. Safety and efficacy of focused ultrasound thalamotomy for patients with medication-refractory, tremor-dominant Parkinson disease a randomized clinical trial. JAMA Neurol. 2017, 74, 1412–1418. [Google Scholar] [CrossRef]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A randomized trial of focused ultrasound thalamotomy for essential tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef]
- Horisawa, S.; Yamaguchi, T.; Abe, K.; Hori, H.; Sumi, M.; Konishi, Y.; Taira, T. A single case of MRI-guided focused ultrasound ventro-oral thalamotomy for musician’s dystonia. J. Neurosurg. 2018, 131, 384–386. [Google Scholar] [CrossRef]
- Krishna, V.; Sammartino, F.; Rezai, A. A review of the current therapies, challenges, and future directions of transcranial focused ultrasound technology advances in diagnosis and treatment. JAMA Neurol. 2018, 75, 246–254. [Google Scholar] [CrossRef]
- Masri, R.; Quiton, R.I.; Lucas, J.M.; Murray, P.D.; Thompson, S.M.; Keller, A. Zona incerta: A role in central pain. J. Neurophysiol. 2009, 102, 181–191. [Google Scholar] [CrossRef]
- Pasco, P.M.; Jamora, R.D.; Rosales, R.L.; Diesta, C.C.; Ng, A.R.; Teleg, R.A.; Go, C.L.; Lee, L.; Fernandez, H.H. Validation of the XDP-MDSP rating scale for the evaluation of patients with X-linked dystonia-parkinsonism. NPJ Park. Dis. 2017, 3, 24. [Google Scholar] [CrossRef]
- Godinho, F.; Magnin, M.; Filho, P.T.; Reis, P.; Moraes, O.; Nascimento, M.; Costa, C.; de Oliveira, M.O.; Rocha, M.S. Stereotactic lesion in the Forel’s Field H: A 2-years prospective open-label study on motor and nonmotor symptoms, neuropsychological functions, and quality of life in Parkinson disease. Neurosurgery 2019, 85, E650–E659. [Google Scholar] [CrossRef]
- Franzini, A.; Levi, V.; Franzini, A.; Dones, I.; Messina, G. Staged pallidotomy: MRI and clinical follow-up in status dystonicus. Br. J. Neurosurg. 2019, 33, 184–187. [Google Scholar] [CrossRef]
- Gallay, M.N.; Moser, D.; Magara, A.E.; Haufler, F.; Jeanmonod, D. Bilateral MR-guided focused ultrasound pallidothalamic tractotomy for Parkinson’s disease with 1-year follow up. Front. Neurol. 2021, 12, 601153. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, H.M.; Krishna, V.; Elias, W.J.; Cosgrove, G.R.; Gandhi, D.; Aldrich, C.E.; Fishman, P.S. MR-guided focused ultrasound pallidotomy for Parkinson’s disease: Safety and feasibility. J. Neurosurg. 2020, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
| XDP-MDSP Scale | Case 1 | Case 2 | Case 3 | Average | % Improvement Compared to Baseline |
|---|---|---|---|---|---|
| Baseline | |||||
| I—Dystonia | 13 | 34 | 9 | 18.7 | |
| II—Parkinsonism | 20 | 36 | 13 | 23 | |
| IIIA—Behavioral | 0 | 3 | 0 | 3 | |
| IIIB—Nonbehavioral | 1 | 15 | 3 | 1 | |
| IV—Activities of daily living | 12 | 36 | 10 | 19.3 | |
| Total | 46 | 124 | 36 | 68.7 | |
| 1 week | |||||
| I—Dystonia | 6 | 30 | 6 | 14 | 25.1 |
| II—Parkinsonism | 14 | 26 | 13 | 17.7 | 23.0 |
| IIIA—Behavioral | 0 | 1 | 0 | 0.3 | 90.0 |
| IIIB—Nonbehavioral | 0 | 6 | 2 | 2.7 | −170.0 |
| IV—Activities of daily living | 6 | 33 | 4 | 14.3 | 25.9 |
| Total | 26 | 96 | 26 | 49.3 | 28.2 |
| 1 month | |||||
| I—Dystonia | 5 | 27 | 5 | 12.3 | 34.2 |
| II—Parkinsonism | 15 | 29 | 13 | 19 | 17.4 |
| IIIA—Behavioral | 0 | 1 | 0 | 0.3 | 90.0 |
| IIIB—Nonbehavioral | 0 | 6 | 1 | 2.3 | −130.0 |
| IV—Activities of daily living | 6 | 30 | 4 | 13.3 | 31.0 |
| Total | 26 | 93 | 24 | 47.7 | 30.5 |
| 6 months | |||||
| I—Dystonia | 7 | 28 | 10 | 15 | 19.7 |
| II—Parkinsonism | 19 | 23 | 7 | 13 | 43.5 |
| IIIA—Behavioral | 0 | 1 | 0 | 0.3 | 89.0 |
| IIIB—Nonbehavioral | 1 | 6 | 2 | 3 | −200.0 |
| IV—Activities of daily living | 5 | 30 | 2 | 12.3 | 36.2 |
| Total | 33 | 88 | 23 | 48 | 30.1 |
| 9 months | |||||
| I—Dystonia | 14 | 25 | NA | 26.5 | −41.7 |
| II—Parkinsonism | 20 | 23 | NA | 21.5 | 6.5 |
| IIIA—Behavioral | 0 | 1 | NA | 0.5 | 83.3 |
| IIIB—Nonbehavioral | 3 | 6 | NA | 4.5 | −350.0 |
| IV—Activities of daily living | 13 | 30 | NA | 21.5 | −11.4 |
| Total | 50 | 85 | NA | 67.5 | 1.7 |
| 12 months | |||||
| I—Dystonia | 11 | NA | 10 | 10.5 | 43.8 |
| II—Parkinsonism | 23 | NA | 7 | 15 | 34.7 |
| IIIA—Behavioral | 1 | NA | 1 | 1 | 66.7 |
| IIIB—Nonbehavioral | 4 | NA | 5 | 4.5 | −350.0 |
| IV—Activities of daily living | 17 | NA | 10 | 13.5 | 30.0 |
| Total | 56 | NA | 35 | 45.5 | 33.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamora, R.D.G.; Chang, W.-C.; Taira, T. Transcranial Magnetic Resonance-Guided Focused Ultrasound in X-Linked Dystonia-Parkinsonism. Life 2021, 11, 392. https://doi.org/10.3390/life11050392
Jamora RDG, Chang W-C, Taira T. Transcranial Magnetic Resonance-Guided Focused Ultrasound in X-Linked Dystonia-Parkinsonism. Life. 2021; 11(5):392. https://doi.org/10.3390/life11050392
Chicago/Turabian StyleJamora, Roland Dominic G., Wei-Chieh Chang, and Takaomi Taira. 2021. "Transcranial Magnetic Resonance-Guided Focused Ultrasound in X-Linked Dystonia-Parkinsonism" Life 11, no. 5: 392. https://doi.org/10.3390/life11050392
APA StyleJamora, R. D. G., Chang, W.-C., & Taira, T. (2021). Transcranial Magnetic Resonance-Guided Focused Ultrasound in X-Linked Dystonia-Parkinsonism. Life, 11(5), 392. https://doi.org/10.3390/life11050392







