Processing of Flavor-Enhanced Oils: Optimization and Validation of Multiple Headspace Solid-Phase Microextraction-Arrow to Quantify Pyrazines in the Oils
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Standard Solutions
2.3. Sample Preparation
2.4. MHS-SPME Conditions
2.5. GC–MS Conditions
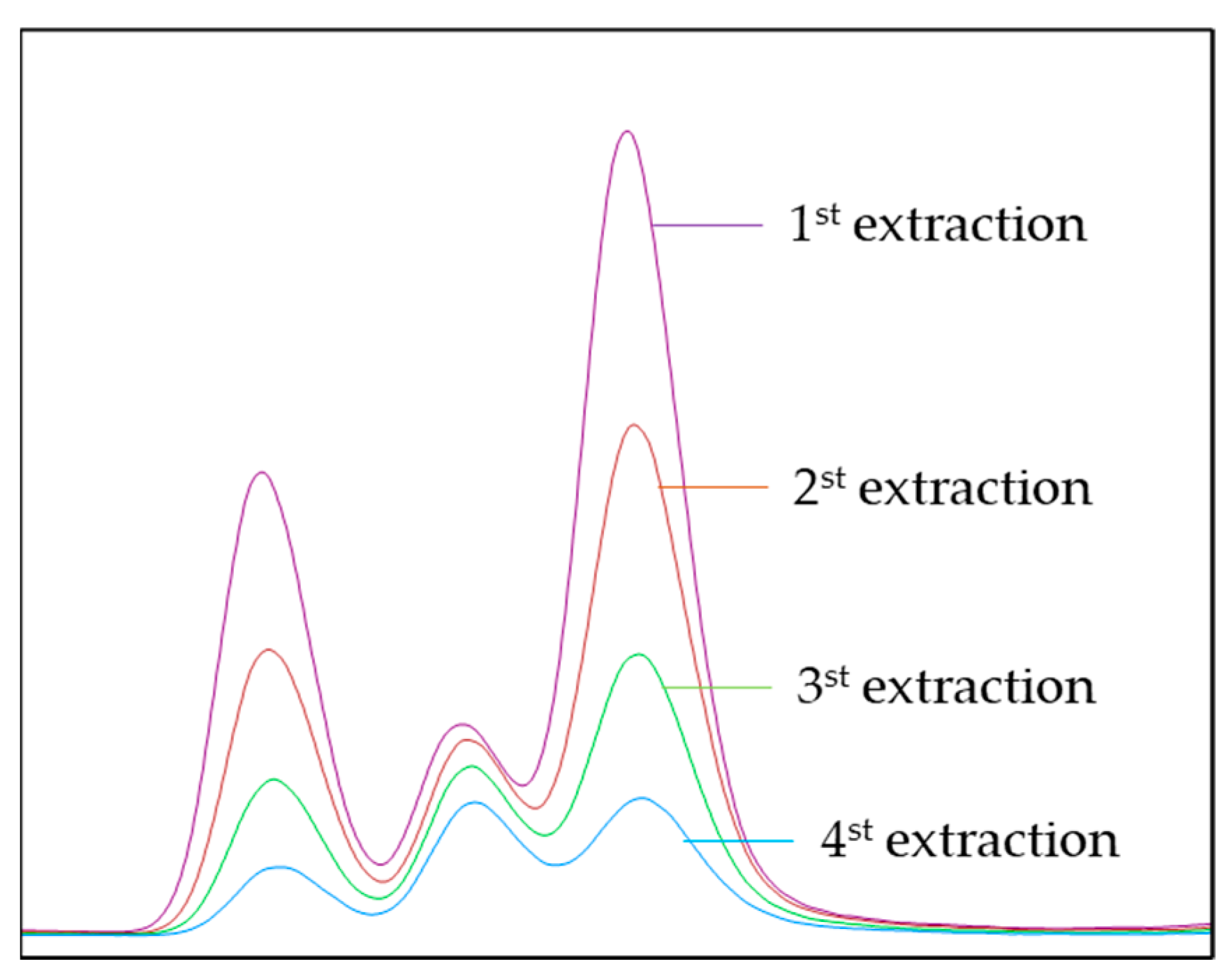
2.6. Quantification of MHS-SPME
2.7. Calculation of ISTD
2.8. Statistical Analysis
3. Results and Discussion
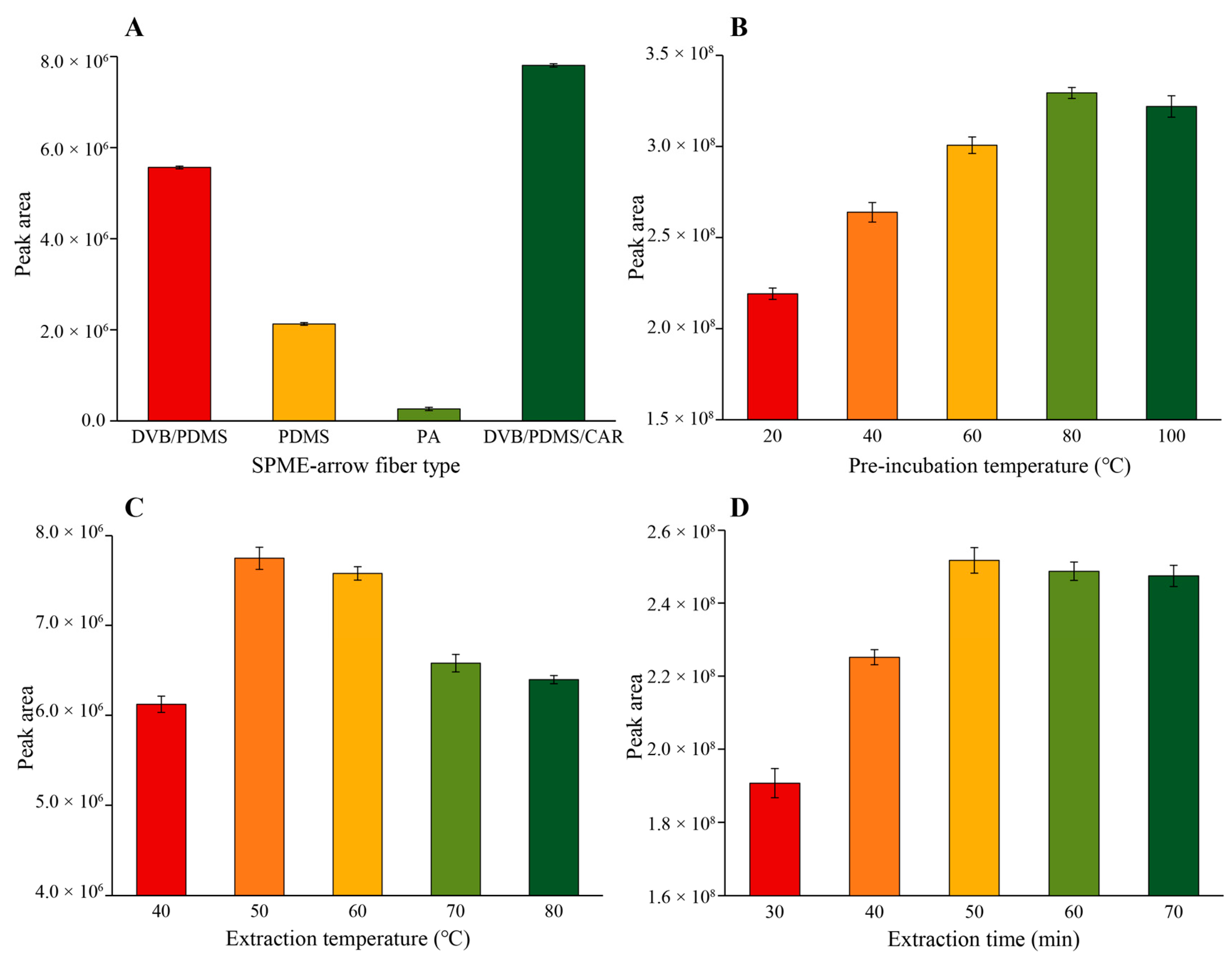
3.1. Optimization of HS-SPME Conditions
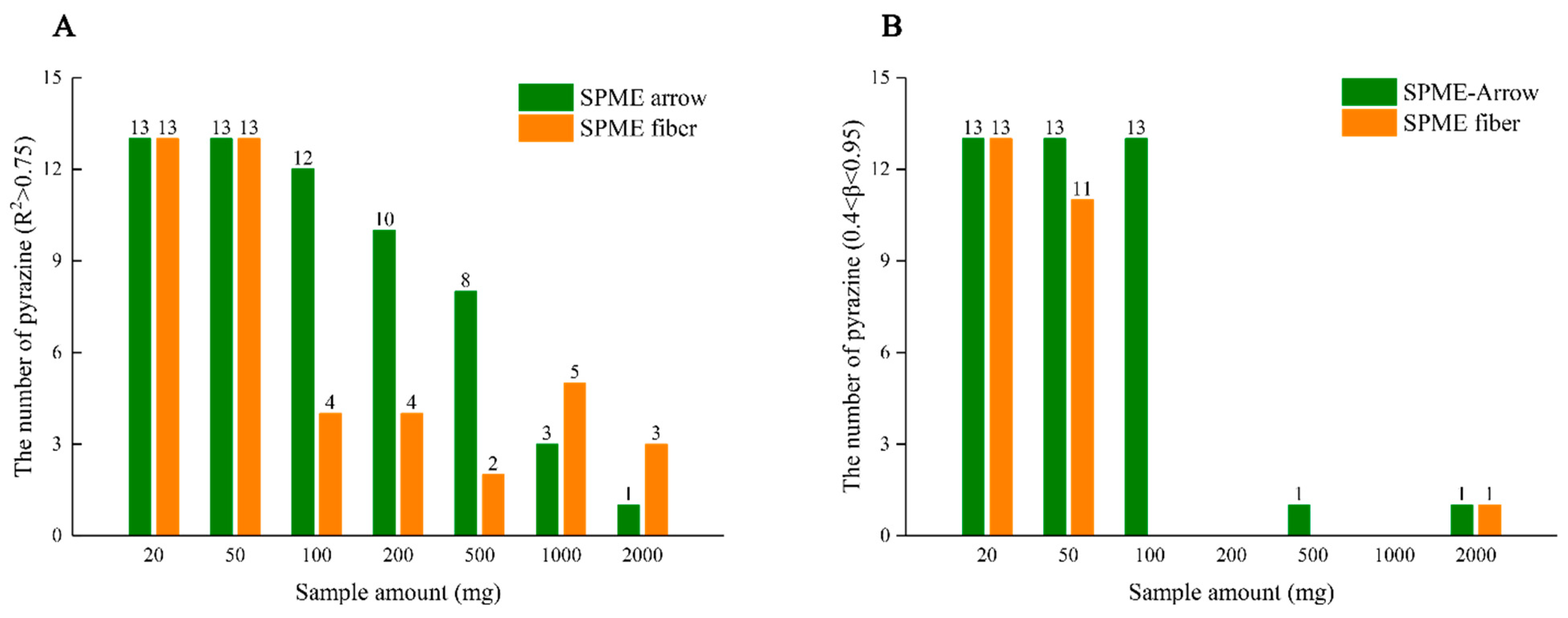
3.2. Amount of Oil Sample
3.3. Validation of MHS-SPME-Arrow Method
| Compound | Retention Time (min) | Selected Ions 1 | LOD 2 (ng/g) | LOQ 2 (ng/g) | Intra-Precision RSD (%) | Inter-Precision RSD (%) | Concentration Level 3 (ng/g) |
|---|---|---|---|---|---|---|---|
| 3-methyl, pyridine 4 | 15.93 | 66, 92, 93 | -- | -- | -- | -- | -- |
| 2-methyl-pyrazine | 14.78 | 67, 53, 94 | 3 | 6 | 7.13 | 8.66 | 907 |
| 2,5-dimethyl-pyrazine | 16.62 | 42, 81, 108 | 5 | 20 | 5.11 | 5.67 | 5011 |
| 2,6-dimethyl-pyrazine | 16.90 | 40, 42, 108 | 8 | 20 | 13.68 | 12.14 | 264 |
| Ethyl-pyrazine | 17.05 | 80, 108, 107 | 2 | 6 | 12.28 | 14.77 | 192 |
| 2-ethyl-6-methyl-pyrazine | 18.93 | 94,122, 121 | 3 | 6 | 9.64 | 12.27 | 363 |
| 2-ethyl-5-methyl-pyrazine | 19.21 | 94, 122, 121 | 3 | 10 | 7.83 | 9.56 | 586 |
| Trimethyl-pyrazine | 19.80 | 42, 81, 122 | 3 | 6 | 10.61 | 11.01 | 622 |
| 2,6-diethyl-pyrazine | 21.04 | 108, 136, 135 | 3 | 10 | 13.69 | 15.10 | 43 |
| 3-ethyl-2,5-dimethyl-pyrazine | 21.41 | 108, 136, 135 | 4 | 20 | 11.28 | 10.78 | 1837 |
| 2-ethyl-3,5-dimethyl-pyrazine | 22.28 | 108, 136, 135 | 5 | 20 | 13.21 | 15.08 | 129 |
| 3,5-diethyl-2-methyl-pyrazine | 23.63 | 122, 135, 149 | 6 | 20 | 13.09 | 12.99 | 177 |
| Isopropenyl-pyrazine | 29.02 | 67, 120, 119 | 60 | 180 | 12.67 | 14.98 | 190 |
| 5H-5-Methyl-6,7-dihydrocyclopenta-pyrazine | 30.18 | 119, 133, 134 | 25 | 50 | 10.00 | 14.02 | 94 |
| Compound | Spiked Level (ng/g) | Recovery 1 (%) | Spiked Level (ng/g) | Recovery 1 (%) | Spiked Level (ng/g) | Recovery 1 (%) |
|---|---|---|---|---|---|---|
| 2-methyl-pyrazine | 342 | 104.4 ± 15.0 | 1710 | 106.1 ± 4.13 | 3420 | 98.5 ± 6.9 |
| 2,5-dimethyl-pyrazine | 642 | 99.8 ± 7.7 | 3210 | 111.3 ± 13.0 | 6420 | 93.9 ± 12.5 |
| 2,6-dimethyl-pyrazine | 382 | 109.8 ± 6.0 | 1910 | 104.3 ± 4.2 | 3820 | 100.9 ± 13.7 |
| Ethyl-pyrazine | 364 | 105.2 ± 2.9 | 1820 | 95.3 ± 9.5 | 3640 | 97.1 ± 14.4 |
| 2-ethyl-6-methyl-pyrazine | 330 | 104.8 ± 8.4 | 1652 | 105.5 ± 6.5 | 3305 | 94.2 ± 10.7 |
| 2-ethyl-5-methyl-pyrazine | 85 | 97.8 ± 8.3 | 425 | 93.4 ± 7.9 | 851 | 113.6 ± 5.5 |
| Trimethyl-pyrazine | 336 | 112.9 ± 3.1 | 1680 | 109.7 ± 5.6 | 3360 | 97.9 ± 10.6 |
| 2,6-diethyl-pyrazine | 256 | 96.1 ± 6.5 | 1280 | 107.8 ± 8.1 | 2560 | 115.4 ± 7.2 |
| 3-ethyl-2,5-dimethyl-pyrazine | 252 | 102.2 ± 6.7 | 1260 | 112.3 ± 15.7 | 2520 | 106.7 ± 7.9 |
| 2-ethyl-3,5-dimethyl-pyrazine | 314 | 112.0 ± 14.0 | 1570 | 106.7 ± 6.3 | 3140 | 91.9 ± 9.4 |
| 3,5-diethyl-2-methyl-pyrazine | 284 | 105.3 ± 10.3 | 1420 | 109.1 ± 6.23 | 2840 | 110.5 ± 6.5 |
| Isopropenyl-pyrazine | 308 | 98.7 ± 7.1 | 1540 | 95.34 ± 9.1 | 3080 | 93.0 ± 10.2 |
| 5H-5-Methyl-6,7-dihydrocyclopenta-pyrazine | 98 | 96.5 ± 5.1 | 490 | 109.2 ± 6.7 | 980 | 111.7 ± 11.0 |
3.4. Analysis of Real Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, X.; Jin, Q.; Liu, Y.; Huang, J.; Wang, X.; Mao, W.; Wang, S. Changes in Volatile Compounds of Peanut Oil during the Roasting Process for Production of Aromatic Roasted Peanut Oil. J. Food Sci. 2011, 76, C404–C412. [Google Scholar] [CrossRef]
- Guerra, P.V.; Yaylayan, V.A. Dimerization of Azomethine Ylides: An Alternate Route to Pyrazine Formation in the Maillard Reaction. J. Agric. Food Chem. 2010, 58, 12523–12529. [Google Scholar] [CrossRef]
- An, A.; Kimpe, N.D. Formation of pyrazines from ascorbic acid and amino acids under dry-roasting conditions—ScienceDirect. Food Chem. 2009, 115, 1417–1423. [Google Scholar]
- Kwon, T.Y.; Park, J.S.; Jung, M.Y. Headspace–Solid Phase Microextraction–Gas Chromatography–Tandem Mass Spectrometry (HS-SPME-GC-MS2) Method for the Determination of Pyrazines in Perilla Seed Oils: Impact of Roasting on the Pyrazines in Perilla Seed Oils. J. Agric. Food Chem. 2013, 61, 8514–8523. [Google Scholar] [CrossRef] [PubMed]
- Gama, A.P.; Adhikari, K. Sensory Characterization of Dominant Malawi Peanut Varieties After Roasting. J. Food Sci. 2019, 84, 1554–1562. [Google Scholar] [CrossRef]
- Jia, X.; Zhou, Q.; Wang, J.; Liu, C.; Huang, F.; Huang, Y. Identification of key aroma-active compounds in sesame oil from microwaved seeds using E-nose and HS-SPME-GC×GC-TOF/MS. J. Food Biochem. 2019, 43, e12786. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Zhu, X.; Ai, Z.; Ai, Y.; Qiu, F.; Ni, D. Effect of different drying methods on the sensory quality and chemical components of black tea. LWT Food Sci. Technol. 2019, 99, 112–118. [Google Scholar] [CrossRef]
- Baker, G.L.; Cornell, J.A.; Gorbet, D.W.; O’Keefe, S.F.; Talcott, S.T. Determination of Pyrazine and Flavor Variations in Peanut Genotypes During Roasting. J. Food Sci. 2010, 68, 394–400. [Google Scholar] [CrossRef]
- Shimoda, M.; Shiratsuchi, H.; Nakada, Y.; Yin, W.; Osajima, Y. Identification and Sensory Characterization of Volatile Flavor Compounds in Sesame Seed Oil. J. Agric. Food Chem. 1996, 44, 3909–3912. [Google Scholar] [CrossRef]
- Poehlmann, S.; Schieberle, P. Characterization of the Aroma Signature of Styrian Pumpkin Seed Oil (Cucurbita pepo subsp. pepo var. Styriaca) by Molecular Sensory Science. J. Agric. Food Chem. 2013, 61, 2933–2942. [Google Scholar] [CrossRef]
- Zhou, Q.; Xiao, J.; Yao, Y.; Wang, B.; Huang, F. Characterization of the Aroma-Active Compounds in Commercial Fragrant Rapeseed Oils via Monolithic Material Sorptive Extraction. J. Agric. Food Chem. 2019, 67, 11454–11463. [Google Scholar] [CrossRef] [PubMed]
- Kanavouras, A.; Kiritsakis, A.; Hernandez, R.J. Comparative study on volatile analysis of extra virgin olive oil by dynamic headspace and solid phase micro-extraction. Food Chem. 2005, 90, 69–79. [Google Scholar] [CrossRef]
- David, F. 4.21—Application of Stir-Bar Sorptive Extraction in Food Analysis. In Comprehensive Sampling and Sample Preparation; Pawliszyn, J., Ed.; Academic Press: Oxford, UK, 2012; pp. 473–493. [Google Scholar] [CrossRef]
- Cecchi, T.; Alfei, B. Volatile profiles of Italian monovarietal extra virgin olive oils via HS-SPME-GC-MS: Newly identified compounds, flavors molecular markers, and terpenic profile. Food Chem. 2013, 141, 2025–2035. [Google Scholar] [CrossRef]
- Matheis, K.; Granvogl, M. Characterization of Key Odorants Causing a Fusty/Musty Off-Flavor in Native Cold-Pressed Rapeseed Oil by Means of the Sensomics Approach. J. Agric. Food Chem. 2016, 64, 8168–8178. [Google Scholar] [CrossRef] [PubMed]
- Pawliszyn, J. Handbook of Solid Phase Microextraction; Elsevier: Waltham, MA, USA, 2012. [Google Scholar]
- Pasqualone, A.; Paradiso, V.M.; Summo, C.; Caponio, F.; Gomes, T. Influence of Drying Conditions on Volatile Compounds of Pasta. Food Bioprocess Technol. 2014, 7, 719–731. [Google Scholar] [CrossRef]
- Giannone, V.; Giarnetti, M.; Spina, A.; Todaro, A.; Pecorino, B.; Summo, C.; Caponio, F.; Paradiso, V.M.; Pasqualone, A. Physico-chemical properties and sensory profile of durum wheat Dittaino PDO (Protected Designation of Origin) bread and quality of re-milled semolina used for its production. Food Chem. 2018, 241, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, S.; Tamogami, S.; Chen, J.; Zhang, H. Volatile Profile and Flavor Characteristics of Ten Edible Oils. Anal. Lett. 2021, 54, 1423–1438. [Google Scholar] [CrossRef]
- Song, N.-E.; Lee, J.-Y.; Lee, Y.-Y.; Park, J.-D.; Jang, H.W. Comparison of headspace–SPME and SPME-Arrow–GC–MS methods for the determination of volatile compounds in Korean salt–fermented fish sauce. Appl. Biol. Chem. 2019, 62, 1–8. [Google Scholar] [CrossRef]
- Nam, T.G.; Lee, J.Y.; Kim, B.K.; Song, N.E.; Jang, H.W. Analyzing volatiles in brown rice vinegar by headspace solid-phase microextraction (SPME)–Arrow: Optimizing the extraction conditions and comparisons with conventional SPME. Int. J. Food Prop. 2019, 22, 1195–1204. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Kim, W.S.; Lee, Y.-Y.; Choi, Y.-S.; Choi, H.; Jang, H.W. Solid-phase microextraction Arrow for the volatile organic compounds in soy sauce. J. Sep. Sci. 2019, 42, 2942–2948. [Google Scholar] [CrossRef]
- Manousi, N.; Rosenberg, E.; Zachariadis, G.A. Solid-Phase Microextraction Arrow for the Sampling of Volatile Organic Compounds in Milk Samples. Separations 2020, 7, 75. [Google Scholar] [CrossRef]
- Kim, S.J.; Yoon, H.N.; Rhee, J.S. The effects of roasting temperatures on the formation of headspace volatile compounds in perilla seed oil. J. Am. Oil Chem. Soc. 2000, 77, 451–456. [Google Scholar] [CrossRef]
- Zhang, W.; Rui, W.; Yuan, Y.; Yang, T.; Liu, S. Changes in volatiles of palm kernel oil before and after kernel roasting. LWT Food Sci. Technol. 2016, 73, 432–441. [Google Scholar] [CrossRef]
- Ezquerro, Ó.; Pons, B.; Tena, M.A.T. Evaluation of multiple solid-phase microextraction as a technique to remove the matrix effect in packaging analysis for determination of volatile organic compounds. J. Chromatogr. A 2003, 1020, 189–197. [Google Scholar] [CrossRef]
- Genovese, A.; Yang, N.; Linforth, R.; Sacchi, R.; Fisk, I. The role of phenolic compounds on olive oil aroma release. Food Res. Int. 2018, 112, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Pickard, S.; Becker, I.; Merz, K.H.; Richling, E. Determination of the alkylpyrazine composition of coffee using stable isotope dilution-gas chromatography-mass spectrometry (SIDA-GC-MS). J. Agric. Food Chem. 2013, 61, 6274–6281. [Google Scholar] [CrossRef]
- Suzuki, M.; Tsuge, S.; Takeuchi, T. Gas chromatographic estimation of occluded solvents in adhesive tape by periodic introduction method. Anal. Chem. 1970, 42, 1705–1708. [Google Scholar] [CrossRef]
- Mcauliffe, C.D. GC Determination of Solutes by Multiple Phase Equilibration. Chem. Technol. 1971, 1, 46–51. [Google Scholar]
- Kolb, B. Multiple headspace extraction—A procedure for eliminating the influence of the sample matrix in quantitative headspace, gas chromatography. Chromatographia 1982, 15, 587–594. [Google Scholar] [CrossRef]
- Ezquerro, Ó.; Pons, B.; Tena, M.A.T. Direct quantitation of volatile organic compounds in packaging materials by headspace solid-phase microextraction–gas chromatography–mass spectrometry. J. Chromatogr. A 2003, 985, 247–257. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.L.; García-Barrera, T.; Lorenzo, F.; Beltrán, R. Use of multiple headspace solid-phase microextraction and pervaporation for the determination of off-flavours in wine. J. Chromatogr. A 2006, 1112, 133–140. [Google Scholar] [CrossRef]
- Canellas, E.; Vera, P.; Nerin, C. Multiple headspace-solid phase microextraction for the determination of migrants coming from a self-stick label in fresh sausage. Food Chem. 2016, 197, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Sgorbini, B.; Cagliero, C.; Acquadro, S.; Marengo, A.; Cordero, C.; Liberto, E.; Bicchi, C.; Rubiolo, P. Evaluation of volatile bioactive secondary metabolites transfer from medicinal and aromatic plants to herbal teas: Comparison of different methods for the determination of transfer rate and human intake. J. Chromatogr. A 2019, 1594, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Xu, S.; Wang, X.; Jin, Q.; Xu, X.; Shen, Y.; Xu, G.; Zhang, H. Analysis of the volatile components of tea seed oil (Camellia sinensis O. Ktze) from China using HS-SPME-GC/MS. Int. J. Food Sci. Technol. 2016, 51, 2591–2602. [Google Scholar] [CrossRef]
- Tena, M.T.; Carrillo, J.D. Multiple solid-phase microextraction: Theory and applications. TrAC Trends Anal. Chem. 2007, 26, 206–214. [Google Scholar] [CrossRef]
- Martínez-Uruuela, A.; González-Sáiz, J.; Pizarro, C. Optimisation of the derivatisation reaction and subsequent headspace solid-phase microextraction method for the direct determination of chlorophenols in red wine. J. Chromatogr. A 2004, 1048, 141–151. [Google Scholar] [CrossRef]
- Schieberle, P.; Grosch, W. Evaluation of the flavour of wheat and rye bread crusts by aroma extract dilution analysis. Z. Lebensm. Unters. Forsch. 1987, 185, 111–113. [Google Scholar] [CrossRef]
- Van Lancker, F.; Adams, A.; De Kimpe, N. Formation of pyrazines in Maillard model systems of lysine-containing dipeptides. J. Agric. Food Chem. 2010, 58, 2470–2478. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, X.; Gao, L.; Jin, J.; Zhong, Q.; Sun, C.; Yan, L.; Liu, R.; Akoh, C.C.; Jin, Q. Quality of Wood-Pressed Rapeseed Oil. J. Am. Oil Chem. Soc. 2017, 94, 767–777. [Google Scholar] [CrossRef]
| Peanut Oil | Sesame Oil | Rapeseed Oil | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (mg/kg) | R2 | Slope | Concentration (mg/kg) | R2 | Slope | Concentration (mg/kg) | R2 | Slope | |
| 2-methyl-pyrazine | 2.87 ± 0.18 | 0.9962 | −0.71 | 15.14 ± 2.46 | 0.9943 | −0.47 | 0.58 ± 0.00 1 | 0.9948 | −0.56 |
| 2,5-dimethyl-pyrazine | 3.23 ± 0.21 | 0.9990 | −0.75 | 9.50 ± 1.01 | 0.9945 | −0.56 | 1.07 ± 0.00 | 0.9979 | −0.73 |
| 2,6-dimethyl-pyrazine | 1.10 ± 0.07 | 0.9974 | −0.74 | 3.98 ± 0.41 | 0.9937 | −0.54 | 0.30 ± 0.02 | 0.9991 | −0.65 |
| Ethyl-pyrazine | 0.38 ± 0.02 | 0.9973 | −0.73 | 1.74 ± 0.12 | 0.9930 | −0.49 | 0.09 ± 0.00 | 0.9932 | −0.61 |
| 2-ethyl-6-methyl-pyrazine | 0.76 ± 0.06 | 0.9998 | −0.52 | 3.62 ± 0.71 | 0.9980 | −0.41 | 0.24 ± 0.00 | 0.9991 | −0.50 |
| 2-ethyl-5-methyl-pyrazine | 0.96 ± 0.07 | 0.9999 | −0.47 | 2.21 ± 0.46 | 0.9986 | −0.41 | 0.21 ± 0.00 | 0.9988 | −0.48 |
| Trimethyl-pyrazine | 1.07 ± 0.09 | 1.0000 | −0.48 | 5.49 ± 1.16 | 0.9989 | −0.38 | 0.29 ± 0.00 | 0.9995 | −0.46 |
| 2,6-diethyl-pyrazine | 0.06 ± 0.01 | 1.0000 | −0.34 | 0.26 ± 0.06 | 0.9996 | −0.27 | 0.02 ± 0.00 | 0.9997 | −0.32 |
| 3-ethyl-2,5-dimethyl-pyrazine | 0.83 ± 0.07 | 0.9998 | −0.31 | 5.08 ± 1.22 | 0.9996 | −0.25 | 0.55 ± 0.00 | 0.9999 | −0.29 |
| 2-ethyl-3,5-dimethyl-pyrazine | 0.12 ± 0.01 | 0.9997 | −0.29 | 0.54 ± 0.13 | 0.9991 | −0.24 | 0.03 ± 0.00 | 0.9897 | −0.28 |
| 3,5-diethyl-2-methyl-pyrazine | 0.05 ± 0.01 | 0.9965 | −0.19 | 0.23 ± 0.05 | 0.9970 | −0.16 | 0.02 ± 0.00 | 0.9959 | −0.17 |
| Isopropenyl-pyrazine | ND 2 | -- 3 | -- | 0.93 ± 0.22 | 0.9994 | −0.27 | ND | -- | -- |
| 5H-5-Methyl-6,7-dihydrocyclopenta-pyrazine | 0.06 ± 0.01 | 0.9964 | −0.21 | 0.47 ± 0.10 | 0.9862 | −0.18 | ND | -- | -- |
| Total pyrazines | 11.51 ± 0.78 | 49.18 ± 3.25 | 3.42 ± 0.03 | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Zhou, C.; Shi, H.; Zhang, H.; Bi, Y.; Xu, X. Processing of Flavor-Enhanced Oils: Optimization and Validation of Multiple Headspace Solid-Phase Microextraction-Arrow to Quantify Pyrazines in the Oils. Life 2021, 11, 390. https://doi.org/10.3390/life11050390
Xu Z, Zhou C, Shi H, Zhang H, Bi Y, Xu X. Processing of Flavor-Enhanced Oils: Optimization and Validation of Multiple Headspace Solid-Phase Microextraction-Arrow to Quantify Pyrazines in the Oils. Life. 2021; 11(5):390. https://doi.org/10.3390/life11050390
Chicago/Turabian StyleXu, Ziyan, Chuan Zhou, Haiming Shi, Hong Zhang, Yanlan Bi, and Xuebing Xu. 2021. "Processing of Flavor-Enhanced Oils: Optimization and Validation of Multiple Headspace Solid-Phase Microextraction-Arrow to Quantify Pyrazines in the Oils" Life 11, no. 5: 390. https://doi.org/10.3390/life11050390
APA StyleXu, Z., Zhou, C., Shi, H., Zhang, H., Bi, Y., & Xu, X. (2021). Processing of Flavor-Enhanced Oils: Optimization and Validation of Multiple Headspace Solid-Phase Microextraction-Arrow to Quantify Pyrazines in the Oils. Life, 11(5), 390. https://doi.org/10.3390/life11050390







